INTRODUCTION
Periphytic ciliates are a primary component of the periphyton microfauna and play an important role in the functioning of the microbial food webs in many aquatic ecosystems, for example, by importing bacteria, microalgae and other organic particles into the benthos (Coppellotti & Matarazzo, Reference Coppellotti and Matarazzo2000; Eisenmann et al., Reference Eisenmann, Letsiou, Feuchtinger, Bersker, Mannweiler, Hutzler and Arnz2001; Norf et al., Reference Norf, Arndt and Weitere2009a, Reference Norf, Arndt and Weitereb; Xu et al., Reference Xu, Zhang, Jiang, Zhu, Al-Rasheid, Warren and Song2011a, Reference Xu, Zhang, Jiang, Min and Choib, Reference Xu, Zhang, Jiang and Yang2014). Furthermore, periphytic ciliates have been widely accepted as useful indicators of water quality due to their rapid responses to environmental stress, ease of sampling, relative immobility, the increasing availability of user-friendly taxonomic guides and keys, and the potential for standardizing methods of collection and enumeration, thus allowing temporal and spatial comparisons (Weitere et al., Reference Weitere, Schmidt-Denter and Arndt2003; Norf et al., Reference Norf, Arndt and Weitere2007; Mieczan, Reference Mieczan2010; Xu et al., Reference Xu, Zhang, Jiang, Zhu and Al-Resheid2012a, Reference Xu, Zhang, Jiang, Zhu and Al-Rasheidb; Zhang et al., Reference Zhang, Xu, Jiang, Zhu and Al-Resheid2012).
Several investigations have demonstrated that taxonomic relatedness is a useful indicator to evaluate both marine biodiversity and environmental quality status, mainly because of its weak dependence on natural habitat type and sampling effort, and its high sensitivity to environmental stress and anthropogenic impacts (Warwick & Clarke, Reference Warwick and Clarke2001; Leonard et al., Reference Leonard, Clarke, Somerfield and Warwick2006; Prato et al., Reference Prato, Morgana, Valle La, Finoia and Lattanzi2009; Xu et al., Reference Xu, Jiang, Al-Rasheid, Al-Farraj and Song2011c, Reference Xu, Choi, Min and Zhud, Reference Xu, Zhang, Jiang, Zhu and Al-Rasheid2012c; Zhang et al., Reference Zhang, Xu, Jiang, Zhu and Al-Resheid2013; Jiang et al., Reference Jiang, Xu and Warren2014).
Our previous investigations have documented the temporal variations in species biodiversity indices (e.g. species richness and diversity) that are traditionally used as indicators of water quality (Xu et al., Reference Xu, Zhang, Jiang, Zhu, Al-Rasheid, Warren and Song2011a, Reference Xu, Zhang, Jiang, Min and Choib, Reference Xu, Zhang, Jiang, Zhu and Al-Resheid2012a). However, little information is available with regard to patterns of taxonomic relatedness of periphytic ciliates during their colonization periods.
In this study, temporal variations in taxonomic relatedness of periphytic ciliates were investigated, based on a dataset of communities with different ages in coastal waters of the Yellow Sea, near Qingdao, northern China collected during May and June 2010. Our aims were: (1) to document the taxonomic composition patterns of the ciliate microfauna during colonization; (2) to reveal the temporal dynamics of taxonomic relatedness during colonization; and (3) to compare the differences in taxonomic structure/relatedness of the periphytic ciliate communities at the two depths.
MATERIALS AND METHODS
Study site and data collection
The study site was located in the harbour of the Olympic Sailing Centre at Qingdao, northern China (Figure 1). This is a typical coastal area of the Yellow Sea, with an average depth of ~8 m and an average tidal range of 3 m.
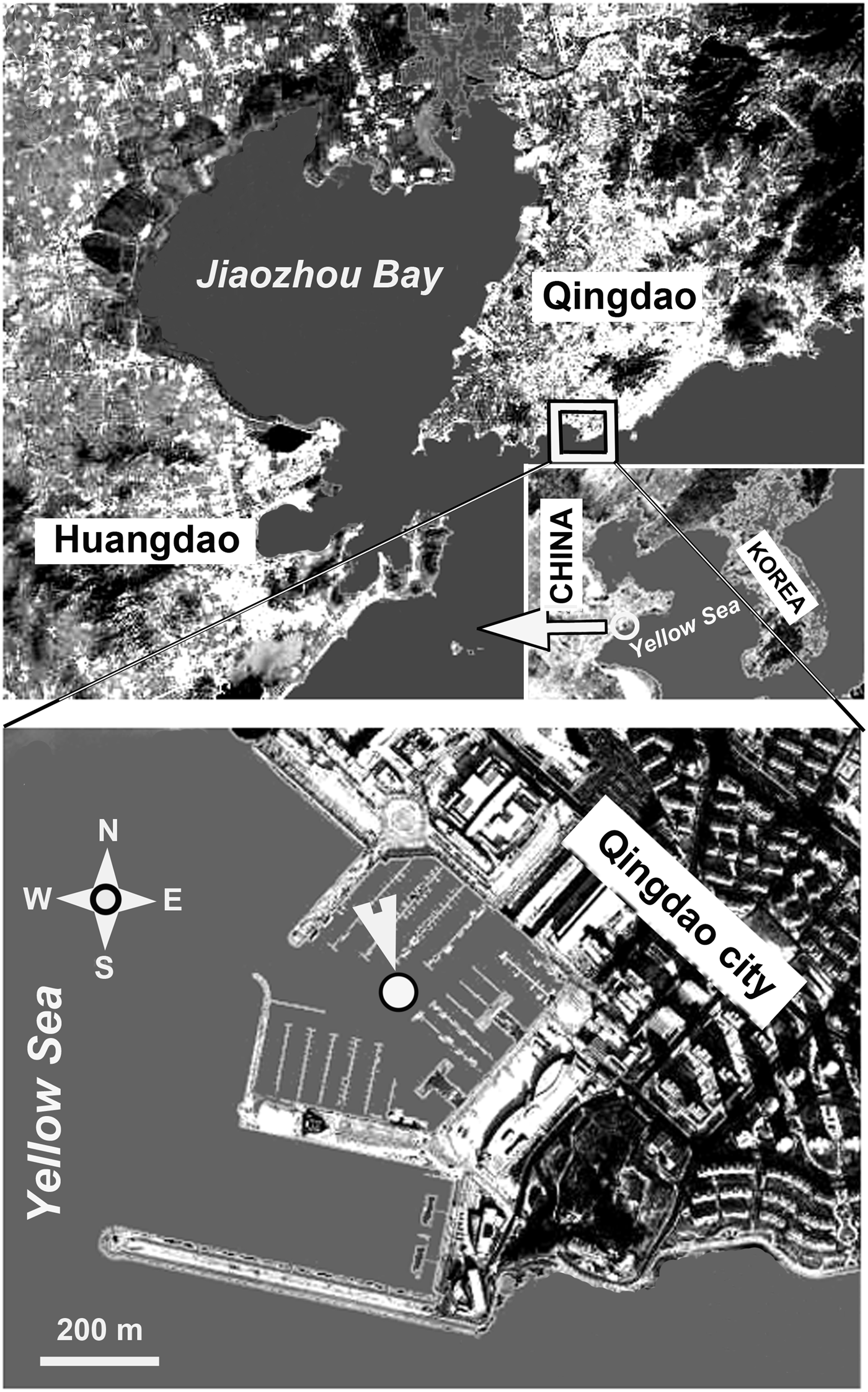
Fig. 1. Map of the sampling station, which was located in the harbour of the Olympic Sailing Centre at Qingdao, on the Yellow Sea coast of northern China.
