INTRODUCTION
Environmental factors can cause differences in the morphology of bivalves (Laudien et al., Reference Laudien, Flint, van der Bank and Brey2003; Ubukata, Reference Ubukata2003). Also, morphological characteristics reflect the phylogenetic history, functionality, behaviour and life habits of an individual (Stanley, Reference Stanley1975). Consequently, the size and shape of bivalve shells could be useful as environmental indicators for differentiating between phenotypic stocks and species (Stanley, Reference Stanley1970; Soares et al., Reference Soares, Callahan and De Ruyck1998; Crampton & Maxwell, Reference Crampton, Maxwell, Harper, Taylor and Crame2000; Palmer et al., Reference Palmer, Pons and Linde2004; Sousa et al., Reference Sousa, Freire, Rufino, Méndez, Gaspar, Antunes and Guilhermino2007; Márquez et al., Reference Márquez, Robledo, Escati Peñaloza and Van der Molen2010).
Some previous studies have registered morphological variations in modern and fossil specimens of the same species by analysing the size and shape of their preserved remains (Stanley & Yang, Reference Stanley and Yang1987; Crampton & Maxwell, Reference Crampton, Maxwell, Harper, Taylor and Crame2000; Gordillo et al., Reference Gordillo, Márquez, Cárdenas and Zubimendi2011; Boretto et al., Reference Boretto, Baranzelli, Gordillo, Consolini, Zanchetta and Moran2014).
In Northern Patagonia, Argentina, particularly in the San Matías Gulf (SMG) (Figure 1), the shells of the bivalve Amiantis purpurata are most abundant and well preserved as fossil (Pleistocene and Holocene) specimens in the marine coastal assemblages as well as in the modern beaches (Bayer et al., Reference Bayer, Gordillo and Morsan2014). During the Patagonian Quaternary, glaciations and high-temperature interglacials produced palaeoenvironmental and geomorphological changes in littoral areas (Schellmann, Reference Schellmann and Kelletat1998; Schellmann & Radtke, Reference Schellmann and Radtke2003, Reference Schellmann and Radtke2010; Rabassa, Reference Rabassa and Rabassa2008; Ponce et al., Reference Ponce, Rabassa, Coronato and Borromei2011). One of the main global events registered in Patagonia was the Last Glacial Maximum at 24,000 cal. years bp (Rabassa, Reference Rabassa and Rabassa2008), when temperature and sea level were distinctly lower than at present (Clapperton, Reference Clapperton1993; Clark & Mix, Reference Clark and Mix2002). The Argentine Atlantic coast would have changed considerably, with the exposure of a large portion of the Argentine Continental Shelf and the advance of ice (Ponce et al., Reference Ponce, Rabassa, Coronato and Borromei2011). Most species, including A. purpurata, were able to survive these drastic environmental changes (Bayer et al., Reference Bayer, Gordillo and Morsan2014). However, other species, including at least three from the SMG – Chama iudicai (Pastorino, Reference Pastorino1991), Tegula atra (Lesson, 1830) and Glycymeris sanmatiensis (Bayer & Gordillo, Reference Bayer and Gordillo2013) – became extinct, apparently during the glaciations, and were only found in the oldest Quaternary deposits surrounding this gulf (Pastorino, Reference Pastorino1991; Bayer & Gordillo, Reference Bayer and Gordillo2013; Gordillo et al., Reference Gordillo, Bayer, Boretto and Charó2014; Charó et al., Reference Charó, Gordillo, Fucks and Giaconi2014). A warmer period called the Hypsithermal occurred during the middle Holocene (between 6000 and 4500 years bp), in which temperatures were slightly higher (Schellmann & Radtke, Reference Schellmann and Radtke2010) than during the rest of the Holocene and the sea level transgression produced geomorphological changes along the Patagonian coastline (Kokot et al., Reference Kokot, Codignotto and Elissondo2004; Favier-Dubois & Kokot, Reference Favier-Dubois and Kokot2011). This global event is reflected in changing marine mollusc assemblages (Gordillo et al., Reference Gordillo, Márquez, Cárdenas and Zubimendi2011; Charó et al., Reference Charó, Gordillo and Fucks2013).

