1. Introduction
The Eocene–Oligocene transition corresponds to a global climate cooling associated with a high turnover of terrestrial and marine faunas, including extinctions and diversifications (Stehlin, Reference Stehlin1909; Lopez & Thaler, Reference Lopez and Thaler1974; Prothero & Berggren, Reference Prothero and Berggren1992; Prothero Reference Prothero1994; Blondel & Mourer-Chauviré, Reference Blondel and Mourer-Chauviré1998; Hooker, Collinson & Sille, Reference Hooker, Collinson and Sille2004; Zachos, Dickens & Zeebe, Reference Zachos, Dickens and Zeebe2008; Houben et al. Reference Houben, Van Mourik, Montanari, Coccioni and Brinkhuis2012; Goldner, Herold & Huber, Reference Goldner, Herold and Huber2014). Knowledge of the late Eocene fossil record makes it possible to document the impact of global climate cooling on the evolutionary and biogeographical histories of the faunas. The interest of the fauna from the Samlat Formation results in the extreme rarity of late Eocene and Oligocene faunas in the relatively unexplored area of northwestern Africa, halfway between the well-known Egyptian (Fayum) and Libyan (Dur At-Talah) Eocene–Oligocene localities in northeastern Africa and those of the eastern North American coast.
Late Eocene – Oligocene deposits of the Samlat Formation crop out in southwestern Moroccan Sahara, south of Ad-Dakhla city, along a transect from El Argoub city to the fishing village of Punta Chica. These deposits have yielded a rich marine vertebrate fauna including abundant chondrichthyans (Adnet, Cappetta & Tabuce, Reference Adnet, Cappetta and Tabuce2010) and archaeocete whales (Field, Racicot & Uhen, Reference Field, Racicot and Uhen2011; Zouhri et al. Reference Zouhri, Gingerich, Elboudali, Sebti, Noubhani, Rahali and Meslouh2014), as well as fragmentary remains of actinopterygians (Adnet, Cappetta & Tabuce, Reference Adnet, Cappetta and Tabuce2010) and sirenians (Zouhri et al. Reference Zouhri, Gingerich, Elboudali, Sebti, Noubhani, Rahali and Meslouh2014). Adnet, Cappetta & Tabuce (Reference Adnet, Cappetta and Tabuce2010) also reported an isolated proboscidean tooth tentatively attributed to Numidotherium. Archaeocetes are represented by at least five basilosaurid species including Basilosaurus isis, Dorudon atrox, cf. Dorudon sp., cf. Saghacetus sp. and cf. Stromerius sp. The dugongid sirenian is identified as cf. Eosiren sp. More recently, in the sector of Porto Rico and El Argoub Marivaux et al. (Reference Marivaux, Adnet, Benammi, Tabuce and Benammi2017) reported an early Oligocene fossiliferous level with estuarine and continental fauna and described in detail the anomaluroid rodents.
The purpose of this paper is to provide a more complete picture of the fossil assemblage from the upper Eocene deposits of the Samlat Formation in order to compare faunas and establish correlations with contemporaneous strata of the Fayum Depression in Egypt and Dur At-Talah in Libya. Here we describe the rest of this marine vertebrate fauna, including actinopterygians, turtles, eusuchian crocodiles, marine snakes and seabirds. Additionally, we discuss the palaeobiogeographical and palaeoenvironmental significances of this diverse marine fauna from the upper Eocene – Oligocene strata of northwestern Africa.
2. Geological setting
The Samlat Formation deposits are well exposed in coastal cliff sections bordering the Atlantic Ocean, south of the city of Ad-Dakhla in southwestern Morocco (Fig. 1). This formation belongs to the Tarfaya–Laâyoun–Ad-Dakhla coastal basin developed in a stable passive margin, and which comprises two discrete sub-basins separated by the Dakhla Fracture Zone (Davison, Reference Davison2005): the northern Boujdour sub-basin and the southern Ad-Dakhla sub-basin. In the middle of the Ad-Dakhla sub-basin along the Atlantic Coast, the Palaeogene Samlat Formation overlies Cretaceous outcrops (Ratschiller, Reference Ratschiller1967; von Rad et al. Reference von Rad, Ryan, Arthur, Cepek, Cita, Cornford, Garifal, Hamilton, Lopatin, Lutze, McCoy, Mountain, Sarnthein, Weser, Whelan and Wind1979). The Samlat Formation includes three members (Ratschiller, Reference Ratschiller1967): the Paleocene Itgui Member with well-preserved foraminifera; the Eocene Guerran Member that consists of white earthy siliceous carbonates interbedded with silty coarse sandstones and conglomerate; and the Neogene Morcba Member. This sequence was described in detail by Adnet, Cappetta & Tabuce (Reference Adnet, Cappetta and Tabuce2010) at Garitas sector SE of Ad-Dakhla, and was divided into three units (Fig. 2). More recently two other units (Unit 4 and Unit 5) of this sequence have been defined in the uppermost section of the sequence in Porto Rico and El Argoub sectors, which are situated north of Garitas on the mainland shoreline (Marivaux et al. Reference Marivaux, Adnet, Benammi, Tabuce and Benammi2017, fig. 1). These five units clearly show lateral variations of facies that could be explained by a slight northwards dip of strata.

Figure 1. Priabonian palaeogeographic map of North Africa showing the position of (1) Ad-Dakhla, (2) Dur at-Talah and (3) Fayum localities. ODSN Plate Tectonic Reconstruction Service (2011), modified.
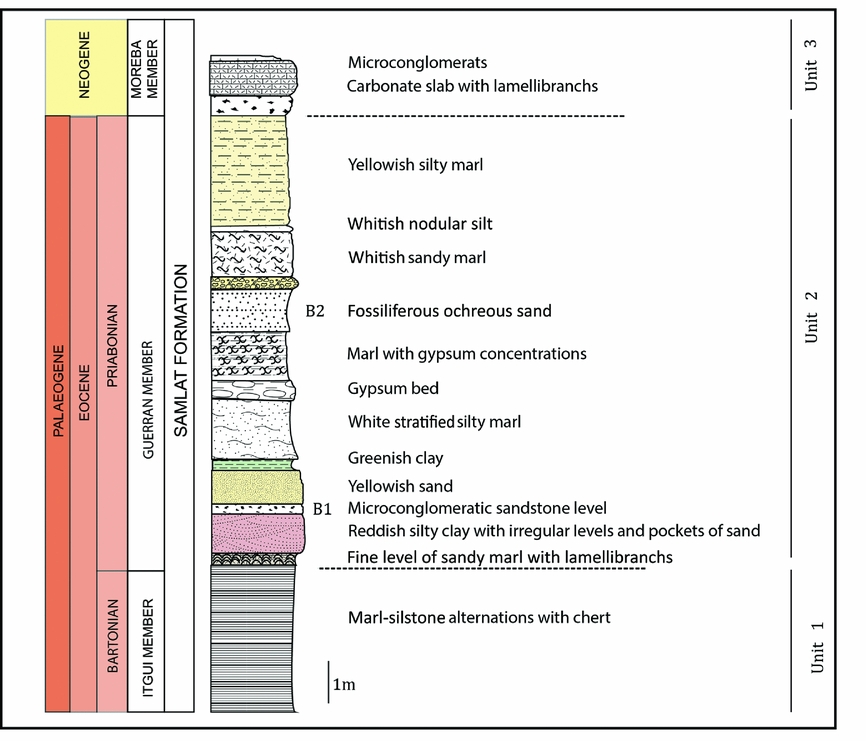
Figure 2. Stratigraphical section at Garitas, south of Ad-Dakhla. Modified from Zouhri et al. (Reference Zouhri, Gingerich, Elboudali, Sebti, Noubhani, Rahali and Meslouh2014).
In Garitas sector, the lower unit (Unit 1) includes some 22 m of rhythmically bedded, chert-rich marly siltstone and marls. Unit 2 includes (from bottom to top): a 1–1.5 m thick level of vertebrate-bearing conglomeratic sandstone; 4–8 m of rhythmically bedded siltstone and marl; and a second 3–6 m thick level of vertebrate-bearing muddy sandstone. Unit 3 consists of a 2–3 m thick interval of sandy to bioclastic limestone of Neogene age in Garitas sector, but this unit can exceed 30 m in thickness in the Porto Rico sector (Marivaux et al. Reference Marivaux, Adnet, Benammi, Tabuce and Benammi2017, fig. 1).
Bonebeds B1 and B2, where most fossils studied here come from, are both located in Unit 2, as shown in the stratigraphical section of the locality Garitas (Fig. 2). Adnet, Cappetta & Tabuce (Reference Adnet, Cappetta and Tabuce2010) assigned a late Eocene (Priabonian) age to Unit 2 including bonebeds B1 and B2, based on the selachian fauna. This age was confirmed by the fauna of archaeocete whales discovered in these bonebeds (Zouhri et al. Reference Zouhri, Gingerich, Elboudali, Sebti, Noubhani, Rahali and Meslouh2014), which comprises at least five basilosaurid species (including Basilosaurus isis) also present in the Birket Qarun Formation of early Priabonian age in Egypt (Gingerich, Reference Gingerich1992).
3. Material and methods
Fossils were collected during several field trips between 2012 and 2014. They come from different localities including ‘Garitas’ and ‘Dafyia’, which are located c. 130 km south of the city of Ad-Dakhla. Dafyia corresponds to a set of fossiliferous outcrops located 500 m south of Garitas. Specimens described in this work were found in bonebeds B1 and B2, with a large majority of specimens recovered from bonebed B1. The material consists of centimetric macroremains that were collected on the surface, without sieving of sediment. This material is housed in the Paleontological Collections of the Department of Geology of the Faculty of Sciences Aïn Chock, Hassan II University of Casablanca, Morocco.
Institutional abbreviations: FSAC DAK – Ad-Dakhla collection from Faculty of Sciences Aïn Chock, Casablanca, Morocco; MNHN – Muséum National d'Histoire Naturelle, Paris, France; SMNS – Staatlichen Museum für Naturkunde, Stuttgart, Germany.
4. Systematic palaeontology
4.a. Actinopterygii
Teleostei Müller, Reference Müller1845
Acanthomorpha Rosen, Reference Rosen, Greenwood, Miles and Patterson1973
Perciformes sensu Johnson & Patterson, Reference Johnson and Patterson1993
Scombroidei sensu Carpenter, Collette & Russo, Reference Carpenter, Collette and Russo1995
Scombroidei incertae sedis
Material: FSAC DAK-407/2, hypural plate (Fig. 3f, g).