Samples were collected using glass slides as artificial substrates, as described by Xu et al. (Reference Xu, Min, Choi, Jung and Park2009a, Reference Xu, Min, Choi, Kim, Jung and Limb). A total of 280 glass slides (2.5 × 7.5 cm) were used for collecting periphytic ciliates at depths 1 and 3 m below the water surface. For each depth, a total of seven PVC frames were used to hold the 140 slides, which were used as two parallel samples. From each parallel sample, 10 slides were randomly collected at time intervals of 1, 3, 7, 10, 14, 21 and 28 days. Samples were collected simultaneously at both depths.
Ciliate identification and enumeration were conducted following the methods outlined by Xu et al. (Reference Xu, Min, Choi, Jung and Park2009a, Reference Xu, Min, Choi, Kim, Jung and Limb). Protargol staining was performed for species identification (Berger, Reference Berger1999). Taxonomic classification of ciliates was based on the published references to keys and guides such as Song et al. (Reference Song, Warren and Hu2009).
The enumeration of ciliates in vivo was conducted at 100× magnification under an inverted microscope within 4–8 h after sampling in order to prevent significant changes in species composition (Xu et al., Reference Xu, Min, Choi, Jung and Park2009a, Reference Xu, Min, Choi, Kim, Jung and Limb). For recovering all species colonizing the glass slides, whole slides were examined using bright field illumination and both occurrences and individual abundances were recorded. After 14 days exposure time, 20 fields of view per slide were randomly chosen for enumerating the dominant ciliates. Two replicate sets of 10 glass slides were examined in order to confirm the average abundance (ind cm−2) of each species.
The following physico-chemical parameters were recorded in situ with appropriate sensors (WTW): water temperature (Temp); salinity (Sal); pH; and dissolved oxygen (DO). Water transparency was measured in situ using a transparent scale in a unit of metre (m).
Data analysis
Taxonomic diversity (Δ), taxonomic distinctness (Δ*), average taxonomic distinctness (Δ+) and variation in taxonomic distinctness (Λ+) were computed following the equations:
where x i (i = 1, 2,…, S) denotes the abundance of the ith species; N is the total number of individuals in the sample; ω ij is the ‘distinctness weight’ given to the path length linking species i and j (with i < j); and S is the number of species (Warwick & Clarke, Reference Warwick and Clarke1995).
The distinctness weights used in this study were according to Clarke & Warwick (Reference Clarke and Warwick1998), i.e. ω = 1 (species in the same genus), 2 (same family but different genera), 3 (same order but different family), 4 (same class but different order) and 5 (same phylum but different class). The distinctness of two species connected at the highest taxonomic level is set equal to 100 (Warwick & Clarke, Reference Warwick and Clarke2001). The taxonomic scheme follows that of Lynn (Reference Lynn2008).
The community structures of samples were analysed using the PRIMER 6.1.16 package and the PERMANOVA+ v.1.0.6 for PRIMER (Clarke & Gorley, Reference Clarke and Gorley2006; Anderson et al., Reference Anderson, Gorley and Clarke2008). The matrices of the ciliate taxon numbers were analysed by cluster analysis and non-metric multidimensional scaling (MDS) ordination on Euclidean distance matrices from log-transformed data (Clarke & Gorley, Reference Clarke and Gorley2006). The canonical analysis of principle coordinates (CAP) was use to discriminate the differences in species composition patterns of the ciliate communities, based on the Sørensen similarity matrices from the species presence/absence data (Anderson et al., Reference Anderson, Gorley and Clarke2008). The relationship between similarity matrices was analysed using the Spearman rank correlation coefficient (ρ value) which was computed by the routine RELATE (Clarke & Gorley, Reference Clarke and Gorley2006). The SE of the community parameters between both enumeration schemes were summarized using the coefficient of variation (CV) (Xu et al., Reference Xu, Zhang, Jiang, Zhu and Al-Rasheid2012b). The t-test was used to evaluate the differences in the taxonomic relatedness parameters between depths of 1 and 3 m at the 0.05 level, using the software SPSS (v.16.0). Data were log-transformed before analysis (Liu et al., Reference Liu, Zhang and Xu2013).
RESULTS
Environmental conditions during the study
During the period of study the following water conditions were recorded: transparency ~3 m; Temp 14–19°C; pH ~8; Sal 30–31 psu; and DO 6–7 mg l−1.