Fig. 1. Location map showing late Quaternary deposits in San Antonio Bay, San Matías Gulf (Argentina).
The main objective of this study is to compare the size and shape of Amiantis purpurata shells from different stages of the late Quaternary (the last 100,000 years) in the SMG with respect to the environmental changes that took place during this interval in Northern Patagonia, Argentina.
Autoecology of A. purpurata
Amiantis purpurata is a suspension feeder which lives infaunally on fine sandy or silty-sand bottoms. This venerid is a warm-temperate water species, inhabiting the intertidal zone up to 15 m deep (Morsan, Reference Morsan2007). Its modern distribution extends from Espiritu Santo (Brazil) to the northern SMG in Argentina (Carcelles, Reference Carcelles1944; Castellanos, Reference Castellanos1967; Scarabino, Reference Scarabino1977; Morsan & Kroeck, Reference Morsan and Kroeck2005). Its southernmost known living population is at Villarino Beach (San Antonio Este, SMG), where the population occurs in high densities which reach 10 kg m−2 at some sites (Morsan, Reference Morsan2003). The fossil record of A. purpurata extends back to the late Pleistocene in San Antonio Bay, located in the SMG (Figure 1; Feruglio, Reference Feruglio1950; Angulo et al., Reference Angulo, Fidalgo, Gomez Peral and Schnack1978; Rutter et al., Reference Rutter, Schnack, del Rio, Fasano, Isla and Radtke1989; Morsan, Reference Morsan1997). A recent revision by Bayer et al. (Reference Bayer, Gordillo and Morsan2014) concluded that the species formed its richest southern population in the SMG throughout the whole Quaternary. The individuals from the SMG are slow-growing and can live for over 40 years (Morsan, Reference Morsan2000; Morsan & Kroeck, Reference Morsan and Kroeck2005).
The San Matías Gulf
This gulf exhibits a multiplicity of geomorphological features and littoral deposits assigned to two main Quaternary transgressive episodes which occurred during the late Pleistocene and Holocene (Angulo et al., Reference Angulo, Fidalgo, Gomez Peral and Schnack1978; Martínez et al., Reference Martínez, Nañes, Lizuain, Dal Molin and Turel2001; Fucks et al., Reference Fucks, Schnak and Charó2012).
San Antonio Bay is located in the north-western area of the SMG and is a tidal delta (40°42′/40°50′ S and 64°43′/65°07′ W) (Figure 1) that was flooded repeatedly by marine transgressions (Rutter et al., Reference Rutter, Schnack, del Rio, Fasano, Isla and Radtke1989; Ponce et al., Reference Ponce, Rabassa, Coronato and Borromei2011). This bay has a particular circulation pattern characterized by a low rate of water renewal (Mazio & Vara, Reference Mazio and Vara1983; Lanfredi & Pousa, Reference Lanfredi and Pousa1988). This feature is also characteristic of the northern SMG, and is the main cause of the increase in water temperature. Additionally, the lack of rain, the absence of natural freshwater input and the high evaporation rate all contribute to higher salinities, which contrast the colder and less saline waters from the southern sector of the gulf (Scasso & Piola, Reference Scasso and Piola1988; Rivas & Beier, Reference Rivas and Beier1990; Gagliardini & Rivas, Reference Gagliardini and Rivas2004).
MATERIALS AND METHODS
Amiantis purpurata shells and study sites
Fossil and modern specimens of Amiantis purpurata (Figure 2) were collected at localities along the SMG, in the northern Argentine Patagonia (Figure 1). Fossil shells from the late Pleistocene (MIS5e, i.e. before the Last Glacial Maximum; 107,000–42,500 years bp; Rutter et al., Reference Rutter, Radke and Schnack1990) and the late Holocene (3730–2880 years bp), as well as from modern beaches were taken randomly from the exposed marine deposits. In total, 419 shells were used in the linear morphometric analyses, while 88 right valves were studied by elliptic Fourier analysis (EFA).