Figure 3. Actinopterygii from Ad-Dakhla. (a–c) ?Ariidae. DAK-189, dorsal spine, in (a) anterior, (b) posterior and (c) right lateral views. (d, e) ?Scomberomorini, DAK-12, left dentary, in (d) left lateral and (e) dorsal views. (f, g) Scombroidei indet. DAK-407/2, hypural plate, in (f) anterior and (g) lateral views. (h, i) ?Scomberomorini. DAK-353, premaxilla, in (h) lateral and (i) ventral views. Scale bar equals 20 mm.
Description. Hypural plate corresponding to the fusion of at least the four first hypurals and probably the parhypural. The plate is broken anteriorly and posteriorly, precluding the observation of fused ural centra or a hypothetical hypural notch. A superficial ornamentation, formed by oblique straight grooves parallel to the anterior sides of the bone, corresponds to the marks left by the insertion of the caudal fin rays. A strong lateral crest, with a well-developed posterior process, is present on each side of the complex; this crest is interpreted as the parhypurapophysis. However, no foramen for the caudal artery is observed.
Comparison and discussion. Various patterns of fusion of hypurals are found in teleosts, and they can show an important intraspecific variability (see for example Monod, Reference Monod1968; Tyler et al. Reference Tyler, Johnson, Nakamura and Collette1989; Taverne & Gayet, Reference Taverne and Gayet2005; Castro Leal & Brito, Reference Castro Leal and Brito2007). The fusion of hypurals into a single plate associated with an extensive hypurostegy is present in different taxa such as the acanthuroids Luvarus imperialis and Arambourgthurus scombrurus (both with a free parhypural; Tyler et al. Reference Tyler, Johnson, Nakamura and Collette1989; Tyler, Reference Tyler2000) and in many scombroids. According to Johnson (Reference Johnson1986), this feature is considered as diagnostic of Scombridae (except Scombrini) and billfishes among Scombroidei, with some exceptions. Within these taxa, a complex including the fusion of the parhypural to the hypural plate is found in istiophorids, in scombrids like Acanthocybium, Gymnosarda, Katsuwonus (in some specimens) and Gasterochisma (Collette & Russo, Reference Collette and Russo1984; Kohno, Reference Kohno1984; Johnson, Reference Johnson1986; Carpenter, Collette & Russo, Reference Carpenter, Collette and Russo1995; Monsch, Reference Monsch2004) and also in numerous fossil forms such as Neocybium, Aglyptorhynchus or Palaeorhynchus (Leriche, Reference Leriche1910; Monsch & Bannikov, Reference Monsch and Bannikov2011; BK, pers. obs.). However, in most of these extant and fossil scombroids, the ‘neck’ of the complex located between the hypurals and the ural centra is thick, whereas it seems to be narrower on the specimen DAK-407/2 and better matches the proportions of a complex with a free parhypural. An assessment of the morphology of the anterior part of this bone will have to await the discovery of more complete material. The specimen DAK-407/2 is interpreted as an indeterminate Scombroidei based on the fusion of the parhypural to the hypural plate and the extensive hypurostegy.
Scombroid hypural plates are not rare in Eocene–Oligocene localities. Concerning the Moroccan fossil record, Arambourg (Reference Arambourg1952) described a small hypural plate from Ypresian deposits of the Oulad Abdoun and considered it as a possible ‘Cybium’. In this specimen the parhypural is fused to the hypural plate, as indicated by the presence of the foramen for the caudal artery, but the parhypurapophyses are probably broken. The ornamentation of caudal fin rays is comparable to that observed in DAK-407/2. Various other hypural plates, mainly assigned to scombrids and billfishes (Leriche, Reference Leriche1905, Reference Leriche1906, Reference Leriche1910; Casier, Reference Casier1966; Monsch, Reference Monsch2004), are known from Eocene and Oligocene deposits of Belgium, France and England.
Scombridae Rafinesque, Reference Rafinesque1815
? Scomberomorini Starks, Reference Starks1910
Material: FSAC DAK-12, incomplete left dentary (Fig. 3d, e); FSAC DAK-353, fragment of posterior part of right premaxilla (Fig. 3h, i); FSAC DAK-89, fragment of posterior part of premaxilla.
Description. DAK-12 (Fig. 3d, e) is an incomplete left dentary bearing a single row of 22 deeply inserted teeth. The teeth are subequal in size, broad, triangular, laterally compressed and slightly convex on their external and internal faces. They show a smooth surface and a sharp dorsal edge. Their section is subcircular at the base and devoid of pulp cavity. The space between the teeth is much reduced. The dentary is stout and slightly curved in dorsal view at its anterior extremity. The bone is broken at its ventral border but shows remains of a large longitudinal groove that probably corresponds to the insertion of the angulo-articular.
DAK-353 (Fig. 3h, i) is a fragment of the posterior part of the right premaxilla. The bone is slightly curved in dorsal and ventral views. The dorsal part is thicker than the ventral part, especially in the anterior portion. Some broken teeth are observed, with a subcircular section at their base and no pulp cavity. The teeth are deeply inserted in the bone and the diameter of their section slightly decreases posteriorly.
DAK-89 is a fragment of tooth-bearing bone, probably posterior part of premaxilla. The bone is small and curved along its anteroposterior axis in dorsal and lateral views. It is pierced by ovoid tooth sockets.
Comparison and discussion. The disposition and shape of the teeth in the specimen DAK-12 are comparable with those of the dentary or premaxilla of the extant scomberomorin Acanthocybium solandri or the Eocene Aramichthys dammeseki Signeux, Reference Signeux1959. In both species, the teeth are triangular and not much elongated, with a reduced interspace. Similar isolated teeth from Ypresian deposits of the Oulad Abdoun (Morocco) were assigned to ‘Cybium’ (=Scomberodon) dumonti by Arambourg (Reference Arambourg1952). Paleocybium proosti from Ypresian strata of the London Clay (UK) shows similar teeth but differs from the Ad-Dakhla specimen in the presence of a second row of minute teeth (Monsch, Reference Monsch2004). The specimen DAK-12 is considered as an indeterminate Scombridae, probably a Scomberomorini. In the specimen DAK-353, the tooth morphology (i.e. subcircular basal section, absence of pulp cavity and reduced interspace) fits well with that of DAK-12. This premaxilla could belong to the same taxon. It is considered as an indeterminate Scombridae, possibly a Scomberomorini. The specimen DAK-89 is not very diagnostic. It could correspond to the premaxilla of a large Scombridae, according to the shape of DAK-353.
Ostariophysi Sagemehl, Reference Sagemehl1885
Siluriformes Cuvier, Reference Cuvier1817
? Ariidae Bleeker, Reference Bleeker1858
Material: FSAC DAK-189, incomplete dorsal spine (Fig. 3a–c).
Description. DAK-189 (Fig. 3a–c) is a dorsal spine with broken distal extremity. A median crest ornamented with tubercles is located on the anterior face of the bone. These tubercles are irregularly disposed on the proximal part of the crest, while they form a single row more distally. Laterally, the ornamentation varies from granulous tubercles at the anterior rim to deep ridges giving a serrated surface more posteriorly. The posterior face of the spine shows a deep median groove with no denticles. The proximal part of the spine, which forms the articular head, bears a large subcircular median foramen that is slightly higher than wide. The lateral wings are stout but not very elongated. The posterior wings are well marked. With the exception of the angle between the articular head and the rest of the bone, the spine is subrectilinear in lateral view.
Comparison and discussion. The shape of the articular head (with a large foramen and well-developed lateral wings) and the ornamentation of the spine (formed by tubercles on the anterior crest) is reminiscent of the dorsal spine of many extant ariids, such as the genera Arius (e.g. A. gigas, A. latiscutatus; Gayet & van Neer, Reference Gayet and van Neer1990; BK, pers. obs.) or Sciades (e.g. S. dowii). A similar granular ornamentation is also found in late Eocene catfishes of Egypt, such as the genera Fajumia and Socnopaea from Qasr El-Sagha (Peyer, Reference Peyer1928) or Qarmoutus hitanensis from the Birket Qarun Formation (El-Sayed et al. Reference El-Sayed, Kora, Sallam, Claeson, Seiffert and Antar2017). However, as pointed out by Gayet & Meunier (Reference Gayet, Meunier, Malabarba, Reis, Vari, Lucena and Lucena1998, Reference Gayet, Meunier, Arratia, Kapoor, Chardon and Diogo2003) and Longbottom (Reference Longbottom2010), the morphology of the dorsal spine does not constitute a sufficient criterion for inclusion into ariids. Numerous fossil taxa with spines showing ornamentation with tubercles or a similar articular head were included into Arioidea (sensu Lundberg, Reference Lundberg and Goldblatt1993) or Silurifomes incertae sedis rather than Ariidae, such as Fajumia (Gayet & Meunier, Reference Gayet, Meunier, Arratia, Kapoor, Chardon and Diogo2003; Longbottom, Reference Longbottom2010). The precise affinities of the specimen DAK-189 from Ad-Dakhla remain unclear due to its fragmentary nature. We assign this specimen to Siluriformes incertae sedis, possibly related to Ariidae.
In the African fossil record, at least five to six siluriform families (Ariidae, Bagridae, Clariidae, Claroteidae, Mochokidae and possibly Schilbeidae) and numerous unidentified or incertae sedis forms have been described from Paleocene and Eocene localities, mainly in Egypt and Nigeria (see Gayet & Meunier, Reference Gayet, Meunier, Arratia, Kapoor, Chardon and Diogo2003 for review; Otero et al. Reference Otero, Pinton, Cappetta, Adnet, Valentin, Salem and Jaeger2015). Siluriform remains are rare in Morocco. Some spines are known from Thanetian deposits of the Ouarzazate basin (Gheerbrant et al. Reference Gheerbrant, Cappetta, de Lapparent de Broin, Rage, Tabuce and Zouhri2017) and an indeterminate fish spine was described from the Bathonian continental levels of the Anoual Formation by Haddoumi et al. (Reference Haddoumi, Allain, Meslouh, Metais, Monbaron, Pons, Rage, Vullo, Zouhri and Gheerbrant2016), but its identification is uncertain.
