Introduction
Subglottic stenosis is defined as a narrowing of the subglottic airway, which can present as a life-threatening airway emergency. Subglottic stenosis is a result of abnormal wound healing processes that lead to hypertrophic scar formation and obstruction of the airway lumen by excess granulation tissue. Unfortunately, wound healing is a dynamic and complex process mediated by a wide range of co-ordinated cellular reactions. These reactions can be influenced by both local and systemic parameters, such as infection, pressure, tissue necrosis, age and other patient co-morbidities.Reference Hirshoren and Eliashar1 Treatments have been investigated in animal studies and clinical practice for a number of years. The most widely practised treatments include: systemic antibiotics and systemic steroids, hyperbaric oxygen, anti-inflammatory agents, anti-reflux therapy, and, finally, open or endoscopic surgical repair.Reference Nakagishi and Morimoto2
A number of animal species, including porcine and rabbit, have been used in the creation and examination of subglottic stenosis, as it is essential to establish experimental animal models in which pathological changes are similar to those observed in clinical cases. Rabbit models have been studied using laser-induced or direct scraping subglottic injury; this resulted in stenosis in all injured rabbits compared with controls.Reference Roh, Lee and Park3 Histological examination of injured tissues has revealed mucosal ulceration, inflammation and granulation tissue formation during the acute phase after wounding.Reference Branski, Sandulache, Dohar and Hebda4 Similarly, a porcine model has been studied in which piglets underwent repeated injury with a sharpened metal rod, with the result that stenosis was induced, and stenosis increased with repeated injuries.Reference Mitskavich, Rimell and Shapiro5 These investigations make apparent the need for a direct penetrating mucosal injury that disrupts the epithelium in order to create a reliable model of acquired subglottic stenosis. At this point in time, there is no standard animal model for the study of airway stenosis.
Unfortunately, limitations in animal size and airway calibre prevent the use of many smaller animal species for in situ examination of subglottic disease. Recently, a heterotopic model of transplantation was described to study obliterative bronchiolitis, which is a fibrotic process causing airway luminal obliteration.Reference Hertz, Jessurun, King, Savik and Murray6 In this model, donor mouse tracheas were transplanted and placed into the subcutaneous tissue of recipient immunocompetent mice. In similar models, it has been demonstrated that airway epithelial injury in heterotopic transplantation appears to enhance the rejection process, causing formation of obliterative tissues, which can then be used to further study immune processes and histochemical tissue changes.Reference Kuo and Bharat7
Obliterative bronchiolitis shares many of the same fibrotic features of subglottic stenosis, so it follows that such an ex situ approach in a murine model might be similarly productive. One prior murine model has been documented, in which intense and focal injury to the posterior cricoid using cautery was carried out before transplantation of one donor laryngotracheal complex into the subcutaneous tissue of a genetically equivalent recipient, with the result that lamina propria thickness seemed to be greatest in previously injured tracheas.Reference Richter, Mehta, Albert and Elluru8 The authors concluded that heterotopic transplanted laryngotracheal complexes in the mouse may be a reasonable model for investigating subglottic stenosis. This animal model may provide a mechanism for investigating subglottic stenosis with reduced experimental cost and increased flexibility in experimental manipulation compared with in vivo models of subglottic stenosis.Reference Richter, Mehta, Albert and Elluru8
We aimed to develop a functional model of subglottic stenosis using heterotopic transplanted mouse tracheas. We induced airway irritation through the use of acid, employed to simulate the effect of reflux, and wire brush trauma in order to replicate the effect of endotracheal intubation, thus allowing for a clinically relevant model of airway injury. We hypothesised that this irritation would result in the formation of granulation tissue, providing a model which can then be used to develop effective prevention strategies. Through this technique, we hoped to provide an efficient tool for investigating the development and treatment of subglottic stenosis, by cultivating granulation tissue for further characterisation and allowing the consideration of new ways to stabilise airway epithelium in order to prevent such an outcome.
