Introduction
The shallow benthos along the western Antarctic Peninsula supports brown macroalgal forests with dense amphipod assemblages, commonly including Gondogeneia antarctica (Amsler et al. Reference Amsler, McClintock and Baker2014). Gondogeneia antarctica and most other amphipods are chemically deterred from consuming the macroalgae (Amsler et al. Reference Amsler, McClintock and Baker2014). They primarily consume diatoms, other microalgae, filamentous macroalgae and a few undefended macroalgal species, including Palmaria decipiens (Aumack et al. Reference Aumack, Lowe, Amsler, Amsler, McClintock and Baker2017). Although unpalatable when alive, G. antarctica and other amphipods will consume the chemically defended brown algae Himantothallus grandifolius and Desmarestia anceps within a few weeks of death (Amsler et al. Reference Amsler, McClintock and Baker2014).
Fatty acids (FAs) may be used as ‘biomarkers’ for tracing trophic pathways in basal consumers (Galloway et al. Reference Galloway, Eisenlord, Dethier, Holtgrieve and Brett2014, Aumack et al. Reference Aumack, Lowe, Amsler, Amsler, McClintock and Baker2017), although this is poorly characterized in Antarctica. This experiment compared the FA composition of G. antarctica after nine weeks of feeding on diatoms, P. decipiens and aged (freeze-killed) D. anceps and H. grandifolius.
Materials and methods
Desmarestia anceps, H. grandifolius, P. decipiens, epilithic diatom assemblages and G. antarctica were collected near Palmer Station (64°46'S, 64°03'W) in March 2017. Living D. anceps and H. grandifolius are not palatable, so the material fed to amphipods was freeze-killed. Macroalgae were then thawed in vented plastic bags in 19 l buckets maintained at 1–2°C until being offered to the amphipods. Macroalgal samples were collected for FA analysis prior to ('live’ throughout) and following the freeze-kill procedure ('dead’ throughout) for D. anceps and H. grandifolius. Only live diatoms and P. decipiens tissue were used.
Haphazardly selected subsets (n = 15) of adult amphipods were starved for one week before allocation to 250 ml bottles in their respective diet treatments, with another subset analysed in order to characterize ‘wild’ amphipod FA profiles. Amphipods were initially maintained in an environmental room (1.0 ± 0.5°C) with tissue from dead D. anceps or H. grandifolius or with live diatoms or P. decipiens. Due to high mortality rates with the dead algae after 3.5 weeks, all of the amphipods were pooled by diet and transferred to 4 l bottles fitted with mesh screens in a flow-through ambient seawater table (1–2°C) for an additional 5.5 weeks, then starved for three days to clear their digestive systems before being frozen at -80°C. No growth or ingestion metrics were recorded.
Samples of each alga and three sets of pooled samples of amphipods fed on each diet were lyophilized, homogenized, lipid extracted and transesterified to produce quantitative measurements (μg mg−1) of FA methyl esters for analysis by gas chromatography mass spectrometry using internal and external standards (Taipale et al. Reference Taipale, Hiltunen, Vuorio and Peltomaa2016). Proportional FA profiles were analysed using permutational multivariate analysis of variance (PERMANOVA), similarity percentage (SIMPER) and non-metric multidimensional scaling (nMDS) in Primer v.6.1.13 with PERMANOVA+ v.1.0.3, as described in Kelly & Scheibling (Reference Kelly and Scheibling2012). Differences in the untransformed FA profiles of fresh and dead macroalgae and amphipods fed dead macroalgae were analysed with PERMANOVA (9999 permutations, type III sum of squares). The FA results were not sensitive to transformation and all analyses used Euclidean distance.
Results and discussion
The FAs of G. antarctica differed according to algal diet (Fig. 1). The FA profiles of the algae, dead and alive, differed from those of amphipods that had been maintained on a given alga (Fig. 1 & Table S1; P = 0.001). Detailed PERMANOVA results are shown in Table S1 and FA proportion and concentration tables are shown in Tables S2–S5. The macroalgae and amphipod FAs had little overlap with those of wild amphipods, and those maintained on monoculture diets fell inside the resource triangle of the diets tested (Fig. 1). Organismal FA profiles commonly group based on phylogenetic relationships (Kelly & Scheibling Reference Kelly and Scheibling2012), so the lack of overlap between macroalgae and amphipods was not surprising.