4.b. Chelonii
Pleurodira Cope, Reference Cope1864
Podocnemididae Cope, Reference Cope1868
Erymnochelyinae de Broin, Reference de Broin1988
Stereogenyina Gaffney et al., Reference Gaffney, Meylan, Wood, Simons and De Almeida Campos2011 partim
Subgroup Shweboemys nov.: Shweboemys group sensu de Broin, Reference de Broin1988 partim
Cf. Cordichelys Gaffney et al., Reference Gaffney, Meylan, Wood, Simons and De Almeida Campos2011
Aff. Cordichelys antiqua (Andrews, Reference Andrews1903)
Material: FSAC DAK-58/3 (Fig. 4i, j), plastral fragment; FSAC DAK-58/4 and FSAC DAK-121, possible plastral fragments; FSAC DAK-58/6, FSAC DAK-58/9-10 (Figs 4k, l, 5), FSAC DAK-128, FSAC DAK-132, FSAC DAK-133 (Figs 4a–c, 5), FSAC DAK-136 (Figs 4d–f, 5), FSAC DAK-185 (Figs 4o–r, 5), FSAC DAK-325/2, peripheral plates (complete or fragmentary); FSAC DAK-131 (Figs 4m, n, 5), FSAC DAK-407/1 (first right costal (Fig. 4g, h); and FSAC DAK-325/2, costal plates (fragmentary). Possible unlocated fragments: FSAC DAK-122, FSAC DAK-139.

Figure 4. Chelonii from Ad-Dakhla, Pleurodira (1). Aff. Cordichelys antiqua (Andrews, Reference Andrews1903). (a–c) DAK-133, anterior bridge peripheral (third or fourth), in (a) dorsal, (b) ventral and (c) posterior views. (d–f) DAK-136, first left peripheral in (d) dorsal, (e) ventral and (f) proximal views. (g, h) DAK-407/1, first left costal 1 in (g) dorsal and (h) ventral views. (i, j) DAK-58/3, axillary notch part of left hyoplastral fragment in (i) dorsal and (j) ventral views. (k, l) DAK-58/9-10, right costal 5 in (k) ventral and (l) dorsal views; (m, n) DAK-131, even right costal (fourth or sixth), medial part, in (m) ventral and (n) dorsal views. (o–r) DAK-185, right peripheral 8, posterior extremity of bridge in (o) ventral, (p) anterior, (q) posterior and (r) dorsal views. Scale bar equals 20 mm.
Description (Figs 4, 5). The surface of the specimens (various individuals of different sizes) is most often slightly worn, smooth as a whole and without clear dichotomic sulci and radiation marks; the scute sulci are not visible or barely so.
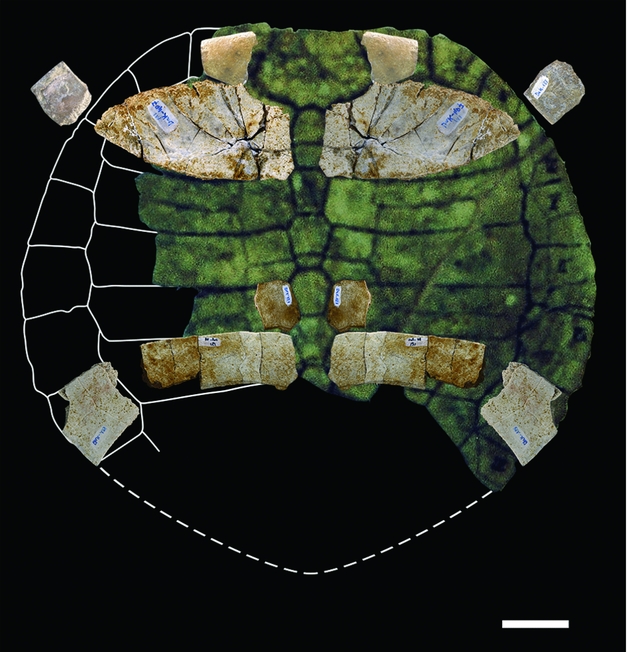
Figure 5. Chelonii from Ad-Dakhla, Pleurodira (2). Cordichelys antiqua, dorsal shell SMNS 87722 from Dacqué (Reference Dacqué1912, fig. 6), partially reconstructed with the Ad-Dakhla dorsal shell specimens of Figure 4 in anatomical position, using symmetry. Scale bar equals 20 mm.
Dorsal shell: Periphery: DAK-136 is a first left peripheral plate that is relatively short; its dorsal face exhibits some pits possibly representing injuries caused by aquatic fungi or bacteria. It is slightly rounded, its external border being rounded and thick internally (Figs 4d–f, 5). DAK-133 is the 3rd or 4th peripheral plate that includes the anterior bridge extremity; its external border is straight. As in the previous specimen, the ventral external border is rounded and not curled up and the plate is not wide (Figs 4a–c, 5). DAK-132 is a fragmentary bridge peripheral (5th?). DAK-58/6 is a partial bridge peripheral, probably the 6th. DAK-325/2 is a partial bridge peripheral toward the extremity, probably the 7th. DAK-128 is a fragmentary bridge peripheral at the posterior extremity, probably the 7th. DAK-185 is a right bridge peripheral 8, which is the last one closing the bridge at its posterior extremity; it is flat and large compared with the more anterior peripherals, with a thin and elongated free border forming an acute section (Figs 4o–r, 5); its scute sulci are visible. Pleural disk: DAK-407/1 is a complete right first costal plate that is twice as wide as it is long (Figs 4g, h, 5). In ventral view, it shows the large reniform scar cavity for the axillary process in lateral position and the lip with the two united first and second ribs in medial position. In dorsal view, the vertebral scutes 1 and 2 meet medially, and the first vertebral is wider than the nuchal suture, extending up to the mid-peripheral 1 suture. In spite of slight post-mortem flattening, the reduced curvature indicates a broad flattened shell at that level. The nuchal contact at costal 1 border and the moderate length of the rounded peripheral 1 make it possible to deduce that the nuchal was relatively short and wide, as shown in Figure 5. DAK-131 is a right costal plate fragment: the medial part without sulcus between vertebral scutes shows that it is an even plate, the 4th or the 6th (Figs 4m, n, 5). The short anterior peripherals and nuchal associated with a short pleural 1 indicate a wide anterior carapace. DAK-58/9-10 is a nearly complete right costal 5 (Figs 4k, l, 5) showing, ventrally and laterally, the short and rounded concave scar for the inguinal process; it is also poorly curved and wide, like the first one. The bridge peripheral plates are anteriorly flat without dorsal concave curvature before the lateroposterior bridge extremity, where they widen and flatten with a slight dorsal concave curve, making water flow easier during locomotion (Renous et al. Reference Renous, de Lapparent de Broin, Depecker, Davenport, Bels, Wyneken, Godfrey and Bels2007).
Plastron: DAK-58/3 is a fragment of left hyoplastron at the axillary notch part (Fig. 4i, j). Its anterior lobe borders are convergent anteriorly, and its slight bend results in a rather wide axillary notch. In littoral forms, this is linked with the shortness and particular width of the anterior lobe (not preserved here) and the short anterior dorsal carapace. DAK-58/4 and DAK-121 are possible plastral fragments (hyo-hypoplastra main parts?). No posterior lobe is preserved that would allow the observation of structures that facilitate swimming, such as posterior convergence of edges and wide inguinal notches that provide more space for hindlimbs. In transverse section, shell bones exhibit the characteristic histology of non-marine turtle dermal plates.
Unlocated fragments on the shell: at least DAK-122 and DAK-139.
Comparison and discussion. The Podocnemididae have a Gondwanan origin. Their fossil record dates back to Cretaceous time and extends until Oligocene time in western Europe and the Holocene–Anthropocene epoch in Africa-Madagascar and in the New World. Regarding the Erymnochelyinae de Broin, Reference de Broin1988 (Old World): (1) most forms have a continental shell pattern, including the Erymnochelys group sensu Pérez-García, de Lapparent de Broin & Murelaga (Reference Pérez-García, de Lapparent de Broin and Murelaga2017), which is mainly defined by the shortened intergular in front of the linked gulars; and (2) another fossil group, the subtribe Stereogenyina Gaffney et al. Reference Gaffney, Meylan, Wood, Simons and De Almeida Campos2011 partim, being here limited to the redefined Shweboemys new subgroup by the secondary palate morphotype, exhibits a littoral shell pattern. The Stereogenyina Gaffney et al. Reference Gaffney, Meylan, Wood, Simons and De Almeida Campos2011 is based on the alleged shared presence of an identical secondary palate, but many independent turtle clades have one which is not constructed in the same way, and which shows various morphotypes (among Bothremydidae, Cheloniidae, etc.).
The Stereogenyina is here separated into two subgroups (defined here): the first, Stereogenys new subgroup, includes Stereogenys cromeri Andrews, Reference Andrews1901, initially based on a single skull from the Qasr El-Sagha Formation (late Eocene). Stereogenys cromeri differs from all the Shweboemys new subgroup members in its autapomorphic secondary palate and the contours of the skull and lower jaw that provide a distinct and stronger crushing pattern. In Stereogenys, the lateral borders of the pterygoids are parallel to the axial line and the palatine part of the seconday palate is much shorter than the pterygoid part; the secondary palate is longer than in the second subgroup, and it reaches the posterior part of the processus trochlearis pterygoideus. In relation to these features, the lateral borders of the skull are more vertical, giving a more trapezoidal shape to the skull. The lower jaw also has a trapezoidal contour and a longer medial symphysis (see Andrews, Reference Andrews1901, Reference Andrews1906; Gaffney et al. Reference Gaffney, Meylan, Wood, Simons and De Almeida Campos2011, figs 77, 79, 80 but not fig. 75 and not the jaw in fig. 81, that belongs to a Shweboemys subgroup member; references with figures in Weems & Knight, Reference Weems, Knight, Brinkman, Holroyd and Gardner2013; Ferreira et al. Reference Ferreira, Rincón, Solórzano and Langer2015).