Materials and methods
Experimental design
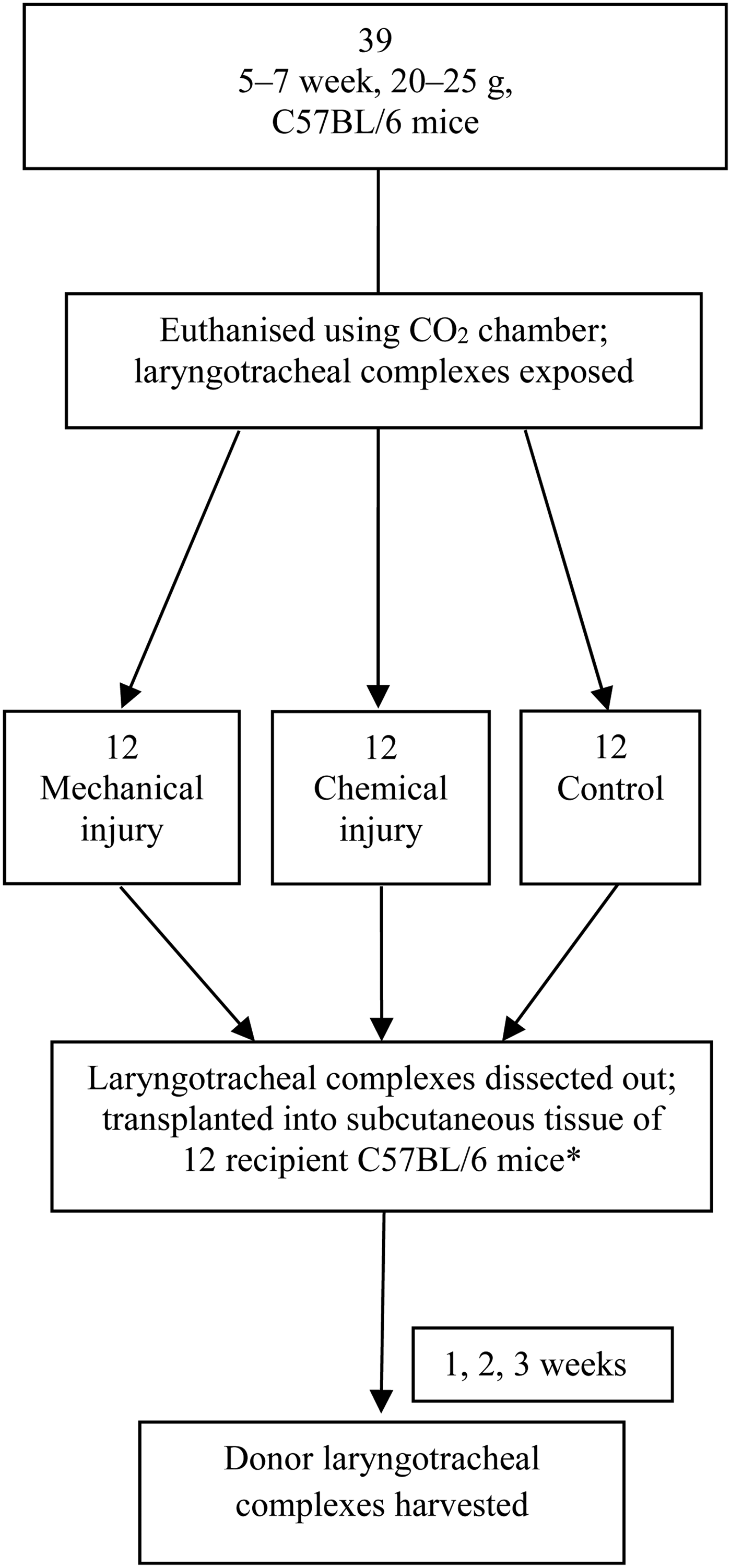
Laryngotracheal complex transplants were performed using donor tracheal segments from five- to seven-week-old, 20–25 g, common, inbred laboratory type C57BL/6 mice (Jackson Laboratories, Bar Harbor, Maine, USA). Laryngotracheal complexes from the 39 donor C57BL/6 mice were harvested and divided into 3 groups: an uninjured group, a group mechanically injured using a wire brush and a group chemically injured using hydrochloric acid.