Taxonomic composition and temporal variation
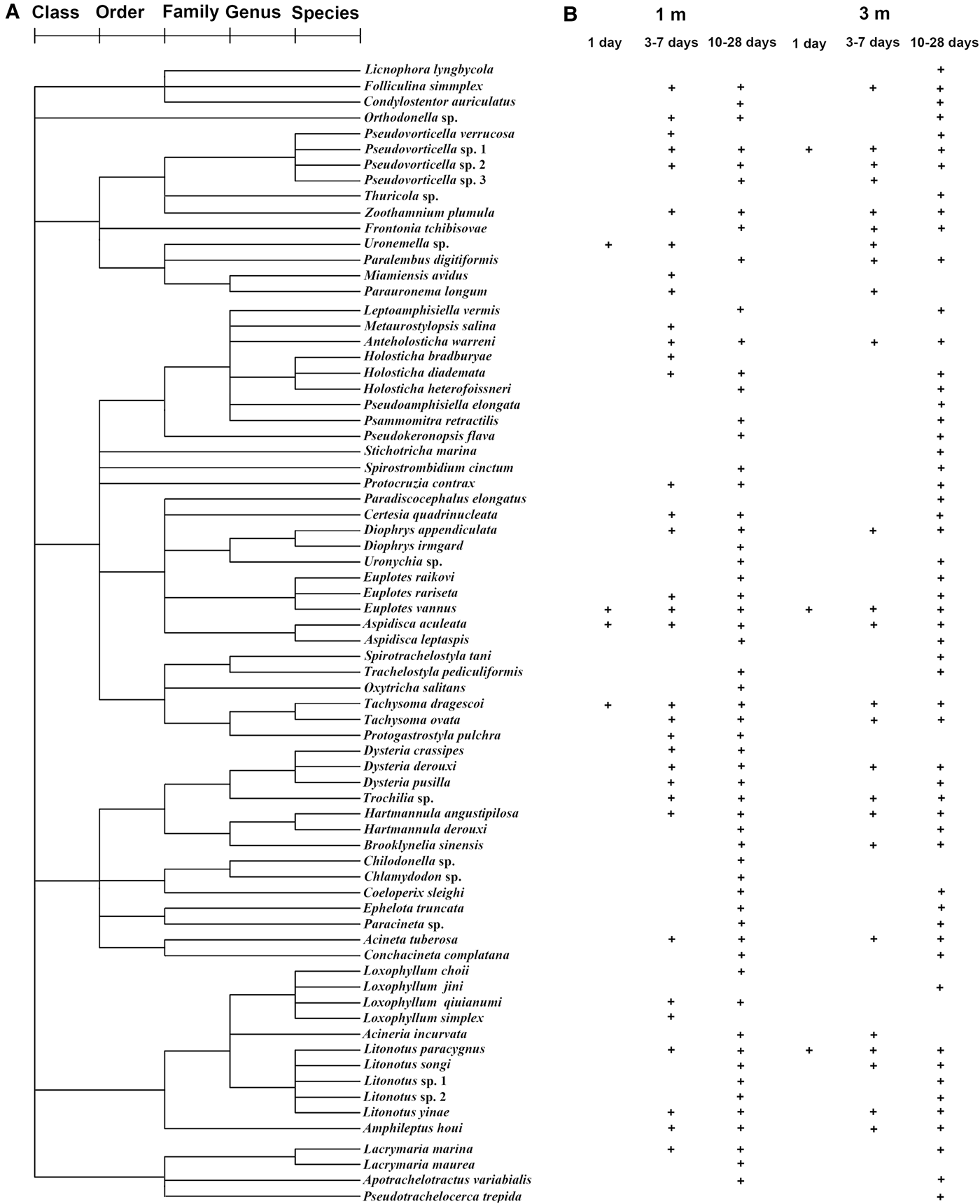
A total of 72 periphytic ciliate species, belonging to 51 genera, 36 families, 18 orders and 7 classes, were recorded at depths of 1 and 3 m throughout the period of study (Table 1 and Figure 2A, for details see Supplementary Material). Of these taxa, 91 and 82% species were identified at 1 and 3 m, respectively, while 73% species occurred at both depths (Table 1).
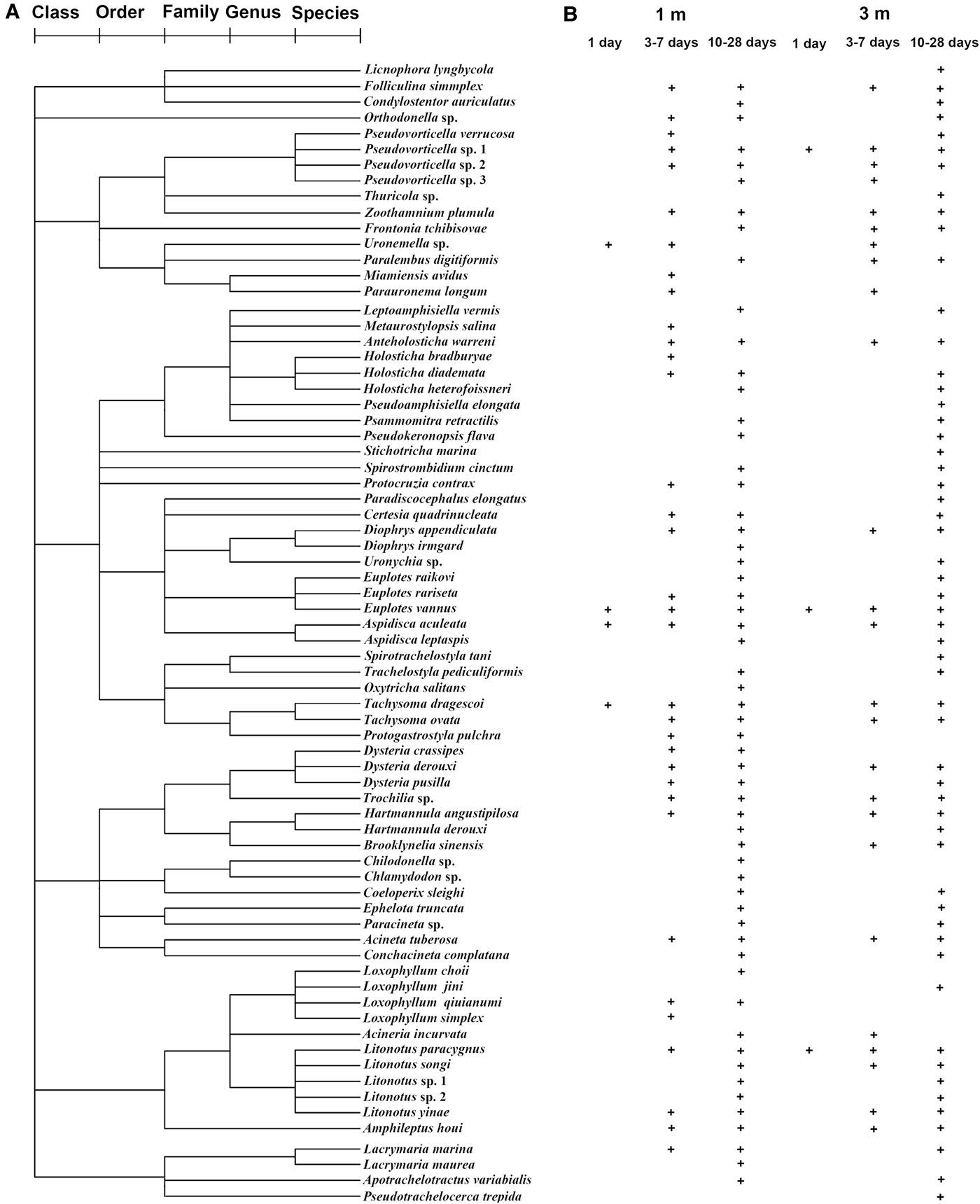
Fig. 2. Classification of periphytic ciliates into five ranks, i.e. species, genus, family, order and class (A), and their temporal and spatial occurrence (presence/absence) pattern (B), during the 1–28-day colonization periods at depths of 1 and 3 m in coastal waters of the Yellow Sea, northern China.