The second subgroup of the Stereogenyina consists of the Shweboemys division (newly defined here), which is characterized by the following features: crushing secondary palate that corresponds to a skull outline with rounded lateral borders; palate showing anteriorly diverging lateral borders of palatine; palate longer than the lateral pterygoid borders of the secondary palate, which has a moderate length and ends anteriorly to the processus trochlearis pterygoideus; jaw symphysis not reaching the articular process (Gaffney et al. Reference Gaffney, Meylan, Wood, Simons and De Almeida Campos2011, fig. 81; Thomas et al. Reference Thomas, Sen, Khan, Battail and Ligabue1982; material, MNHN.F deposit). The associated shells are adapted to the littoral pattern for enhanced swimming abilities: wide flattened shape; anterior part short with relatively wide nuchal (more or less long but always wide in the shell) and wide anterior peripherals, the latter having a rounded edge; posterior part of shell wider with lateroposteriorly expanded peripherals having an acute border; plastron showing wide axillary and inguinal notches, short and wide anterior lobe and convergent non-rounded lateral borders of posterior lobe. The plates are somewhat smooth-granulated or decorated with small polygones and short divided sulci. Some of these littoral adaptations (shell, secondary palate) appear occasionally and homoplastically in the littoral Bothremydidae (Antunes & de Broin, Reference Antunes and de Broin1988; Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006). The Shweboemys subgroup includes: Shweboemys s.l. auct. before Gaffney et al. Reference Gaffney, Meylan, Wood, Simons and De Almeida Campos2011 (i.e. Pocdonemis/Cordichelys antiqua, Shweboemys pilgrimi and Shweboemys/Brontochelys gaffneyi), ‘Podocnemis’ bramlyi, Lemurchelys diasphax and Latentemys plowdeni, all from the Old World (from Africa to Burma); and Bairdemys spp. from north and central South America (see Pérez-García et al. Reference Pérez-García, de Lapparent de Broin and Murelaga2017). Most of them are known only from the skull, except Cordichelys and Bairdemys. All species of Bairdemys include the skull, and few of them also preserve the lower jaw and the shell. Several ‘Shweoboemys s.l. sp.’ specimens from Miocene strata of Saudi Arabia (Thomas et al. Reference Thomas, Sen, Khan, Battail and Ligabue1982; MNHN.F. deposit), including the lower jaw and shell parts, are also referrable to the Shweboemys new subgroup because of their shared characteristics with Bairdemys (lower jaws) and with Bairdemys and Cordichelys (shells). The species ‘Podocnemis’ antiqua Andrews, Reference Andrews1903 (senior synonym of ‘P.’ stromeri von Reinach, Reference von Reinach1903 and Dacqué, Reference Dacqué1912) comes from the upper Eocene Qsar El-Sagha beds of the Fayum (Egypt) and was initially based on shells. Gaffney et al. (Reference Gaffney, Meylan, Wood, Simons and De Almeida Campos2011) defined Cordichelys antiqua (Andrews, Reference Andrews1903) based on additional material including shell (similar to ‘P. antiqua/stromeri’) associated with skull. The Ad-Dakhla specimens are tentatively referred to this species, although cranial material is not preserved. The reconstructed dorsal shell from Ad-Dakhla (Fig. 5) fits well with the original figures of Cordichelys antiqua and particularly with one specimen of Dacqué (Reference Dacqué1912), in spite of post-mortem flattening of Ad-Dakhla plates. The littoral adaptive characters mentioned above are present: shape of the nuchal, rounded anterior peripheral borders, outlines/relative proportions of elements, and identical plastral process insertions. These features confidently exclude all the other forms from the Fayum (see following paragraph). Between late Eocene and Plio-Pleistocene time, the littoral Shweboemys subgroup nov. (without Stereogenys) expanded from Africa to the Arabian Peninsula (Thomas et al. Reference Thomas, Sen, Khan, Battail and Ligabue1982, Reference Thomas, Roger, Sen, Dejax, Schuler, Al Sulaimani, Bourdillon de Grissac, Breton, de Broin, Camoin, Carpetta, Carriol, Cavelier, Chaix, Crochet, Farjanel, Gayet, Gheerbrant, Lauriat-Rage, Noel, Pickford, Poignant, Rage, Roman, Rouchy, Secrétan, Sigé, Tassy and Wenz1991; Roger et al. Reference Roger, Pickford, Thomas, de Lapparent de Broin, Tassy, Van Neer, Bourdillon De Grissac and Al-Busaldi1994) up to Burma on the one hand (Shweboemys, Brontochelys) and up to North America – northern South America on the other (Bairdemys) (de Lapparent de Broin, Reference de Lapparent de Broin2001; Gaffney et al. Reference Gaffney, Meylan, Wood, Simons and De Almeida Campos2011; Weems & Knight, Reference Weems, Knight, Brinkman, Holroyd and Gardner2013; Ferreira et al. Reference Ferreira, Rincón, Solórzano and Langer2015; Pérez-García et al. Reference Pérez-García, de Lapparent de Broin and Murelaga2017). The presence of the Shweboemys subgroup in Ad-Dakhla is a supplementary milestone in its African distribution, in relation to Egypt.
The other Fayum turtles differ from the Ad-Dakhla species. Stereogenys cromeri Andrews, Reference Andrews1903 (upper Eocene of the Fayum) was based on an isolated skull. Isolated shell elements were attributed to this species (Andrews, Reference Andrews1903). These were later distinguished from the genus Stereogenys (Gaffney et al. Reference Gaffney, Meylan, Wood, Simons and De Almeida Campos2011) because they are not associated with the skull and remain without any new generic attribution, although they were the only available shells that could be attributed to the Stereogenys skull in the upper Eocene Fayum beds. Consequently, ‘Stereogenys’ libyca Andrews, Reference Andrews1903 (lower Oligocene of the Fayum), also based on an isolated shell and basically similar to the previous one, was removed from the genus Stereogenys. Shells from both species share an apomorphic gular-intergular complex (distinct from those of Erymnochelys group and Shweboemys subgroup) and a narrow nuchal shape, in contrast to the Ad-Dakhla form. The tandem “‘Stereogenys’ libyca/‘S’. cromeri shell” belongs to the continental erymnochelyines, as do the African Erymnochelys group members (fig. 1 in Pérez-García et al. Reference Pérez-García, de Lapparent de Broin and Murelaga2017). Along with ‘Stereogenys’ podocnemoides von Reinach, Reference von Reinach1903, another shell from the upper Eocene beds, these Fayum ‘species’ show a continental shell morphotype that is adapted for a better protection of limbs and body on ground: carapace narrower and more domed, not (‘cromeri’, podocnemoides) or slightly (libyca) posteriorly expanded; anterior borders not rounded; and plastral lobes longer and wider, the posterior lobes being rounded with their lateral borders not directly convergent posteriorly, particularly in ‘cromeri’/libyca as in the African continental box-turtle Pelusios and in contrast to the Ad-Dakhla littoral specimens.
Cryptodira Cope, Reference Cope1868
Dermochelyoidea Fitzinger, Reference Fitzinger1843
Dermochelyidae Fitzinger, Reference Fitzinger1843
Cf. Egyptemys Wood et al. Reference Wood, Johnson Gove, Gaffney and Maley1996
Cf. Egyptemys sp.
Material: FSAC DAK-58/7 (Fig. 6i, j), FSAC DAK-138 (Fig. 6k–m), smooth ossicles.

Figure 6. Chelonii from Ad-Dakhla, Testudinidae indet. (a–d) DAK-116/1, right peripheral plate, posterior extremity of bridge in (a) external, (b) medial, (c) anterior and (d) posterior views. (e, f) DAK-130, octogonal neural in (e) dorsal and (f) ventral views. Cheloniidae indet. (g, h) DAK-123, first neural in (g) dorsal and (h) ventral views. Dermochelyidae Cf. Egyptemys sp., (i–o) smooth ossicles, (i, j) DAK-58/7, complete dorsal ossicle in (i) dorsal and (j) ventral views. (k–m) DAK-138, incomplete dorsal ossicle in (k) dorsal, (l) ventral views and (m) cross-section; decorated ossicles (n, o) DAK-135, dorsal ossicle in (n) dorsal and (o) ventral (o) views. Scale bar equals 20 mm.
Description (Fig. 6i–m). DAK-58/7 and DAK-138 are incomplete ossicles (or platelets) from unkeeled rows (or ridges) in the epithecal shell, which may be adjacent to keeled ridges. They are externally smooth (i.e. without tubercles organized in radiate ridges), and the histological structure of the bone is spongy (Fig. 6m) as described in Psephophorus (Delfino et al. Reference Delfino, Scheyer, Chesi, Fletcher, Gemel, Macdonald, Rabi and Salisbury2013). DAK-58/7 is polygonal, elongated (nearly complete), relatively smooth, without either carina or radiate ornamentation (Fig. 6i, j). The dorsal surface is poorly undulated, roughly marked by polygons, delimited by fine sulci, and bears minute nutritive foramina. The visceral side is slightly concave with some tiny and some larger foramina. Laterally, seven preserved concave edges (probably nine in the complete ossicle) articulate with other ossicles. DAK-138 is widened and thicker than the previous ossicle (Fig. 6k–m), and six of the eight articular sides are preserved. The surface of both faces is marked by small polygons. The dorsal face bears minute foramina. The slightly concave visceral face is denser with one bigger hole and one visible small foramen.
Material: FSAC DAK-135 (Fig. 6n, o), decorated ossicle.
Description (Fig. 6n, o). DAK-135 is a complete polygonal ossicle. It is roughly hexagonal, elongated, longitudinally keeled, and forms part of a keeled ridge (or row) in the shell. It is slightly shallower than the two previous smooth ossicles. It measures c. 4 cm at its widest point. The dorsal face has a medial longitudinal carina, and a weak ornamentation which consists of small aligned rounded tubercles that diverge radially from the carina and form ridges separated by sulci. The visceral side is smooth as a whole, with fine radiating lines, nutritive foramina and few small scattered cupules or undulations. The anterior and the posterior sides are straight, perpendicular to the carina, and contacted identical plates in a keeled ridge. The lateral sides are irregular and form three concave edges separated by protrusion points, in order to interlock with ossicles of other unkeeled ridges.
Comparison and discussion. Dermochelyidae are open sea turtles with rigid flippers. They show a significant osseous reduction leading to changes in the skull and shell, including formation of an epithecal carapace made of a superficial mosaic of ossicles taken in the hard skin (leather), and reduction of the dermal thecal carapace in more recently developed forms. The family is tentatively recorded (Bardet et al. Reference Bardet, Jalil, De Lapparent De Broin, Germain, Lambert and Amaghzaz2013) from Late Cretaceous time (Hirayama & Chitoku, Reference Hirayama and Chitoku1996), and is known with certainty from Eocene time onwards (Wood et al. Reference Wood, Johnson Gove, Gaffney and Maley1996; Delfino et al. Reference Delfino, Scheyer, Chesi, Fletcher, Gemel, Macdonald, Rabi and Salisbury2013). The Tertiary occurrences of dermochelyids include all continents (see Wood et al. Reference Wood, Johnson Gove, Gaffney and Maley1996 for review; Zvonok, Danilov & Syromyatnikova, Reference Zvonok, Danilov and Syromyatnikova2013). Dermochelyid genera or species without epithecal shell exist, such as Eosphargis from Eocene deposits of England, Belgium and Denmark (Quintart & Plisnier-Ladame, Reference Quintart and Plisnier-Ladame1968; Wood et al. Reference Wood, Johnson Gove, Gaffney and Maley1996; de Lapparent de Broin, Reference de Lapparent de Broin2001). Cardiochelys rupeliensis (van Beneden, Reference van Beneden1883) from Oligocene deposits of Belgium (Moody, Reference Moody1993; Wood et al. Reference Wood, Johnson Gove, Gaffney and Maley1996; de Lapparent de Broin, Reference de Lapparent de Broin2001) and Natemys peruvianus Wood et al. Reference Wood, Johnson Gove, Gaffney and Maley1996 from Oligocene strata of Peru differ from Ad-Dakhla specimens in the shape of the smooth ossicles, those of C. rupeliensis being very thick.