The C57BL/6 donor tracheal segments were transplanted into the deep subcutaneous tissue of 12 recipient wild-type C57BL/6 mice (Jackson Laboratories); 1 donor laryngotracheal complex from each group was placed in deep dorsal subcutaneous pockets of recipient C57BL/6 mice, for a total of 3 transplanted laryngotracheal complexes per recipient mouse (Figure 1). One set of donor tracheal segments was not transplanted and was instead used for week zero time point data.

Fig. 1 Experimental design. *One set of tracheal specimens taken for week zero assessment. CO2 = carbon dioxide
Recipient mice were monitored daily for evidence of infection or extrusion of transplanted donor tracheas.
All animal studies were performed in accordance with the Institutional Animal Care and Use Committee of the Philadelphia Veterans' Affairs Medical Center guidelines. All C57BL/6 mice were housed in an Association for Assessment of Laboratory Animal Care approved facility, within a pathogen-free environment, with climate-controlled rooms and free access to standard pelleted food and sterile water.
Laryngotracheal complex transplantation
Donor C57BL/6 mice were euthanised using a compressed carbon dioxide (CO2) chamber. Once mice were confirmed dead, a vertical mental to sternal incision was made and the laryngotracheal complex was exposed through careful dissection.
The uninjured group served as controls, with direct harvesting and transplanting of the laryngotracheal complexes. Laryngotracheal complexes in the injured groups were kept in situ and exposed to direct subglottic insult through one of two methods: mechanical or chemical injury. The mechanical injury group underwent direct mucosal scraping by passing a wire brush with a diameter of 0.1778 mm through a pharyngotomy incision back and forth a total of 15 times, thus abrading the subglottic mucosa. The chemical injury group received an injection of 0.5 ml hydrochloric acid titrated to a pH of 4 to the subglottic mucosa. After 5 minutes, the acid was irrigated out of the trachea using a saline injection. Uninjured and injured laryngotracheal complexes were dissected out and placed in saline to await transplantation into the recipient mouse.
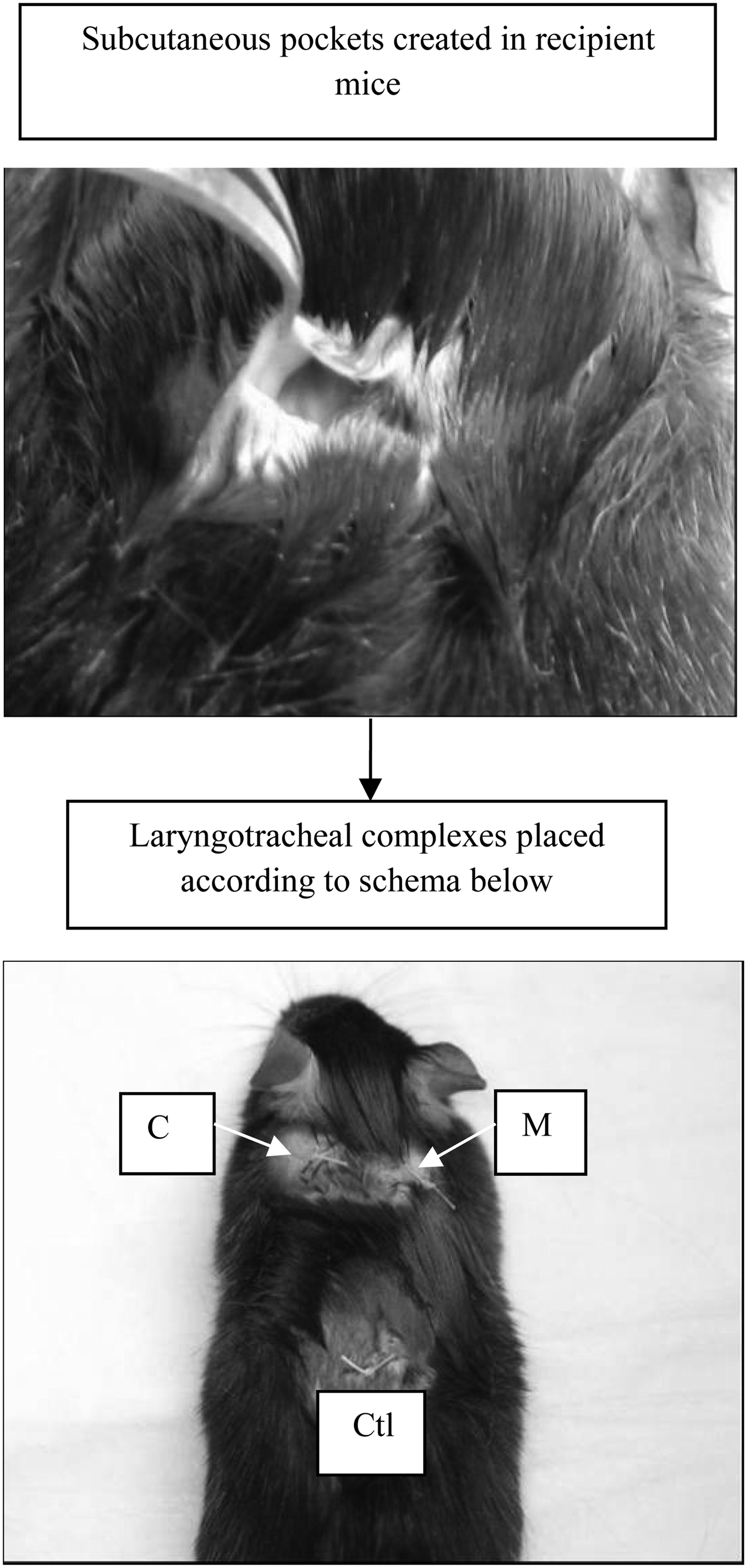