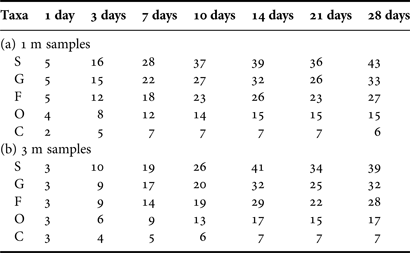
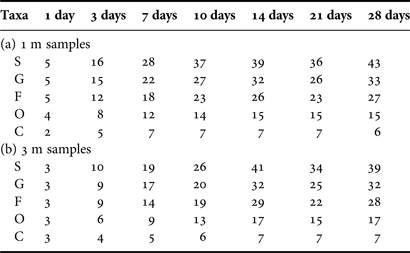
Table 1. Numbers of taxa at five taxonomic levels of species (S), genus (G), family (F), order (O) and class (C) of total periphytic ciliate communities and their distribution at depths of 1 m and 3 m, and both in coastal waters of the Yellow Sea, northern China during the study period.

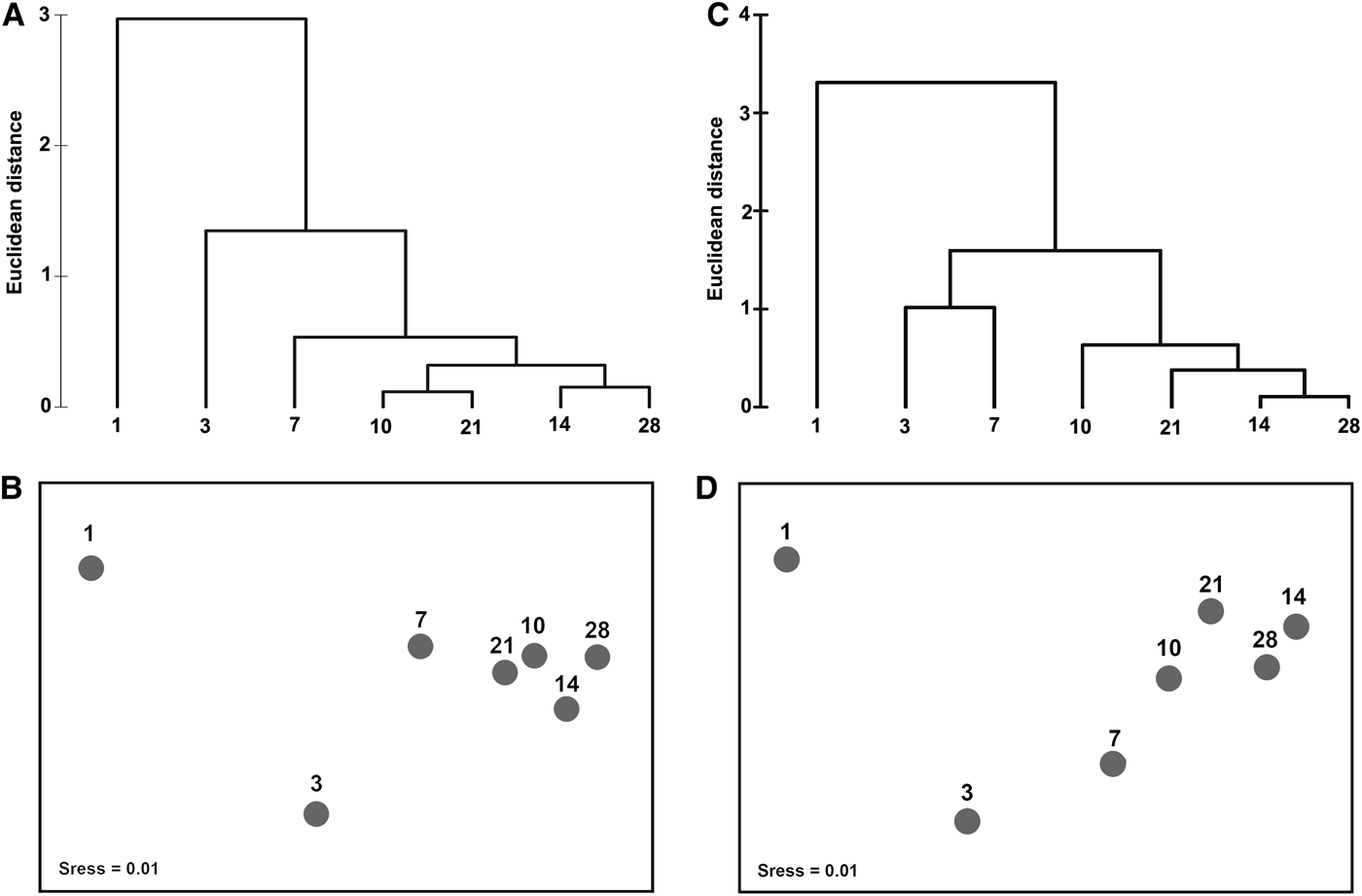
The numbers of the ciliate taxa at 1-, 3-, 7-,…, 28-day colonization times increased asymptotically (Table 2). It should be noted that these counts were comparatively higher at 1 m than at 3 m, especially in the 1–7-day colonization periods (Table 2). The clustering and MDS ordination resulted in the matrices of taxon numbers of the mature communities (≥10 days) falling into a same group at a 1-Euclidean distance level at both depths (Figure 3). Mental (RELATE) test demonstrated a significant correlation between the matrices for the two depths (ρ value = 0.932, P < 0.05).