The extant Dermochelys Blainville, Reference Blainville1816 has a worldwide oceanic distribution. It shows very thin ossicles arranged in sutured keeled and unkeeled ridges (Gervais, Reference Gervais1872; de Broin & Pironon, Reference de Broin and Pironon1980; Wood et al. Reference Wood, Johnson Gove, Gaffney and Maley1996; Delfino et al. Reference Delfino, Scheyer, Chesi, Fletcher, Gemel, Macdonald, Rabi and Salisbury2013). Keeled ridges of large ossicles (shape similar to that of the decorated plate DAK-135 but with a larger size) alternate with irregular ridges of flat irregular tiny ossicles (shape less similar to those of Ad-Dakhla flat ossicles DAK-58/7 and DAK-138). Dermochelys plates are smooth as in DAK-58/7 and DAK-138, but they are much thinner than in the Ad-Dakhla specimens and not decorated as in DAK-135.
Comparable fossil taxa: Cosmochelys dolloi Andrews, Reference Andrews1919, from middle Eocene strata of Nigeria, has polygonal thin ossicles ornamented by ridges of low small tubercles. Its keeled ossicles show similarities only to the complete plate DAK-135 from Dakhla. However, this specimen is relatively longer and deeper, with wider tubercles and narrower spaces between sulci. DAK-135 therefore belongs to a different taxon, more similar in shape to keeled ossicles of Ps. polygonus than to the thin Cosmochelys plates, as in DAK-58/7 and DAK-138.
Specimens of Eocene–Pliocene age from Europe, New Zealand, North Africa including Fayum (Egypt) and America (Wood et al. Reference Wood, Johnson Gove, Gaffney and Maley1996) that can be compared with the material from Ad-Dakhla have been attributed to Psephophorus von Meyer, Reference von Meyer1847 (type species P. polygonus von Meyer, Reference von Meyer1847, from middle Miocene deposits of Slovakia) (Mlynarski, Reference Mlynarski1976; Wood et al. Reference Wood, Johnson Gove, Gaffney and Maley1996; de Broin & Pironon, Reference de Broin and Pironon1980; Delfino et al. Reference Delfino, Scheyer, Chesi, Fletcher, Gemel, Macdonald, Rabi and Salisbury2013). The two smooth plates DAK-58/7 and DAK-138 are similar to ossicles of unkeeled ridges of Psephophorus polygonus in the shape, depth, histology and smooth ornamentation (see Delfino et al. Reference Delfino, Scheyer, Chesi, Fletcher, Gemel, Macdonald, Rabi and Salisbury2013, figs 1, 4). However, there are no decorated plates as in DAK-135. Based on the few isolated plates, it is not possible to compare at the specific level because the shape of ossicles and the arrangements in ridges – with or without carina, and more or less regular – is very variable within the genus and type species, as well as in the other genera. However, the two smooth plates from Ad-Dakhla might correspond to the variability within the genus. DAK-135 is similar in shape to one keeled ossicle of Ps. polygonus (see Delfino et al. Reference Delfino, Scheyer, Chesi, Fletcher, Gemel, Macdonald, Rabi and Salisbury2013, fig. 1m), this ossicle being possibly slightly decorated as in DAK-135.
Egyptemys was erected by Wood et al. Reference Wood, Johnson Gove, Gaffney and Maley1996 for Psephophorus eocaenus Andrews, Reference Andrews1901, from upper Eocene strata of the Fayum. Egyptemys eocaenus (Andrews, Reference Andrews1901) was originally based on a single humerus. Wood et al. (Reference Wood, Johnson Gove, Gaffney and Maley1996) attributed additional shell material to this species. The shell material comprises unkeeled and keeled rows including ossicles comparable in shape to those of Ad-Dakhla and Psephophorus, although the depth is not given. Wood et al. (Reference Wood, Johnson Gove, Gaffney and Maley1996) indicated that ‘evidence of dimpling . . . on the external surface indicate that its fresh, not eroded surface was textured with some kind of decoration, perhaps akin to that of Cosmochelys’. However, illustrations are not helpful for comparison with Ad-Dakhla plates, and decoration is individually variable according to the position of plates in the shell. Nevertheless, it is important to notice that both Ad-Dakhla specimens and Egyptemys are of late Eocene age and occur in the same African littoral environment, whereas Psephophorus s.s., which has similar plates (keeled and unkeeled, but possibly never decorated), is known with certainty only from Miocene time in Europe. The Ad-Dakhla material could therefore belong to Egyptemys if, as hypothesized previously, a decoration was originally present only in some places, as in Psephophorus (Delfino et al. Reference Delfino, Scheyer, Chesi, Fletcher, Gemel, Macdonald, Rabi and Salisbury2013, fig. 1m).
Isolated dermochelyid smooth plates, such as those of middle Eocene age from Crimea (Zvonok, Danilov & Syromyatnikova, Reference Zvonok, Danilov and Syromyatnikova2013), are not comparable with the Ad-Dakhla material and remain indetermined.
Arabemys Tong et al. Reference Tong, Buffetaut, Thomas, Roger, Halawani, Memesh and Lebret1999, from Paleocene Saudi Arabian deposits, is not a Dermochelyidae because its isolated dermal plates strongly differ in shape (outline and depth) and histological structure from those of all known dermochelyids.
Chelonioidea Oppel, Reference Oppel1811
Cheloniidae Oppel, Reference Oppel1811
Cheloniidae indet.
Material: FSAC DAK-123 (Fig. 6g, h), isolated neural plate; FSAC DAK-127, fragment of neural plate.
Description (Fig. 6g, h). DAK-123 is a nearly complete first neural plate that is thick and relatively wide (the width is nearly two-thirds of the length). A possible indentation of the anterior side is located just medial to the left anterior corner. The anterior part is slightly widened and the lateral borders are slightly rounded. The posterior side is rounded and originally contacted the concave anterior border of the second neural. The dorsal side is roughly smooth and somewhat eroded, but actually shows an undulated surface with very low pits. The posterior part of the dorsal side exhibits short minute rounded ridges that radiate toward the posterior border. DAK-123 has no preserved transversal groove for the contact of vertebrals 1 and 2, although such a groove is possibly partly preserved on the right side lateral to the posterior catalogue number. The ventral side shows an elongated ovoid imprint of the neural arch of the first thoracic vertebra that is located medially on two-thirds of the length, without anterior and posterior extension except a thin axial line. DAK-127 is a partly preserved first neural that is eroded dorsally and smooth. It has the same size and thickness as DAK-123. Broken sides indicate substantial bone porosity.
Comparison and discussion. The plates belong to the Cheloniidae and are similar to those of Lepidochelys or Caretta, due to the relative width of DAK-123 and the shape of both DAK-123 and DAK-127. The bone porosity is consistent with the Cheloniidae. Frequent in Chelonioidea (de Lapparent de Broin et al. Reference de Lapparent de Broin, Murelaga, Farrés and Altimiras2014, Supplementary data), the marked ornamentation generally consists of ridges and tubercles and rarely pits. The present morphology with pits is not recorded in a named chelonioid taxon in Eocene times. The cheloniid ‘Thalassochelys’ libyca Andrews, Reference Andrews1901 (Andrews, Reference Andrews1906) has been found in the upper Eocene Qasr El-Sagha beds of the Fayum (Egypt). This cheloniid is figured only by one of the crushed skulls and is not generically determined.
Testudinoidea Batsch, Reference Batsch1788
Testudinidae Batsch, Reference Batsch1788
Testudinidae indet.
Material: FSAC DAK-116/1 (Fig. 6a–d), right seventh peripheral plate, posterior extremity of the bridge. FSAC DAK-130 (Fig. 6e, f), possible neural plate.
Description (Fig. 6a–f). The plate DAK-116/1 is high for its anteroposterior width, with an open angle over 90°. The bone is thick. The external surface is irregular and shows rough concentric and tuberculate ridges towards the angle. The inner face (Fig. 6b) and the anterior face (Fig. 6c) show the cavity that closes the bridge posteriorly thanks to the 8th plate, their common suture being high on the common face. The maximum height is about 7 cm. DAK-130 (Fig. 6e, f) is a small plate with five preserved sides. The anterior border is angular and represents the bone section. The ventral face shows the medial scar for the neural arch of a thoracic vertebra, which consists of a small canal on two-thirds of the bone and longer than wide. The broken side may represent one or two sides for the free borders of the differentiated neural of a terrestrial testudinid (as presented in Fig. 6e, f), which was heptagonal or octogonal. The plate is crossed dorsally by a vertebral scute junction; the sulcus is possibly between vertebral scutes 4/5 or 5/6, but it is sinuous and has an unusual trajectory.
Comparison and discussion. Due to the open angle of the bridge and the annuli ondulations, DAK-116/1 belongs to a large-bodied, fully terrestrial undetermined testudinid. DAK-130 belongs to a testudinid due to the alternative neural differentiation (de Broin, Reference de Broin1977). In Fayum (Egypt), the lower Oligocene Qatrani Formation has yielded the giant species Gigantochersina ammon (Andrews, Reference Andrews1903) (in Andrews & Beadnell, Reference Andrews and Beadnell1903; de Lapparent de Broin, Reference de Lapparent de Broin2000), which was the first certain record of the Testudinidae in Africa. Terrestrial testudinids were also present during early Oligocene time in the Arabian area at Taqah (Thomas et al. Reference Thomas, Roger, Sen, Dejax, Schuler, Al Sulaimani, Bourdillon de Grissac, Breton, de Broin, Camoin, Carpetta, Carriol, Cavelier, Chaix, Crochet, Farjanel, Gayet, Gheerbrant, Lauriat-Rage, Noel, Pickford, Poignant, Rage, Roman, Rouchy, Secrétan, Sigé, Tassy and Wenz1991). The presence of the Testudinidae in Priabonian strata of Ad-Dakhla widens the stratigraphical range of this taxon in Africa.