Recipient mice were weighed and anaesthetised with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (16 mg/kg). Once anaesthetised, the mice were shaved along the dorsum and hindside. After surgical preparation and using a sterile technique, small 0.5 cm horizontal incisions were made on the left and right dorsum and on the hindside. Connective tissue was pulled back to create three deep subcutaneous pockets, into which a transplanted trachea was placed (Figure 2). After each trachea was placed into its respective pocket, incisions were carefully sutured with 4.0 Vicryl® sutures, averaging one to two stitches per incision. The recipient mouse was then placed on a warmer and observed until it began to regain consciousness.

Fig. 2 Donor trachea transplantation into recipient mice. C = chemical injury; M = mechanical injury; Ctl = control
Recipient mice were treated with oral acetaminophen for post-operative pain and monitored daily for signs of infection. At time points of one, two and three weeks after surgery, transplanted laryngotracheal complexes were harvested from the C57BL/6 recipient mice. This was done by euthanising the recipient mice in a compressed CO2 chamber and opening the previously made incisions to carefully dissect out each transplanted specimen (Figure 3).

Fig. 3 Donor trachea harvest. *Indicates the gross appearance of intact trachea with surrounding fibrotic capsule and angiogenesis.
Histopathological analysis
Once donor tracheas were removed, specimens were placed in a 10 per cent formalin solution for fixation. Each specimen was placed in a separate paraffin block, from which four slides of 5 µm thickness were made per 1 mm of tissue. Slides were stained with haematoxylin and eosin. Representative slides from each layer were then assessed by pathologists blinded to the injury status of the laryngotracheal complex to determine the formation of granulation. Granulation tissue was identified by the presence of fibroblasts and angiogenesis in the lamina propria. At the time of microscopic examination, digital photos of each set of slides were taken at a number of magnifications, and the thickness of the lamina propria was determined under 40× magnification. For each specimen, thickness was recorded at three discrete points at the thickest portions of the lamina propria, uniformly measured from the medial surface of the tracheal cartilaginous ring to the basement membrane of the epithelium.
Statistical analysis
Lamina propria thickness data in this study are presented as means ± standard deviations. For each experimental group of tracheas, an analysis of variance (ANOVA) was performed and differences between each time point were examined. Furthermore, for each time point, an ANOVA was performed, and differences in trachea lamina propria thickness between experimental groups and control group were assessed. Statistical significance in all cases was defined as p < 0.05.