Fig. 3. Clustering analysis and non-metric multidimensional scaling ordination for the matrices of taxon numbers of ciliates during the 1–28-day colonization periods at depths of 1 and 3 m in coastal waters of the Yellow Sea, northern China.
Table 2. Accumulative numbers of taxa at five taxonomic levels of species (S), genus (G), family (F), order (O) and class (C) of total periphytic ciliate communities with 1-, 3-, 7-, 10-, 14-, 21-, 28-day ages at depths of 1 m (a) and 3 m (b) in coastal waters of the Yellow Sea, northern China.
Colonization dynamics in community pattern
The temporal variations in species composition pattern of the ciliate communities during the colonization periods of 1–28 days were discriminated by CAP ordination on Sørensen similarity matrices from the species presence/absence data (Figure 4). The first canonical axis (CAP 1) mainly separated the immature (1–7-day) ciliate communities (on the left) from the mature (10–28-day) (on the right), while the second canonical axis (CAP 2) mainly discriminated those collected at 3 m (upper) from those collected at 1 m (lower) (Figure 4A).

Fig. 4. Canonical analysis of principle coordinates (CAP) ordination with correlations of ciliate species with correlation coefficients >0.35 CAP axes, showing the differences in taxonomic pattern of the ciliate microfauna during the 1–28-day colonization periods at depths of 1 and 3 m in coastal waters of the Yellow Sea, northern China.
A vector overlay of Pearson correlations of seven species (correlation coefficients >0.35) with the CAP axes is shown in Figure 4B. Vectors for six ciliate species (Tachysoma dragescoi, Aspidisca aculeata, Litonotus paracygnus, Pseudovorticella sp.1, Trochilia sp. and Uronema sp.) pointed toward the mature sample cloud (right) and for one species (Anteholostcha warreni) toward the immature samples from a depth of 1 m (lower) (Figure 4B).
Temporal patterns of taxonomic relatedness measures
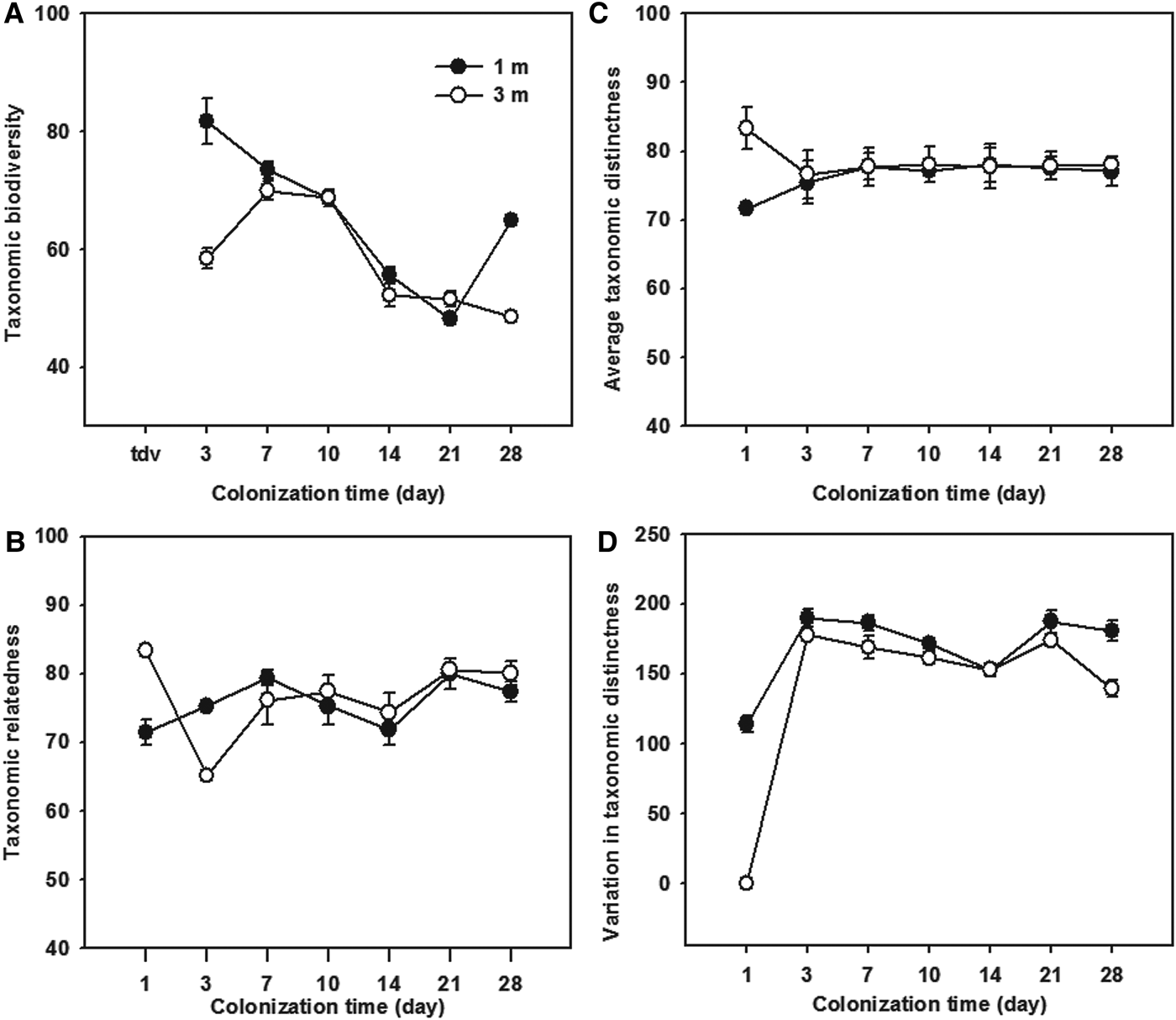
The temporal variations of taxonomic relatedness indices of the ciliate communities colonizing the glass slides during study period are shown in Figure 5. The taxonomic diversity (Δ) represented a high variability (coefficients of variation >10%) among both immature and mature communities (Figure 5A), whereas the taxonomic distinctness (Δ*), average taxonomic distinctness (Δ+) and the variation in taxonomic distinctness (Λ+) showed a high stability (coefficients of variation <10%) during the colonization times of 3–28 days (Figure 5B–D).