Fig. 2. Views of Amiantis purpurata. (A) external view of a modern shell; (B) internal view of a modern shell; (C) internal view of a Holocene shell; (D) internal view of a Pleistocene shell. Scale bars: 1 cm.
Linear morphometric analysis
Length and height measurements of shells from the different ages were compared with ANOVA. A Tukey test was conducted in order to identify which set of shells showed shell length and height differences.
In order to evaluate whether there were shape changes during ontogeny, differences in allometric relationships at the three sets of shells were assessed by the model H t = a L t b (Seed, Reference Seed, Rhoads and Lutz1980), where Ht is shell height and Lt is shell length. In order to estimate parameters of the model at each site, log-transformed shell height data were regressed against log-transformed shell length and significant departures from isometry were evaluated with t-tests (Zar, Reference Zar2010).
Outline analysis (contour shape)
From the total number of shells (N = 419), 88 right valves (41 Pleistocene, 22 Holocene and 25 modern) were used for the outline analysis.
The shell shape variation was studied by EFA, which consists of decomposing a curve into a sum of harmonically related ellipses (Lestrel, Reference Lestrel1997). For each valve, images with the inner region upward were photographed using a Sony Cyber-shot (DSC-W610) digital camera. The closed contours of each valve outline were obtained as chain-coded data from the digital images (Freeman, Reference Freeman1974). The number of harmonics (n) was calculated following Crampton (Reference Crampton1995). The Fourier series was truncated at n = 10 with an average cumulative power of 99.98% of the total average power. The orientation, size and starting point of the different outlines were standardized (Kuhl & Giardina, Reference Kuhl and Giardina1982) so that three of the four elliptic Fourier coefficients describing the first harmonic ellipse were constant for all outlines. The software Shape v.1.3 (Iwata & Ukai, Reference Iwata and Ukai2002) was used for all the analyses. Principal component analysis (PCA) of the variance–covariance matrix (Rohlf & Archie, Reference Rohlf and Archie1984; Crampton, Reference Crampton1995) was applied to summarize shape variation based on harmonic coefficients for each valve. Differences in the coefficients between ages (Pleistocene, Holocene and Modern) were tested using multivariate analysis of variance (MANOVA) and a Hotelling Bonferroni test (P < 0.05) in order to identify which assemblage was different. The average +2 standard deviation (SD) shape for each group was reconstructed from the mean values of Fourier coefficients using the inverse Fourier transformations (provided by SHAPE-PrinPrint).
An analysis of covariance (ANCOVA) was carried out in order to evaluate whether valve shape differs when valve size varies, using the sets of shells (Pleistocene, Holocene and modern beaches) as a classification factor, principal components as variables, and size (geometric mean, i.e. square root (length × height); Kosnik et al., Reference Kosnik, Jablonski, Lockwood and Novack-Gottshall2006) as a covariable. Slope homogeneity was also performed to identify which set of shells registered a different shape variation associated to shell size. Statistical analyses were processed using the software Infostat (Di Rienzo et al., Reference Di Rienzo, Casanoves, Balzarini, Gonzalez, Tablada and Robledo2011).
RESULTS
Linear morphometry
Shell size variation from different geological sets of shells was significant in length (ANOVA, F = 26.11; P < 0.0001) and height (ANOVA, F = 30.66; P < 0.0001), where in both cases Pleistocene shells showed variations with respect to Holocene and modern ones in Tukey comparisons (Figure 3).
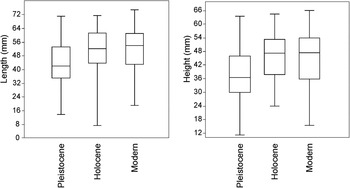
Fig. 3. Box plots for length and height of Pleistocene, Holocene and modern A. purpurata shells.
The shell size geometric means were significantly different (ANOVA, F = 28.17; P < 0.0001), with the Pleistocene shells smaller than Holocene and modern shells. Allometry indices of shells from different ages did not show significant differences (ANOVA, F = 2.09; P = 0.1249).