Results
Laryngotracheal complex specimens from the control, chemical injury and mechanical injury groups were harvested from C57BL/6 mice. Histologically assessable tissue was recovered from all of the 39 harvested specimens. Furthermore, lamina propria thickness data were taken from 36 harvested specimens (after transplantation for one, two or three weeks).
Histological analysis
Week zero
Week zero specimens (n = 1) were used to assess the degree of damage immediately following injury and to demonstrate the absence of any precursors to granulation at baseline. Uninjured control tracheas showed preserved epithelium immediately after dissection; these specimens were histologically normal (Figure 4a and 4b). Attenuated epithelium and exposure of basement membrane was seen in the experimental specimens immediately following both mechanical and chemical injury (Figure 4d–f); however, these specimens did not show evidence of immune infiltrate or fibroblasts at this early stage of injury.

Fig. 4 Histopathological analysis (H&E staining) at week zero. Low (a) and high (b) magnifications of uninjured control trachea immediately after dissection; note preserved epithelium. Low (c) and high (d) magnifications of harvested trachea immediately after chemical injury; note attenuated epithelium and exposed basement membrane. Low (e) and high (f) magnifications of harvested trachea immediately after mechanical injury; note attenuated epithelium and exposure of basement membrane. E = epithelium; L = lumen; BM = basement membrane; C = cartilage
Week one
Control tracheas harvested one week after explant (n = 2) appeared to have continued preservation of epithelium (Figure 5a and 5b) and no evidence of tissue remodelling. In contrast, high and low magnification of chemically injured tracheas showed epithelium that appeared denuded and basement membrane that was exposed; this was also seen in mechanically injured tracheas harvested at one week (Figure 5c–f). While the experimental groups did not have demonstrable tissue remodelling at this time, it was clear that an immune infiltrate had begun to appear as a result of exposed basement membrane; this is an important precursor to granulation formation.

Fig. 5 Histopathological analysis (H&E staining) at week one. Low (a) and high (b) magnifications of control trachea harvested one week after explant; note continued preservation of epithelium. Low (c) and high (d) magnifications of chemically injured trachea harvested at one week; note that epithelium appears denuded and basement membrane is exposed. Low (e) and high (f) magnifications of mechanically injured trachea harvested at one week; note continued attenuation of epithelium and exposure of basement membrane. C = cartilage; E = epithelium; L = lumen; BM = basement membrane
Week two
Evidence of granulation tissue began to appear. Although high and low magnifications of control tracheas harvested two weeks after explant still appeared histologically normal (Figure 6a and 6b), chemically injured tracheas harvested at this time showed evidence of tissue remodelling, with extrusion of granulation tissue (composed primarily of fibroblasts) into tracheal lumen from underneath previously disrupted epithelium (Figure 6c and 6d). There was also evidence of angiogenesis in these specimens. Similarly, mechanically injured tracheas harvested at two weeks showed invagination of granulation (with fibroblasts and evidence of angiogenesis) into the tracheal lumen from underneath disrupted airway epithelium (Figures 6e and 6f).

Fig. 6 Histopathological analysis (H&E staining) at week two. Low (a) and high (b) magnifications of control trachea harvested two weeks after explant show preservation of epithelium. Low (c) and high (d) magnifications of chemically injured trachea harvested at two weeks show extrusion of fibroblastic and granulation tissue into tracheal lumen from underneath previously disrupted epithelium; note angiogenesis. Low (e) and high (f) magnifications of mechanically injured trachea harvested at two weeks show invagination of granulation into the tracheal lumen from underneath disrupted airway epithelium. E = epithelium; L = lumen; C = cartilage; G = granulation; A = angiogenesis
Week three
Of the three-week specimens taken from the C57BL/6 recipient mice (n = 7), the control specimens appeared histologically normal. Apart from luminal debris of sloughed epithelium and inflammatory cells, airway epithelium was notably preserved, and there was no evidence of tissue remodelling (Figures 7a and 7b). In the chemically and mechanically injured specimens, there was evidence of 25–75 per cent obstruction of the tracheal lumen, with granulation tissue in the lamina propria beneath notably attenuated airway epithelium (Figures 7c–f). Subepithelial granulation was demonstrated by the presence of fibroblasts, angiogenesis, and inflammation with a predominantly lymphocytic infiltrate. In some cases, as demonstrated in Figure 7c, early fibroproliferative changes were noted in the lamina propria.