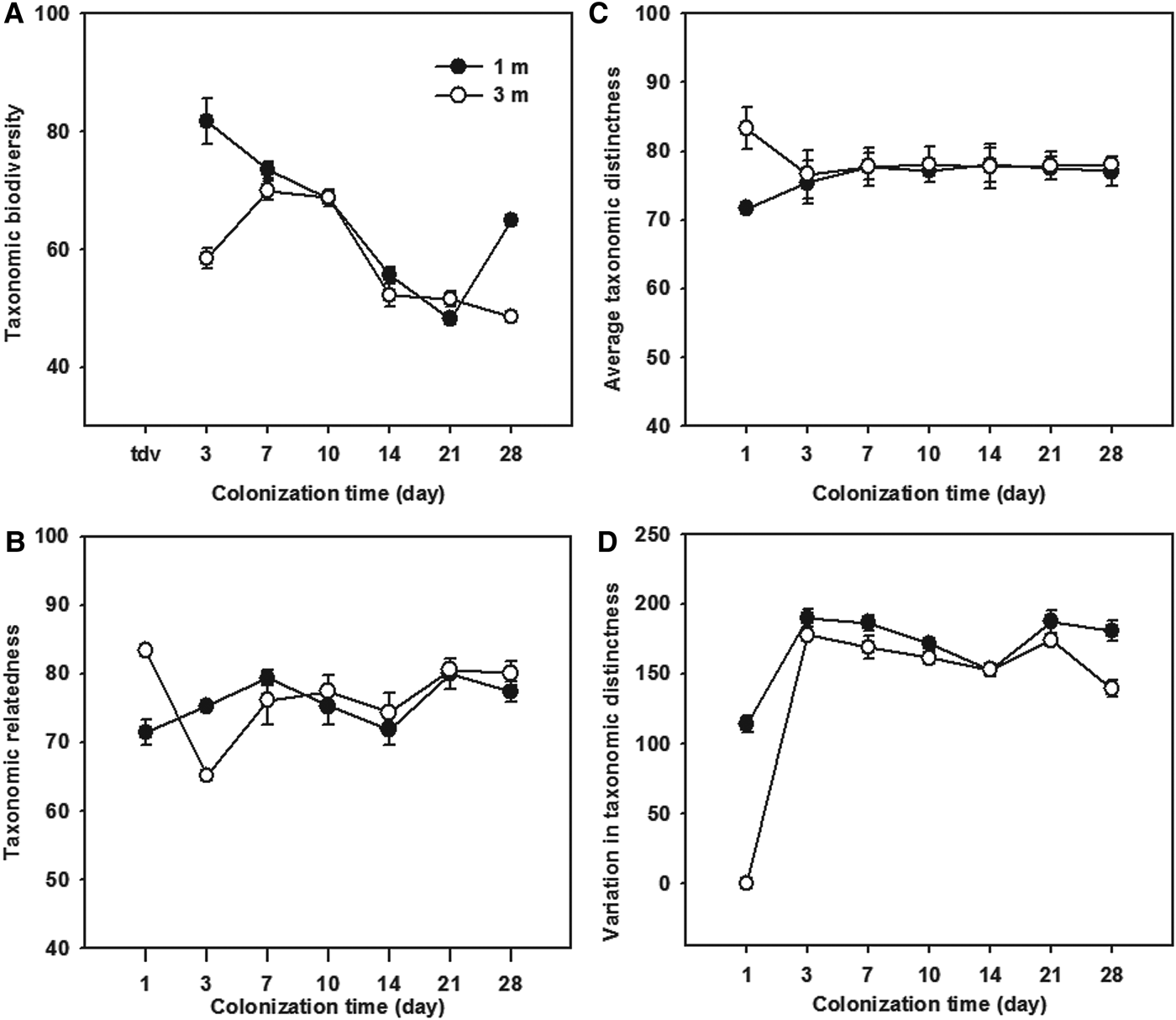
Fig. 5. Temporal variations of the taxonomic diversity (A), taxonomic distinctness (B), average taxonomic distinctness (C), and variation in taxonomic distinctness (D), of periphytic ciliate communities during the 1–28-day colonization periods at depths of 1 and 3 m in coastal waters of the Yellow Sea, northern China.