Each linear regression model between height and length showed a good fit (R 2 Pleistocene = 0.982; R 2 Holocene = 0.968; R 2 Modern = 0.983) (Figure 4). It was shown that valve length covaried with valve height with no differences between sets of shells (ANCOVA F = 1.368; P = 0.255).
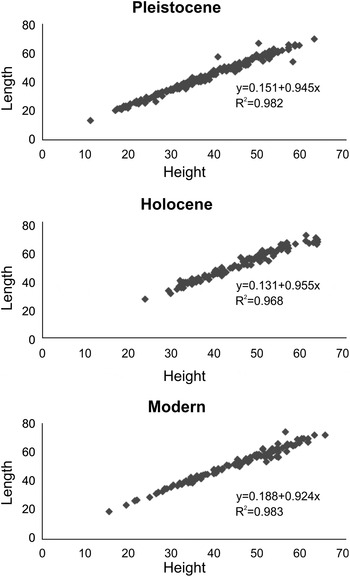
Fig. 4. Height and length (mm) relationship on shells from Pleistocene, Holocene and modern beaches.
The height–length relationships of the A. purpurata shells match close to linear. However, they were negatively allometric (b < 1) for all populations (t-tests: P < 0.001), thus indicating that this species grows slightly faster in length than in height, and a slight change of shape takes place during ontogeny. This pattern was the same in all analysed sets of shells.
Outline analysis
Elliptic Fourier analysis of A. purpurata shells revealed contour differences between shells of different ages, although there were considerable overlaps. The first four PCs (Figure 4) explained around 87% of the total variation. Of the four principal components, PC1 (ANOVA, P = 0.0118) and PC4 (ANOVA, P < 0.0001) showed variation between the different sets of shells, and in both cases Pleistocene shells were significantly different in a Hotelling Bonferroni test (Figure 5). However, PC2 and PC3 did not show significant differences between the three sets of shells. When the four coefficients were tested together (MANOVA, F Wilks = 7.46; P < 0.0001), Pleistocene shells showed significant differences using the Hotelling Bonferroni test. Using the extreme shapes of these figures it was possible to assign morphological meaning to the PC axes. The first PC (50.43%) could be explained by the degree of roundness. Although high variation was observed, the average shape of Holocene and modern shells were more closely related than the Pleistocene shells. The last ones exhibit a more elliptical and elongated shape than the Holocene/ modern ones, which were more rounded. The second PC (27.18%) represented the position of the umbo. Pleistocene and modern shells had a more prosogyrous umbo than the Holocene ones. The third PC (7.17%) did not follow any pattern which permitted a differentiation between assemblages. Although the fourth PC (6.05%) represented a low variation, it was related to the depth of the lunule, and showed that average Holocene and modern shells had deeper lunules than the Pleistocene shells.

Fig. 5. Principal component plots from the elliptic Fourier analysis of A. purpurata from Quaternary assemblages, with indication of reconstructed extreme configurations.
In Pleistocene, Holocene and modern shells, shape variation was not independent from size changes (ANCOVA, F = 2.52; P = 0.0866). Additionally, the relationship between the shape variation and the size changes showed no significant differences in an F-test on the Pleistocene, Holocene and modern shells slopes (Homogeneity of slopes, F = 0.9915; P = 0.3755).
DISCUSSION
The relationship between the length and height of the A. purpurata shell is almost linear. However, b was weakly but significantly less than 1 in all assemblages, indicating weak allometry. Shape variation was observed throughout the late Quaternary, although differences in allometric indices from different sets of shells were not detected. Shells were negatively allometric, which indicates that during ontogeny the individual growth is expressed more in terms of their length than in terms of their height (Seed & Richardson, Reference Seed and Richardson1999; Gaspar et al., Reference Gaspar, Chícharo, Vasconcelos, García, Santos and Monteiro2002a; Barón et al., Reference Barón, Real, Ciocco and Ré2004). Hence, this elongated shape may be an adaptive strategy that allows improving the efficiency of the burrowing process within substrate (Gaspar et al., Reference Gaspar, Santos, Vasconcelos and Monteiro2002b; Barón et al., Reference Barón, Real, Ciocco and Ré2004).