Fig. 7 Histopathological analysis (H&E staining) at week three. Low (a) and high (b) magnifications of control trachea harvested three weeks after explant show preservation of airway epithelium. Low (c) and high (d) magnifications of chemically injured trachea harvested at three weeks show areas of early-stage healing in the form of granulation and later stage of fibrosis. Low (e) and high (f) magnifications of mechanically injured trachea harvested at three weeks shows significant invagination of granulation into the tracheal lumen from underneath disrupted airway epithelium; note resultant narrowing of lumen; L = lumen; E = epithelium; C = cartilage; G = granulation; A = angiogenesis
Lamina propria thickness
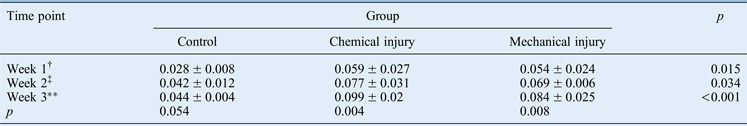
Lamina propria thickness is a measure of subglottic stenosis in both ex situ and in vivo models, depicting cellular infiltration beneath disrupted epithelium. Table I illustrates the mean lamina propria thicknesses of laryngotracheal complexes harvested from C57BL/6 mice at one, two and three weeks post-transplantation.
Table I Time point comparison of lamina propria thickness*

Data represent mean (± standard deviation) lamina propria thicknesses (in millimetres), unless indicated otherwise. *In specimens harvested from C57BL/6 mice. †n = 2; ‡n = 3; **n = 7
There was an observable and statistically significant thickness increase in laryngotracheal complexes harvested from C57BL/6 mice between one and three weeks, in both the chemical injury (p = 0.008) and the mechanical injury groups (p = 0.004). The difference between control laryngotracheal complexes in donor tracheas harvested at one week and those harvested at three weeks was borderline significant (p = 0.054). The statistically significant differences between lamina propria thicknesses in all experimental groups over time are graphically depicted in Figure 8. Furthermore, comparison of the lamina propria thickness in the control trachea and experimentally injured groups revealed a significant difference between each group of specimens within each time point (Figure 8). This demonstrates that the tracheas of both experimental groups had significantly thicker lamina propria than those in the control at any given time point.

Fig. 8 Graphic comparison of granulation formation in specimens harvested from C57BL/6 mice. Note that all tracheal specimens have increasing lamina propria thickness with a longer transplant incubation period, more apparent in the chemical and mechanical injury groups.
Discussion
This experiment presents a novel model to study the granulation that leads to eventual subglottic stenosis. We proposed using a heterotopic mouse transplantation model to study the effect of airway epithelial injury on granulation formation, an early fibroproliferative response to airway irritation, within the subglottic airway. We hypothesised that transplanted laryngotracheal complexes from donor mice subjected to airway epithelial injury would demonstrate histological tissue remodelling, resulting in granulation tissue formation.
The findings obtained indicate that direct injury of the airway epithelium, mechanical or chemical, results in significant granulation formation under the disrupted airway epithelium. These results are seen as early as two weeks following injury, but are pronounced at three weeks, with narrowing of the airway lumen and evidence of early fibrosis. The evolution of the injury through time could be seen in the histological specimens; these initially showed epithelial denuding and basement membrane exposure, followed by polymorphonuclear infiltrate and fibroblast proliferation, and resulted in granulation and evidence of angiogenesis. Thus, the experimental model was successful in showing the effect of airway injury on granulation formation over a three-week period, and is a functional and novel animal model.