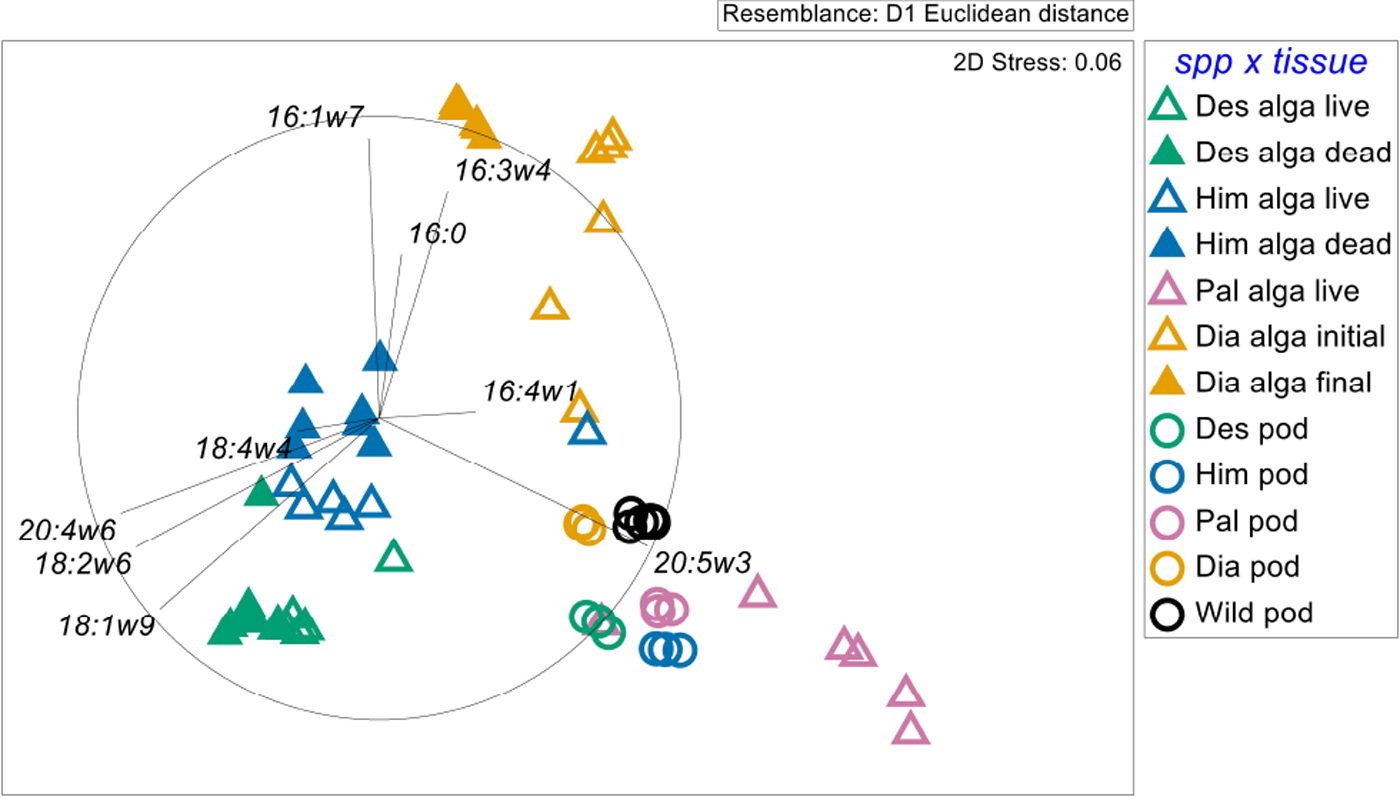
Fig. 1. The nMDS plot of the FA profiles of macroalgae and amphipods fed on experimental diets with a vector overlay indicating the top five FAs identified by SIMPER as contributing to the dissimilarity between treatment groups. Note: the ‘wild pod’ treatment group represents initial collections. alga = algal tissue, Des = Desmarestia anceps, dia = diatoms, Him = Himantothallus grandifolius, Pal = Palmaria decipiens, pod = amphipod tissue.
Within the algal FA profiles, there was clear separation between the FAs of the algal diets (Fig. 1 & Table S1; P = 0.001) and between the live and dead macroalgae (Table S1; P = 0.001). There was a significant interaction between macroalgal species and whether the tissue was alive or dead (Table S1; P = 0.001), indicating that not all of the FAs changed in the same way when macroalgae were freeze-killed. Similarly to the macroalga comparisons, amphipod FAs differed based on diet (Fig. 1 & Table S1; P = 0.001). By the end of the feeding trial, the amphipod FAs had changed from their initial ‘wild’ profiles, and these changes were driven by several FAs that are frequently used as trophic biomarkers (Fig. 1).
Common FAs have been used as biomarkers for differentiating diatoms (16:1ω7, 16:0 and 16:3ω4) and brown (18:1ω9 and 20:4ω6) and red macroalgae (e.g. particularly 20:5ω3; Kelly & Scheibling Reference Kelly and Scheibling2012). Eicosapentaenoic acid (20:5ω3) is an essential FA found in many primary producers, but it often occurs in the highest proportions in red macroalgae (Kelly & Scheibling Reference Kelly and Scheibling2012), and it was a strong driver of the FA results in our experiment (Fig. 1). There was clear separation between amphipods fed on macroalgae or diatoms, but due to their higher proportions of 20:5ω3 than in their diet tissues (Tables S2 & S3), amphipods tended to group more closely to the tissues of P. decipiens (Fig. 1), suggesting that 20:5ω3 can be either synthesized or selectively retained by amphipods. G. antarctica fed on H. grandifolius and P. decipiens also had higher proportions of 20:5ω3 than amphipods maintained on other diets (Table S3 & S5).
The low variation within a treatment group for wild and laboratory-maintained amphipods suggests that wild G. antarctica are consuming similar diets, with high proportions of diatoms, as suggested by Aumack et al. (Reference Aumack, Lowe, Amsler, Amsler, McClintock and Baker2017). The present results suggest that diatoms do not make up the entire diet of G. antarctica, as evidenced by the consistent shift of wild amphipods to the right on the nMDS plot, and similarly correlated with the increased proportion of 20:5ω3 (Fig. 1). This work demonstrates the clear assimilation of dietary FA in an Antarctic amphipod, and it also demonstrates that the amphipods ordinate within the ‘resource library’ of likely algal food sources for wild individuals (e.g. Galloway et al. Reference Galloway, Eisenlord, Dethier, Holtgrieve and Brett2014).
Acknowledgements
The authors are grateful for the science and logistical support of the Palmer Station staff and to an anonymous reviewer. This work was supported by NSF awards PLR-1341333, OPP 1744550 and OPP-1744570 and by University of Oregon start-up funds to AWEG.
Author contributions
AWEG, CDA, MOA and JBM developed the experimental design. MOA and CDA performed field experiments. JBS performed FA extractions, data analysis and wrote the first draft. All authors contributed to preparing the final manuscript.
Supplemental material
A description of the fatty acid extraction method and five tables will be found at https://doi.org/10.1017/S0954102019000397.