4.c. Crocodilia
Eusuchia Huxley, Reference Huxley1875
Eusuchia indet.
Material: FSAC DAK-310 (Fig. 7n–r), posterior cervical vertebra; FSAC DAK-342 (Fig. 7d, e), fragmentary dorsal centrum; FSAC DAK-341 (Fig. 7f–j), lumbar centrum; FSAC DAK-157 (Fig. 7k, l), dorsal osteoderm; FSAC DAK-58/1 (Fig. 7m), partial ventral osteoderm; FSAC DAK-156 (Fig. 7v–y) and FSAC DAK-155 (Fig. 7s–u), teeth; FSAC DAK-134 (Fig. 7a–c), partial femur.

Figure 7. Crocodilia from Ad-Dakhla, Eusuchia indet. (a–c) DAK-134, distal part of right femur in (a) anterior, (b) ventral and (c) posterior views. (d, e) DAK-342, fragment of centrum of procoelous dorsal vertebra in (d) ventral and (e) right lateral views. (f–j) DAK-341, centrum of procoelous lumbar vertebra in (f) ventral, (g) dorsal, (h) cranial, (i) left lateral and (j) caudal views. (k, l) DAK-157, dorsal osteoderm in (k) external and (l) lateral views. (m) DAK-58/1, osteoderm in dorsal view. (n–r) DAK-310, procoelous posterior cervical vertebra in (n) cranial, (o) caudal, (p) ventral, (q) left and (r) right lateral views; (s–u) DAK-155, tooth in (s) lateral, (t) posterobasal and (u) opposite lateral views. (v–y) DAK-156, tooth in (v) lateral, (w) posterior (distal), (y) basal and (x) opposite lateral views. Scale bars equal (a–r) 20 mm and (s–y) 30 mm.
Description. Procoelous vertebrae (Fig. 7d–j, n–r). The best-preserved vertebra DAK-310 (Fig. 7n–r) is a tall (c. 12 cm high) posterior cervical or anterior dorsal vertebra, based on the presence of two features: a wide U-shaped hypapophysis projecting ventrally below the anterior part of the centrum; and a dorsal diapophysis/transverse apophysis and ventral parapophysis for attachment of the bicephalous cervical ribs on the lateral part of the centrum. This specimen is probably a posterior cervical because the transverse apophysis is still directed ventrally, but this ventral projection varies depending on the position on the column in the various families. DAK-342 (Fig. 7d, e) is the partial centrum of a dorsal vertebra that is shorter than DAK-310 and lacks bicephalous rib apophyses. This vertebra is positioned towards the middle of the column because it lacks the slight anteroventral protuberance (remains of the hypapophysis that gradually diminishes in height posteriorly) and because the ventral face is regularly rounded, without longitudinal sulcus separating two longitudinal convexities, a condition that occurs in lumbar vertebrae. DAK-341 (Fig. 7f–j) consists of a centrum shorter than DAK-342. This specimen is a lumbar vertebra because it is devoid of hypapophysis, and because of the medial longitudinal sulcus of the ventral face. All three vertebrae have a marked procoely with a very prominent posterior condyle, and probably belong to the same taxon.
Osteoderms (Fig. 7k–m). DAK-157 (Fig. 7k, l) is regarded as a dorsal osteoderm due to the presence of a flat anterior facet for articulation with the preceding plate and a medial convexity forming a low longitudinal ridge, from which irregular rounded pits radiate out in rows. The pits are smaller in some places, especially in the anteriormost transversal row and towards the borders. They are separated by smooth ridges that are as wide as or slightly narrower than the pits. Laterally, the osteoderm articulated by means of sutures with other osteoderms of the same transversal row. DAK-58/1 (Fig. 7m) is a lateral part of a flat osteoderm showing a dorsal anterior facet and a left lateral suture for articulation with another osteoderm of the same transversal row. Pits and ridges are similar to those of the previous plate. Pits are slightly smaller than in DAK-157 and do not radiate from a medial longitudinal row. They are larger anteriorly and smaller at the left lateral border and posteriorly. Without longtudinal ridge, the specimen belongs to the ventral armor of a crocodile with ventral scutes overlapping each other in the rows, anteriorly to posteriorly.
Teeth (Fig. 7s–y). DAK-156 (Fig. 7v–y) is a tall, slender and curved tooth including the root. It is subcircular in section and slightly flattened laterally, with two weak lingual and labial carinae. The enamel is bright, rather smooth and finely striated. DAK-155 (Fig. 7s–u) is a shorter and stouter tooth including the root. It is circular in basal section, and the enamel is bright and completely smooth with some faint postmortem fractures. The two teeth have no marked neck collar. The apex shows wear surfaces caused by contact with the opposite tooth in both specimens.
Femur (Fig. 7a–c). DAK-134 is a distal half of a right femur. The shaft is curved and widens at the distal extremity, which shows a strong convexity for the tibial facet.
Comparison and discussion. The material may belong to one or several taxa of Eusuchia (vertebral procoely). When compared with a modern Crocodylus niloticus skeleton, all elements could belong to only one taxon. Compared with the fauna from the Fayum, the cervical vertebra is similar to that assigned and figured by Andrews (Reference Andrews1906) to the longirostrine ‘Tomistoma’ africanum Andrews, Reference Andrews1901 from Qasr El-Sagha beds (late Eocene). The skull of this ‘Tomistoma’ was later assigned to the genus Eogavialis Buffetaut, Reference Buffetaut1982. However, the Ad-Dakhla vertebrae differ from those of the Gavialidae in the stronger and more globulous condyle (DAK-310), and resemble the extant Crocodylus niloticus. These Ad-Dakhla vertebrae could match the size of the brevirostrine ‘Crocodylus’ megarhinus Andrews, Reference Andrews1905 (late Eocene Fayum beds, synonymized with the early Oligocene ‘C’. articeps Andrews, Reference Andrews1905) for which the axial column, teeth and armor are not known. On the other hand, without marked neck collar the Ad-Dakhla teeth are not definitely available for the ‘Crocodylus’ lineage (see Prasad & de Lapparent de Broin, Reference Prasad and de Lapparent de Broin2002, pl. 15), and the assemblage of the slender anterior tooth and the short stout tooth possibly better fits with a longirostrine crocodile such as the aforementioned Eogavialis, among all gavialoids (Delfino, Piras & Smith, Reference Delfino, Piras and Smith2005; Brochu, Reference Brochu2006). It seems that the teeth of the Paleocene North American gavialoid Eosuchus spp. could match those from Ad-Dakhla. Its osteoderms (Brochu, Reference Brochu2006) are very similar to the Ad-Dakhla remains and unlike those of the gavialoid Argochampsa from Paleocene-aged deposits of the Moroccan Phosphates (Jouve et al. Reference Jouve, Iarochène, Bouya and Amaghzaz2006), based on pit morphology and the absence of strong carina. Similarly, proportions of cervical vertebrae of Argochampsa differ from those of the other previous taxa, with a less prominent condyle than in Ad-Dakhla specimens and Crocodylus. The crocodile sample from upper Eocene strata of Dur At-Talah (Libya) (Llinás Agrasar, Reference Llinás Agrasar2004) is partly similar to that of the Fayum because of the presence of a Gavialidae indet. and a Tomistominae indet., but it lacks postcranial material to compare with Ad-Dakhla elements. In addition, the only preserved Dur At-Talah vertebra is amphicoelous and constitutes the youngest record of a mesosuchian crocodile in Africa. However, the Mesosuchia are not recorded in the Fayum and Ad-Dakhla, where the crocodile vertebrae are all procoelous.
4.d. Serpentes
Ophidia Brongniart, Reference Brongniart1800
Palaeophiidae Lydekker, Reference Lydekker1888
Palaeophiinae Lydekker, Reference Lydekker1888
Pterosphenus Lucas, Reference Lucas1899
Pterosphenus sp.
Material: FSAC DAK-349 (Fig. 8a–f), FSAC DAK-350 (Fig. 8g–l), isolated vertebrae.

Figure 8. Serpentes from Ad-Dakhla, Pterosphenus sp., vertebrae. (a–f) DAK-349, in (a) anterior, (b) posterior, (c) dorsal, (d) ventral, (e) left lateral and (f) right lateral views. (g–l) DAK-350, in (g) anterior, (h) posterior, (i) dorsal, (j) ventral, (k) left lateral and (l) right lateral views. Scale bar equals 10 mm.
Description. The vertebra DAK-350 provides less information than the vertebra DAK-349, but probably belongs to the same taxon (Fig. 8). The vertebrae are clearly compressed laterally and the prezygapophyses are markedly reduced. The paradiapophyses, only preserved in DAK-349, are strongly displaced ventrally and markedly project below the centrum. The pterapophyses are broken, but their remaining bases show that they were well developed. The condyle and cotyle are broad compared to the section of the neural canal. As is common in palaeophiids the dorsal part of the cotyle is truncated, whereas the condyle shows a more or less triangular shape, its ‘tip’ being ventral. The anteroposterior axis of the condyle is horizontal. The interzygapophyseal constriction is very shallow and its bottom is formed on either side by the straight interzygapophyseal ridge. In both vertebrae, the sagittal area of the centrum is damaged. It is not possible to determine whether an anterior hypapophysis was present. The roof of the zygosphene of DAK-349 and DAK-350 is raised and forms the base of the anterior border of the neural spine. However, in the two Ad-Dakhla vertebrae, this character is not as pronounced as in most vertebrae of Pterosphenus.
Comparison and discussion. Although the material only consists of two isolated vertebrae, the present record is of considerable significance because fossil snakes from the Palaeogene deposits are extremely poorly known in Africa (McCartney & Seiffert, Reference McCartney and Seiffert2016). The two vertebrae DAK-349 and DAK-350 belong indisputably to the Palaeophiinae, a subfamily of the highly aquatic Palaeophiidae. The Palaeophiidae is a family of ?Cenomanian/Maastrichtian – late Eocene age (Rage & Werner, Reference Rage and Werner1999; Rage et al. Reference Rage, Bajpal, Thewissen and Tiwari2003) that includes two subfamilies: the Palaeophiinae Lydekker, Reference Lydekker1888; and the Archaeophiinae Janensch, Reference Janensch1906. Palaeophiidae are characterized on the basis of vertebral morphology: vertebrae more or less compressed laterally; tendency towards reduction of prezygapophyses; presence of pterapophyses in at least part of the vertebrae; and axis of the cotyle and condyle horizontal or nearly horizontal. Variation within the vertebral column is well known only in the trunk (= precloacal) region of the presumed basal Palaeophis, P. maghrebianus (Houssaye et al. Reference Houssaye, Rage, Bardet, Vincent, Amaghzaz and Meslouh2013). Intracolumnar variation is poorly known in other Palaeophiinae, specifically in more advanced species, so that the original position of disarticulated vertebrae along the vertebral column is extremely difficult to infer.