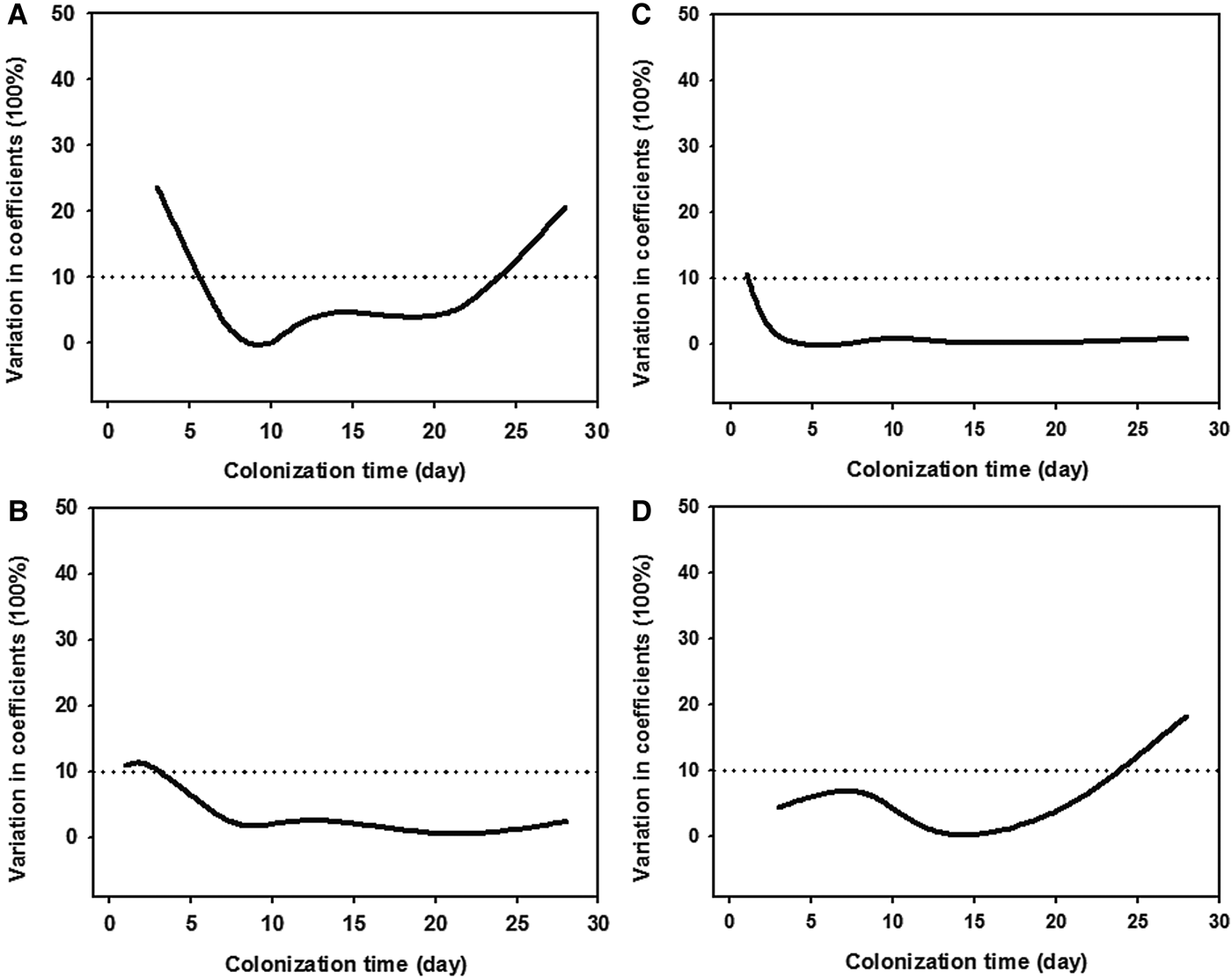
Statistical analyses (t-test) showed that all four measures had higher values of the 1- and 28-day ciliate communities at a depth of 1 m than those at 3 m (t-test: P > 0.05; Figure 5), while there was a high variability (CV values >10%) between the two depths (Figure 6). It should be noted that the average taxonomic distinctness (Δ+) maintained stable values (<10% CV) during the colonization times of 3–28 days at both depths (Figures 5C & 6C).
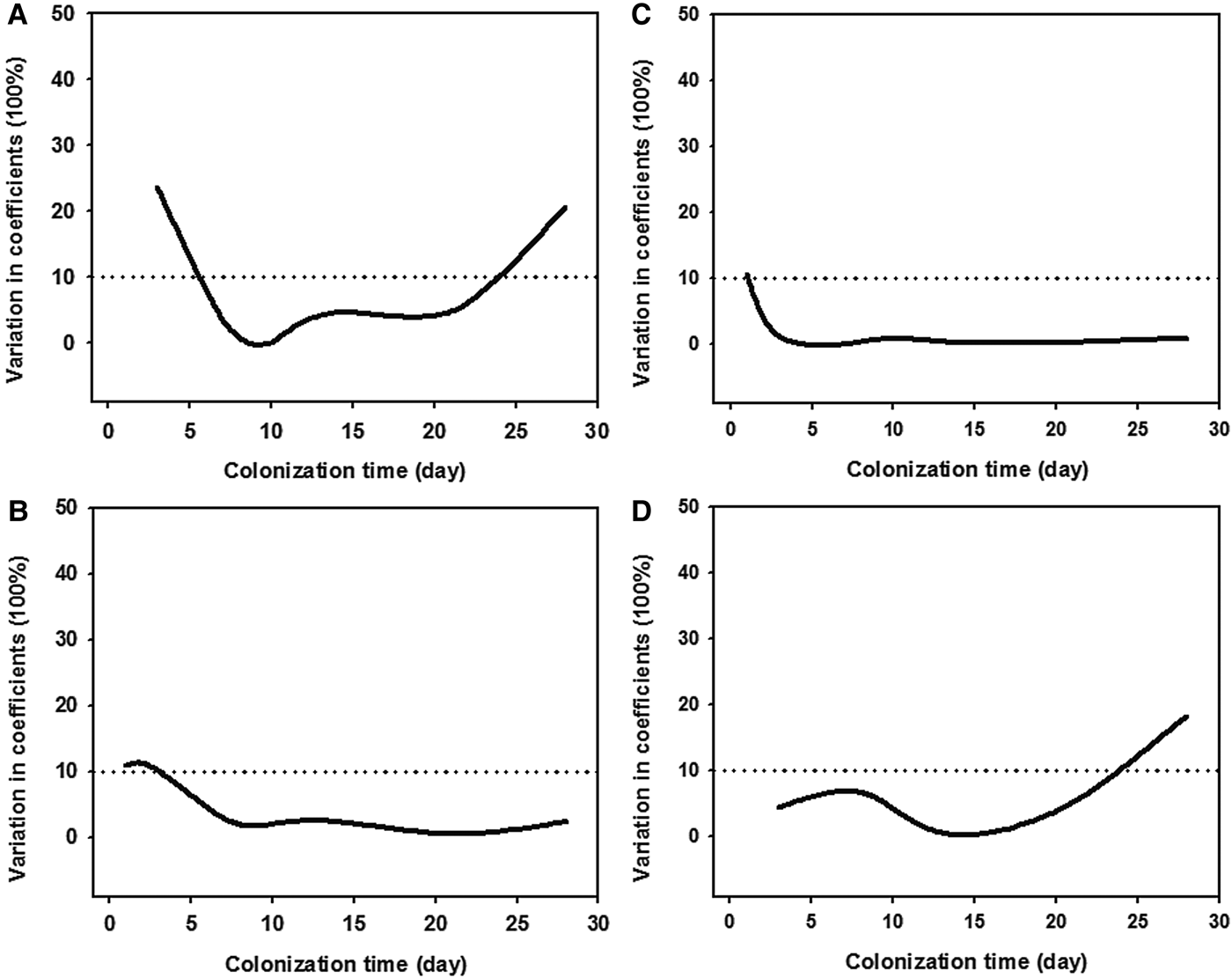
Fig. 6. Coefficients of variations of taxonomic diversity (A), taxonomic distinctness (B), average taxonomic distinctness (C), and variation in taxonomic distinctness (D), of periphytic ciliate communities between depths of 1 m (A, C) and 3 m (B, D) in coastal waters of the Yellow Sea, northern China during 1–28-day colonization periods.