It was therefore necessary to investigate the relationship between the shape and size of A. purpurata shells, and to understand how shape varied when size was modified. In our samples, shell shape from Pleistocene, Holocene and modern beaches was dependent on shell size, and this relationship did not differ between these sets of shells, thus indicating that shape variation is associated with shell size changes. This was constant throughout the late Quaternary.
Environmental changes
Through the late Quaternary the coastal environment of the SMG forced sudden changes in the faunal communities (Ponce et al., Reference Ponce, Rabassa, Coronato and Borromei2011; Fucks et al., Reference Fucks, Schnak and Charó2012) due to modifications in substrates, water exchange with the open sea (Ponce et al., Reference Ponce, Rabassa, Coronato and Borromei2011; Fucks et al., Reference Fucks, Schnak and Charó2012), sea surface temperatures, palaeocirculation and productivity, as seen on a preliminary analysis of isotopes (see Bayer et al., Reference Bayer, Brey, Beierlein and Gordillo2013).
PHYSICAL ENVIRONMENT
It has been proposed that the presence of a deep lunule gives stability to the burrowing process (Ansell, Reference Ansell1962; Stanley, Reference Stanley1975; Camacho, Reference Camacho2007). Indeed, more elliptical and elongated shells with deeper lunules and smaller sizes lessen the resistance to sediment and allow faster and/or easier burrowing for infaunal bivalves (Stanley, Reference Stanley1970, Reference Stanley1975; Seilacher, Reference Seilacher1984; McLachlan et al., Reference McLachlan, Jaramillo, Defeo, Dugan, de Ruyck and Coetzee1995).
During the Pleistocene, the environment of the SMG area was an open sea affected by tidal regimes (Fucks et al., Reference Fucks, Schnak and Charó2012). Pleistocene shells were more exposed to wave action and the influence of tides (compare Neubauer et al., Reference Neubauer, Harzhauser and Mandic2013) than the modern shells and more stability was needed (Stanley, Reference Stanley1970). Consequently, physiological responses to environmental factors has probably led to slightly different contours in Pleistocene shells when compared with Holocene and modern ones. Pleistocene shells tended to be slightly more elliptical and elongated with a deeper lunule than younger Holocene and modern shells, which had more rounded shapes with a shallower lunule. Interestingly, Charó et al. (Reference Charó, Gordillo, Fucks and Giaconi2014) found that throughout the late Quaternary the proportion of taxa adapted to sandy-rocky environments has decreased in favour of taxa preferably colonizing rocky environments. A rounder shell with a shallow lunule, as observed in Holocene and modern shells, is more suitable in environments with a higher proportion of hard substrates and areas more protected from water energy where stability and/or easier burrowing would not be needed. Furthermore, this species attained its largest size in Holocene/modern assemblages compared with the Pleistocene. The larger individuals experienced higher sediment resistance during burrowing (Trueman et al., Reference Trueman, Brand and Davis1966; Stanley, Reference Stanley1970), but had higher mechanical stability in environments with a high proportion of rocky patches in connection with higher water energy (Neubauer et al., Reference Neubauer, Harzhauser and Mandic2013).