Despite the significant trauma and the ischaemic time needed for all transplanted specimens, the model produced viable harvested laryngotracheal complexes, with little evidence of epithelial or cartilaginous necrosis. This is likely to be because of the relatively quick onset of angiogenesis within the recipient subcutaneous tissue. At harvest, the laryngotracheal complex was encapsulated in a stroma of highly vascular fibrotic tissue, allowing survival of the laryngotracheal complex even at the three-week post-transplantation harvest. The viability of the laryngotracheal complex was confirmed on histological evaluation, demonstrating maintenance of the subglottic epithelium, lamina propria and cartilage. Given the evidence of transplanted laryngotracheal complex viability, subglottic lumen measurements of injured and control laryngotracheal complexes could be obtained with confidence.
Importantly, the methods of direct airway injury used in this model simulate physiological mechanisms of epithelial irritation, as opposed to previous models which have focused on injury through electrocautery. Previous investigations revealed the need for a direct and penetrating injury that disrupts the lamina propria and perichondrium, to create a reliable model of acquired subglottic stenosis.Reference Dohar, Klein, Betsch and Hebda9 This model describes the formation of granulation following the manipulation of physiological and common clinically relevant variables such as laryngopharyngeal reflux (chemical injury) and endotracheal intubation (mechanical injury) to create deep subglottic injury; both processes are strongly implicated in the pathogenesis of subglottic stenosis. Thus, through using physiologically relevant variables, we have been able to produce a novel model of subglottic stenosis that may be clinically applicable.
Several potential limitations of this model were evident in this study. The tracheal graft is a large airway located subcutaneously, thereby eliminating the air–epithelium interface, and is not primarily vascularised, which may alter the immunopathogenesis of subglottic stenosis in this model. However, as the model reproduces the histopathology of human subglottic stenosis, these shortcomings do not preclude the use of this model in this study. Furthermore, given the small airway calibre in mice, it is evident that in situ injury of the mouse airway would result in increased morbidity and mortality. The formation of granulation is unpredictable, and could possibly obstruct the tracheal lumen of mice subjected to airway epithelial injury or even lead to the aspiration of granulation specimens, resulting in airway compromise. Airway compromise is the leading reason why larger animal species are not used in models of subglottic stenosis; increased morbidity and mortality leads to increased expense. Thus, this experimental murine model results in comparative ease of animal handling, as well as decreased morbidity and mortality within the subject population, while providing an inexpensive means to study the pathogenesis of subglottic stenosis. Another potential immunopathogenic limitation is the use of one recipient mouse for all three laryngotracheal complexes, wherein the immune response to one laryngotracheal complex may have interfered with the systemic response to the others. This issue will be elucidated in future studies involving the immune cascade within this model.
• A heterotopic mouse transplantation model was used to study the effect of airway epithelial injury on granulation formation within the subglottic airway
• Direct injury of airway epithelium resulted in significant granulation formation under the disrupted airway epithelium
• These effects were seen as early as two weeks following injury, but were pronounced at three weeks, with narrowing of airway lumen and evidence of early fibrosis
• Graft survival, subglottic trilaminar layer changes, and the difference between control and experimental laryngotracheal complexes suggest a reasonable model
• Ease in animal handling, low material costs and flexibility in experimental design are benefits of this model
In summary, the survival of the grafts and the changes in the subglottic trilaminar layer are consistent with other models of subglottic stenosis. Moreover, the significant difference between control and experimental laryngotracheal complexes in our study suggests that heterotopic transplanted laryngotracheal complexes in the mouse may be a reasonable model for investigating subglottic stenosis. Ease in animal handling, low material costs, and flexibility in experimental design are benefits of this model for investigating subglottic stenosis. This animal model holds promise for future investigations of subglottic stenosis and mucosal healing. Future studies of this model may employ immunohistochemical staining and polymerase chain reaction. In addition, knockout mice could be utilised in this model to identify and target wound healing mediators for treatment, as a novel way to stabilise airway epithelium and prevent subglottic stenosis.
Conclusion
This study describes a novel animal model for the study of subglottic stenosis. Increases in lamina propria thickness, the formation of histologically observable granulation tissue and the preservation of subglottic structure are consistent with the development of subglottic stenosis following airway epithelial injury. Ease in animal handling, low material costs and flexibility in experimental design are benefits of this model for investigating subglottic stenosis. This animal model may hold promise for future investigations of subglottic stenosis and mucosal healing.