The Archaeophiinae are distinguished from the Palaeophiinae by their less stout, more elongated vertebrae and the triangular cross-section of the centrum (which is unusual for snakes). In addition, their paradiapophyses (articulations for ribs) are not well developed and the zygapophyseal plane is higher than in Palaeophiinae (Rage et al. Reference Rage, Bajpal, Thewissen and Tiwari2003, pp. 697–698).
The Palaeophiinae includes species of various sizes that form a morphological series from slightly adapted to strongly specialized for aquatic life. They include two genera: Palaeophis Owen, Reference Owen1841 and Pterosphenus Lucas, Reference Lucas1899. However, Rage et al. (Reference Rage, Bajpal, Thewissen and Tiwari2003) recognized three ‘evolutionary stages’ in the Palaeophiinae.
(1) ‘Primitive’ Palaeophis: species with vertebrae weakly compressed laterally; pterapophyses weakly developed; prezygapophyses not very small; and paradiapophyses slightly displaced ventrally. Two African species show this morphology: Palaeophis maghrebianus Arambourg, Reference Arambourg1952 and P. colossaeus Rage, Reference Rage1983a.
(2) ‘Advanced’ Palaeophis: species with laterally compressed vertebrae; pterapophyses developed; prezygapophyses significantly reduced; and paradiapophyses markedly displaced ventrally. An African palaeophiid, P. africanus (of middle Eocene age) appears to be morphologically intermediate between these two Palaeophis assemblages.
(3) Pterosphenus: vertebrae relatively more slender and more laterally compressed, with high pterapophyses; prezygapophyses at least as small as in advanced Palaeophis; and paradiapophyses at least as displaced ventrally as in advanced Palaeophis. In addition, in Pterosphenus, the roof of the zygosphene is arched dorsally and forms the base of the anterior border of the neural spine (Rage, Reference Rage1983b). Generally, the anterior border of the neural spine reaches the anterior border of the zygosphene. In contrast, the distinction between the zygosphenal roof and the neural spine is marked in Palaeophis.
However, rare vertebrae appear to be morphologically intermediate between advanced Palaeophis and Pterosphenus. This is the case for the single vertebra assigned to Palaeophis nessovi from the Priabonian of Kazakhstan (Averianov, Reference Averianov1997) and for the two vertebrae from Ad-Dakhla. In both cases, the vertebrae are morphologically similar to advanced Palaeophis, but the roof of their zygosphene is arched dorsally as in Pterosphenus. Despite the latter feature, the species from Kazakhstan was provisionally retained in Palaeophis by Rage et al. (Reference Rage, Bajpal, Thewissen and Tiwari2003).
Species of questionable validity set aside (see remarks in Rage et al. Reference Rage, Bajpal, Thewissen and Tiwari2003), around a dozen species of the genus Palaeophis are known worldwide (Parmley & deVore, Reference Parmley and Devore2005) including three species in Africa (P. maghrebianus Arambourg, Reference Arambourg1952; P. africanus Andrews, Reference Andrews1924 and P. colossaeus Rage, Reference Rage1983a). The genus Pterosphenus is represented by five recognized species, among which only one (Pt. schweinfurthi Andrews, Reference Andrews1901) is known in Africa, more precisely from the Qasr El-Sagha Formation of Egypt (Andrews, Reference Andrews1906; McCartney & Seiffert, Reference McCartney and Seiffert2016) and Dur At-Talah Formation of Libya (Hoffstetter, Reference Hoffstetter1961).
The overall morphology (except the zygosphene) of the Ad-Dakhla palaeophiid is close to an advanced Palaeophis such as Palaeophis typhaeus from western Europe. However, the palaeophiid from Ad-Dakhla differs from advanced Palaeophis in having an arched zygosphene as in Pterosphenus. The palaeophiid from Ad-Dakhla and P. nessovi are morphologically intermediate between the advanced Palaeophis and Pterosphenus. Consequently, it is difficult to provide a precise taxonomic (generic) assignment to these species. Given that an arched zygosphene characterizes Pterosphenus, we refer the specimens from Ad-Dakhla to this genus. P. nessovi might be transferred to the same genus. However, until intracolumnar variations are known in palaeophiids, assignment of such vertebrae will remain uncertain. As far as Ad-Dakhla is concerned, discovery of new material representing various regions of the vertebral column will certainly provide important taxonomic information.
4.e. Aves
Odontopterygiformes Howard, Reference Howard1957
Pelagornithidae Fürbringer, Reference Fürbringer1888
Material: FSAC DAK-405 (Fig. 9a–c), portion of left mandible bearing pseudo-teeth (two fragments); FSAC DAK-187 (Fig. 9d–f), small fragment of rostral portion of right mandible bearing pseudo-teeth.

Figure 9. Aves from Ad-Dakhla, Pelagornithidae indet. (a–c) DAK-405, portion of left mandible (two fragments) in (a) lateral, (b) dorsal and (c) medial views. White lines indicate the rostralmost fragment. (d–f) DAK-187, fragment of rostral portion of right mandible in (d) lateral, (e) dorsal and (f) medial views. r1, r2, r3 and r4 refer to rank 1, rank 2, rank 3 and rank 4 pseudo-teeth, respectively. Rank 1 to rank 3 pseudo-teeth (PT1-PT8) are numbered consecutively from the most proximal to the most distal. Scale bar equals 20 mm.
Measurements in millimetres (rank 1 to rank 3 pseudo-teeth are numbered consecutively from the most proximal to the most distal). DAK-405: preserved length of proximal portion, 92.4; preserved length of distal portion, 63.0; height of mandibula at the level of PT4, 25.0; PT3, craniocaudal length at base, 4.6; PT3, height, 8.6; PT5, craniocaudal length at base, 8.6; PT5, height, 17.4; PT6, craniocaudal length at base, 4.9; PT6, height, 8.6; PT7, craniocaudal length at base 2.7; PT7, height 5.5; distance between PT1 and PT3, 25.0; distance between PT1 and PT2, 10.0; distance between PT2 and PT3, 10.2; distance between PT3 and PT5, 24.2; distance between PT3 and PT4, 9.1; distance between PT4 and PT5, 10.8; distance between PT6 and PT7, 11.0. DAK-187: Length as preserved, 45.7. PT1, craniocaudal length at base, 41.5; PT1, height as preserved, 20.2; PT3, craniocaudal length at base, 10.5; PT3, height as preserved, 19.8; distance between PT1 and PT3, 20.7; distance between PT1 and PT2, 9.0; distance between PT2 and PT3, 9.4.
Description. Anatomical terminology follows Baumel & Witmer (Reference Baumel, Witmer, Baumel, King, Breazile, Evans and Vanden Berge1993), with English equivalents of the Latin nomenclature. DAK-405 consists of two fragments of left mandibular ramus that belong to the same specimen (Fig. 9a–c). The height of the mandible decreases gradually towards the distal end. As in other pseudo-toothed birds, the lateral surface of the mandibular ramus exhibits a longitudinal neurovascular sulcus, which runs along the ventral third of the ramus and gradually gets closer to the ventral margin. The rostralmost fragment is broken ventral to the neurovascular sulcus. Its tomial crest is not aligned with the tomial crest of the caudalmost fragment. Considering the relatively good state of preservation of the mandible, the lateral side seems nearly flat, while the medial side is slightly convex. The pseudo-teeth are conical, sharp and stand vertically. They are arranged in a regular pattern similar to that of other pelagornithids. The caudalmost fragment exhibits two large pseudo-teeth (rank 1), one complete (PT5) and another one broken at the base (PT1). The large pseudo-teeth are separated by about 53 mm. In the centre of the space between them is a smaller pseudo-tooth (PT3, rank 2). Tiny pseudo-teeth (PT2 and PT4, rank 3) are located in the centre of the spaces between rank 1 and rank 2 pseudo-teeth. The rank 2 pseudo-tooth is complete, whereas rank 3 pseudo-teeth are broken. The rostralmost fragment exhibits the base of one rank 1 pseudo-tooth (PT8), as well as one complete rank 2 pseudo-tooth (PT6) and one complete rank 3 pseudo-tooth (PT7). On both fragments, thin ridge-like pseudo-teeth (rank 4) are located in the centre of the spaces between pseudo-teeth of ranks 1, 2 and 3. The complete rank 1 pseudo-tooth is wide and its height is approximately 3/4 the height of the mandibular ramus. All broken pseudo-teeth show the hollow structure which is characteristic of pelagornithids (Louchart et al. Reference Louchart, Sire, Mourer-Chauviré, Geraads, Viriot and Buffrénil2013).
DAK-187 is a small fragment of rostral portion of right mandibular ramus (Fig. 9d–f). The medial side is damaged, and the mandible is broken just ventral to the longitudinal neurovascular sulcus, which is exposed on the lateral side. Two incomplete pseudo-teeth are preserved, a large one (PT1, rank 1) and a smaller one (PT3, rank 2). These two pseudo-teeth are separated by c. 20 mm. A tiny pseudo-tooth (PT2, rank 3) is located in the centre of the space between rank 1 and rank 2 pseudo-teeth. The pseudo-teeth are vertical, as in DAK-405.