DISCUSSION
Periphyton colonization of an artificial substratum commonly follows an asymptotic dynamic model, i.e. the number of taxa generally increases and then equilibrates (Xu et al., Reference Xu, Min, Choi, Jung and Park2009a, Reference Xu, Choi, Min and Zhu2011d; Zhang et al., Reference Zhang, Xu, Jiang, Zhu and Al-Resheid2012, Reference Zhang, Xu, Jiang, Zhu and Al-Resheid2013). Once the colonization reaches equilibrium, internal factors such as competition and predation pressure become more important (Railkin, Reference Railkin1998; Zhang et al., Reference Zhang, Xu, Jiang, Zhu and Al-Resheid2012). Our previous investigations of Yellow Sea coastal waters have demonstrated that the microperiphyton communities with colonization ages of more than 10 days can be considered as mature (Zhang et al., Reference Zhang, Xu, Jiang, Zhu and Al-Resheid2012, Reference Zhang, Xu, Jiang, Zhu and Al-Resheid2013). In the present study, the clustering analysis and CAP ordination revealed that the matrices of the numbers of the ciliate taxa and those from the species presence/absence data of the mature samples represented high similarities compared to the immature communities.
Compared with traditional biodiversity indices (e.g. species richness, evenness and diversity), taxonomic relatedness measures have proven to be robust indicators for the evaluation of both ecological patterns and anthropogenic impacts, mainly due to their weak dependence on natural habitat type and sampling effort (Warwick & Clarke, Reference Warwick and Clarke2001; Tan et al., Reference Tan, Shi, Liu, Xu and Nie2010; Xu et al., Reference Xu, Jiang, Al-Rasheid, Al-Farraj and Song2011c; Shi et al., Reference Shi, Liu, Liu, Sun and Xu2012). Based on the present data, although all four taxonomic relatedness measures were less sensitive to sample size than analysis of community patterns, they represented different dependence on the communities with different ages, i.e. high sensitivity for the immature communities but low sensitivity for the mature communities; this was most marked in the taxonomic diversity and variation in taxonomic distinctness. In addition, the taxonomic relatedness indices showed low variability in the mature communities of the ciliates compared to the traditional biological indices (Zhang et al., Reference Zhang, Xu, Jiang, Zhu and Al-Resheid2013). This finding implies that taxonomic relatedness indices are powerful bio-indicators for use in monitoring and conservation programs, and have the additional advantages of being less time-consuming and more cost-effective than traditional biodiversity indices (e.g. species richness, evenness and diversity).
It is noteworthy that taxon numbers were higher at a depth of 1 m than at 3 m, even though the periphytic ciliate community structure was similar at the two depths. A possible explanation for this might be that there was weaker sunlight penetration, and therefore less food available, at 3 m than at 1 m. In the 28-day samples collected at 1 m, however, the taxonomic diversity and variation in taxonomic distinctness dropped sharply, mainly due to the intensive immigration of metazoan consumers (e.g. copepods, coelenterates, annelids and barnacles). Thus, it is suggested that the colonization ages of 3–21 days are probably optimal to detect the taxonomic relatedness of the periphytic ciliate microfauna.
In summary, the colonization dynamics of ciliate microfauna on glass slides had similar patterns of species composition during colonization periods at both depths. In the immature communities (1–7 days), the taxonomic patterns showed a high variability compared to the mature ones (10 days and more). However, the taxonomic relatedness parameters differed between the two depths at early colonization times (e.g. 1–3 days). Taxonomic diversity (Δ) was subject to high variability (coefficients of variation >10%) in both immature and mature communities, whereas taxonomic distinctness (Δ*), average taxonomic distinctness (Δ+) and the variation in taxonomic distinctness (Λ+) showed a high stability (coefficients of variation <10%) during colonization times of 3–21 days. These findings suggest that 3–21-day exposure times are sufficient to detect the taxonomic distinctness of periphytic ciliate microfauna at depths of 1–3 m for the purposes of ecological research and monitoring of marine ecosystems. However, further studies on a range of marine waters and over extended time periods are needed in order to verify this conclusion.
ACKNOWLEDGEMENTS
Special thanks are due to Dr X. Fan, Dr J. Jiang and Dr X. Chen, Laboratory of Protozoology, Institute of Evolution and Marine Biodiversity, Ocean University of China, China, for their help with sampling and sample processing.
FINANCIAL SUPPORT
This work was supported by the National Natural Science Foundation of China (No. 41076089) and Scholarship Award for Excellent Doctoral Student granted by the Chinese Ministry of Education.
Supplementary materials and methods
The supplementary material for this article can be found at journals.cambridge.org/MBI.