NUTRIENT AVAILABILITY
The size of the studied shells could be a consequence of multiple variables such as growth rate and age structure of the death assemblages. The growth rate of A. purpurata was studied and modelled on several beaches from Uruguay to the SMG (Morsan, Reference Morsan2000). A latitudinal gradient from north to south in the estimated growth rate was observed, suggesting an influence of temperature on growth. Differences were pronounced between the SMG and the other populations. Slow growth of the SMG population was linked to a food-mediated density-dependence, which was further studied by Morsan et al. (Reference Morsan, Pappalardo and Doldan2011), using 30 years of data. Growth curves were indicative of a density-dependent effect: individuals at low-density sites grew faster than individuals at high-density sites. Differences were stronger between sites than between cohorts. The A. purpurata size is therefore strongly affected by local environmental conditions, probably more influenced by availability or quality of nutrients than temperature (Vermeij, Reference Vermeij1978, Reference Vermeij, Rhoads and Lutz1980; Wikelski & Thom, Reference Wikelski and Thom2000; Regehr et al., Reference Regehr, Amstrup and Stirling2006; Zalizniak et al., Reference Zalizniak, Kefford and Nugegoda2006; Schneider et al., Reference Schneider, Fürsich, Schulz-Mirbach and Werner2010; Kefford et al., Reference Kefford, Marchant, Schäfer, Metzeling, Dunlop, Choy and Goonan2011).
Throughout the Holocene the coastal environment of the SMG would have forced sudden changes, such as modifications in water exchange with the open sea (Ponce et al., Reference Ponce, Rabassa, Coronato and Borromei2011; Fucks et al., Reference Fucks, Schnak and Charó2012), sea surface temperature changes, palaeocirculation and productivity (see Bayer et al., Reference Bayer, Brey, Beierlein and Gordillo2013). Thus, the larger size of Holocene A. purpurata compared with the Pleistocene might be due to increased nutrient uptake following the higher productivity during the Holocene and up to the present time as reported by Ponce et al. (Reference Ponce, Rabassa, Coronato and Borromei2011) and Fucks et al. (Reference Fucks, Schnak and Charó2012).
PREDATION
Another factor that could possibly have triggered the modifications in shell size and shape is predation, since more efficient burrowing ensures a rapid escape from predators (Stanley, Reference Stanley1970; Seilacher, Reference Seilacher1984). For example, the burrowing gastropod Adelomelon brasiliana (Lamarck, 1811) produces high predatory pressure on living A. purpurata from northern Argentine coasts (Cledón, Reference Cledón2004; Penchaszadeh et al., Reference Penchaszadeh, Arrighetti, Cledón, Livore, Botto and Iribarne2006). This gastropod was also found in Pleistocene assemblages in the SMG (Charó et al., Reference Charó, Gordillo, Fucks and Giaconi2014), suggesting a comparable pressure on A. purpurata. Consequently, the bivalves may have developed a suitable shell form that allowed it to burrow deeply and thus escape from A. brasiliana.
During the Holocene the proportion of carnivores increased (Charó et al., Reference Charó, Gordillo, Fucks and Giaconi2014). Amiantis purpurata shells tended to have a rounded shape with a shallow lunule, so they were likely not able to burrow as fast and easily as Pleistocene representatives. Thus, the larger size of Holocene A. purpurata compared with the Pleistocene could be a defence response to possible predators (Hone & Benton, Reference Hone and Benton2005; Schneider et al., Reference Schneider, Fürsich, Schulz-Mirbach and Werner2010).
FINAL REMARKS AND CONCLUSIONS
The environmental changes taking place during the late Quaternary, such as variations in sea level, substrate, water circulation and nutrient availability, as well as interspecific competition, likely affected the morphology and size of A. purpurata.
Amiantis purpurata shell size (length and height, and geometric mean) was smaller in Pleistocene shells than Holocene/modern ones, probably due to variations in productivity.
The Pleistocene shell contours of A. purpurata were slightly different from the Holocene and modern ones. This contour variation was expressed as a more elliptical shape and a deeper lunule, which enabled the bivalves to burrow more easily and efficiently than the Holocene and modern ones.
The high predatory pressure on A. purpurata probably produced a development of elliptical and elongated shells with deep lunules that allowed it to burrow fast and deeply during the Pleistocene. The large size of A. purpurata shells could be also a defence response to possible predators since the Holocene.
ACKNOWLEDGEMENTS
We would like to thank Diego Balseiro (CONICET-CICTERRA) and the reviewer Thomas A. Neubauer for valuable comments. This work is part of Sol Bayer's doctoral thesis.