Comparison and discussion. The Pelagornithidae, also called pseudo-toothed birds, are an extinct group of large seabirds with a long history spanning late Paleocene – late Pliocene time (Harrison, Reference Harrison1985; Averianov et al. Reference Averianov, Panteleyev, Potapova and Nessov1991; Mourer-Chauviré & Geraads, Reference Mourer-Chauviré and Geraads2008; Bourdon, Amaghzaz & Bouya, Reference Bourdon, Amaghzaz and Bouya2010; Boessenecker & Smith, Reference Boessenecker and Smith2011). In parallel with their extensive chronostratigraphic distribution, pelagornithids have been recorded from all continents (e.g. Bourdon, Amaghzaz & Bouya, Reference Bourdon, Amaghzaz and Bouya2010; Mayr & Rubilar-Rogers, Reference Mayr and Rubilar-Rogers2010; Bourdon, Reference Bourdon, Dyke and Kaiser2011; Fitzgerald, Park & Worthy, Reference Fitzgerald, Park and Worthy2012; Cenizo, Hospitaleche & Reguero, Reference Cenizo, Hospitaleche and Reguero2015; Solórzano & Rincón, Reference Solórzano and Rincón2015). The pseudo-toothed birds include gigantic forms with wingspans above 5 m (Mayr & Rubilar-Rogers, Reference Mayr and Rubilar-Rogers2010; Ksepka, Reference Ksepka2014). Pelagornithids are characterized by the presence of pseudo-teeth, which consist of spike-like projections along the tomia of the beak (Louchart et al. Reference Louchart, Sire, Mourer-Chauviré, Geraads, Viriot and Buffrénil2013). These extinct soaring birds have been traditionally linked to the Pelecaniformes and Procellariiformes (Howard, Reference Howard1957; Harrison & Walker, Reference Harrison and Walker1976; Olson, Reference Olson, Farner, King and Parkes1985). Recent phylogenetic analyses suggest that this clade is sister to either Anseriformes (Bourdon, Reference Bourdon2005) or Galloanserae (Mayr, Reference Mayr2011).
Pelagornithid fossil remains have an age range spanning more than 56 million years from late Paleocene through late Pliocene time in Africa (Mourer-Chauviré & Geraads, Reference Mourer-Chauviré and Geraads2008; Bourdon, Amaghzaz & Bouya, Reference Bourdon, Amaghzaz and Bouya2010). Abundant material of pseudo-toothed birds is known from the upper Paleocene – lower Eocene phosphate deposits of the Oulad Abdoun Basin in Morocco (Bourdon, Amaghzaz & Bouya, Reference Bourdon, Amaghzaz and Bouya2010). Fossil pelagornithids from the Oulad Abdoun Basin are assigned to the genus Dasornis Owen, Reference Owen1870, which is characterized by the primitive morphology of the wing bones. An isolated sternum assigned to the genus Gigantornis Andrews, Reference Andrews1916 is known from the middle Eocene Ameki Formation of Nigeria (Andrews, Reference Andrews1916). Fragmentary wing bones and a vertebra tentatively assigned to Gigantornis have been described from the middle Eocene phosphate deposits of Kpogamé-Hahotoé (Togo) (Bourdon & Cappetta, Reference Bourdon and Cappetta2012). Pelagornithid remains assigned to the genus Pelagornis Lartet, Reference Lartet1857 have been discovered in the upper Pliocene deposits of Ahl Al Oughlam, Morocco (Mourer-Chauviré & Geraads, Reference Mourer-Chauviré and Geraads2008). The bird specimens from bonebed B1 of Unit 2 that is part of the Eocene Guerran Member of the Samlat Formation constitute the first record of the pseudo-toothed birds in the upper Eocene deposits of Africa and the second oldest record of this group in North Africa.
The specimens from the Samlat Formation exhibit two diagnostic features of the Pelagornithidae, including the presence of pseudo-teeth arranged in a regular pattern and the occurrence of a longitudinal neurovascular sulcus on the lateral surface of the mandibular ramus (e.g. Bourdon, Amaghzaz & Bouya, Reference Bourdon, Amaghzaz and Bouya2010; Mayr & Rubilar-Rogers, Reference Mayr and Rubilar-Rogers2010; Mayr & Zvonok, Reference Mayr and Zvonok2011, Reference Mayr and Zvonok2012; Louchart et al. Reference Louchart, Sire, Mourer-Chauviré, Geraads, Viriot and Buffrénil2013; Solórzano & Rincón, Reference Solórzano and Rincón2015). The avian remains from Ad-Dakhla exhibit features which may prove diagnostic once more complete material from this locality is discovered: the pseudo-teeth are oriented vertically and widely spaced (especially if the distance between rank 1 pseudo-teeth is considered). In Dasornis toliapicus from upper Palaeogene strata of Morocco and England (Harrison & Walker, Reference Harrison and Walker1976; Bourdon, Amaghzaz & Bouya, Reference Bourdon, Amaghzaz and Bouya2010), the pseudo-teeth are smaller than in the Ad-Dakhla specimens and rostrally slanted. No direct comparison can be made with the genus Gigantornis, which is only known from the middle Eocene deposits of Africa (Andrews, Reference Andrews1916; Bourdon & Cappetta, Reference Bourdon and Cappetta2012). Lutetodontopteryx tethyensis Mayr & Zvonok, Reference Mayr and Zvonok2012 from the middle Eocene deposits of Ukraine (Mayr & Zvonok, Reference Mayr and Zvonok2012) differs from the specimens from Ad-Dakhla in the narrower, rostrally slanted and less widely spaced pseudo-teeth, and in the deeper lateral neurovascular sulcus. The morphology of the Ad-Dakhla specimens matches better with species of the late Oligocene – Neogene genus Pelagornis (Cenizo, Hospitaleche & Reguero, Reference Cenizo, Hospitaleche and Reguero2015), which exhibit vertically oriented and widely spaced pseudo-teeth (Howard, Reference Howard1957; Matsuoka, Sakakura & Ohe, Reference Matsuoka, Sakakura and Ohe1998; Stidham, Reference Stidham2004; Mourer-Chauviré & Geraads, Reference Mourer-Chauviré and Geraads2008; Mayr & Rubilar-Rogers, Reference Mayr and Rubilar-Rogers2010; Solórzano & Rincón, Reference Solórzano and Rincón2015). This might be indicative of a closer affinity between the Ad-Dakhla species and Pelagornis. However, vertically oriented and widely spaced pseudo-teeth are also present in the Oligocene Caspiodontornis kobystanicus Aslanova & Burchak-Abramovich, Reference Aslanova and Burchak-Abramovich1982 (Aslanova & Burchak-Abramovich, Reference Aslanova and Burchak-Abramovich1982, Reference Aslanova and Burchak-Abramovich1999). Vertically oriented pseudo-teeth also occur in the early Eocene Pseudodontornis longidentata Harrison & Walker, Reference Harrison and Walker1976, which is probably a junior synonym of Dasornis emuinus (Bowerbank, Reference Bowerbank1854) (Mayr, Reference Mayr2008). Another potentially diagnostic feature of the specimen DAK-405 is that the rank 1 pseudo-tooth (PT5) is approximately twice as tall as rank 2 pseudo-teeth (PT3 and PT6). This is similar to the condition found in, for example, Pelagornis mauretanicus (Mourer-Chauviré & Geraads, Reference Mourer-Chauviré and Geraads2008) and Pelagornis chilensis (Mayr & Rubilar-Rogers, Reference Mayr and Rubilar-Rogers2010). In Lutetodontopteryx, the height difference between rank 1 and rank 2 pseudo-teeth is much lower (Mayr & Zvonok, Reference Mayr and Zvonok2012). In spite of the presence of a few diagnostic features, we think that the fragmentary nature of the mandibular fragments from the Samlat Formation precludes their assignment beyond the family level. More complete material is needed for a more precise taxonomic assignment.
The dimensions of the specimens and the height of the complete rank 1 pseudo-tooth of DAK-405 indicate that they belong to a large form, distinctly larger than Dasornis toliapicus (Harrison & Walker, Reference Harrison and Walker1976; Bourdon, Amaghzaz & Bouya, Reference Bourdon, Amaghzaz and Bouya2010) and Lutetodontopteryx tethyensis (Mayr & Zvonok, Reference Mayr and Zvonok2012). This provides evidence that a large pelagornithid occurred close the Atlantic coast in the Ad-Dakhla region during late Eocene time.
5. Discussion
The Priabonian fauna from Ad-Dakhla is composed of selachians, actinopterygians (siluriforms and perciforms), sauropsids (turtles, palaeophiid snakes, crocodilians and pelagornithid seabirds) and mammals (archaeocete whales, sirenians and proboscideans). Part of this fauna was previously studied by Adnet, Cappetta & Tabuce (Reference Adnet, Cappetta and Tabuce2010) for fish remains (selachians and actinopterygians) and proboscideans, and by Zouhri et al. (Reference Zouhri, Gingerich, Elboudali, Sebti, Noubhani, Rahali and Meslouh2014) for archaeocete whales and sirenians.
At least 48 species of selachians have been identified, including several new taxa distributed among six orders (Adnet, Cappetta & Tabuce, Reference Adnet, Cappetta and Tabuce2010). This selachian fossil assemblage shows great similarity to the selachian faunas of Qasr El-Sagha, Birket Qarun and Daba'a Formations in Egypt (Underwood et al. Reference Underwood, Ward, King, Antar, Zalmout and Gingerich2011; Zalmout et al. Reference Zalmout, Antar, Abd-El Shafy, Metwally, Hatab and Gingerich2012) and to the faunas of Qa'Faydat ad Dahikiya in the Wadi Esh-Shallala Formation in Jordan (Mustafa & Zalmout, Reference Mustafa and Zalmout2002; Adnet, Cappetta & Tabuce, Reference Adnet, Cappetta and Tabuce2010).
The actinopterygian fauna is only known by incomplete and isolated remains. Adnet, Cappetta & Tabuce (Reference Adnet, Cappetta and Tabuce2010) reported three species belonging to three families (Sphyraenidae, Trichiuridae and Xiphiidae), and regarded them as close to the late Eocene ichthyofauna from the Irwinton Sand Member in Georgia (described by Case & Borodin, Reference Case and Borodin2000). The material described here corresponds to at least two large-sized taxa, a scombroid probably close to the extant Acanthocybium and to the Eocene Aramichthys, and a siluriform probably related to Ariidae. This association is rather comparable to the Eocene ichthyofaunas from the Birket Qarun and Qasr El-Sagha formations of the Fayum Depression, with arioid-like spines and acanthocybin-like teeth (Dames, Reference Dames1883; Priem, Reference Priem1899, Reference Priem1914; Peyer, Reference Peyer1928; Murray, Reference Murray2004; El-Sayed et al. Reference El-Sayed, Kora, Sallam, Claeson, Seiffert and Antar2017). More generally, Scombroidei and Siluriformes are found in numerous marine Eocene or Oligocene localities in Europe including England (Casier, Reference Casier1966; Friedman et al. Reference Friedman, Beckett, Close, Johanson, Johanson, Richter and Smith2015), Belgium (Leriche, Reference Leriche1905, Reference Leriche1910) and France (Leriche, Reference Leriche1906) or in Africa (Murray, Reference Murray2000), including Nigeria (White, Reference White1926, Reference White1934), Angola (Casier, Reference Casier1957) or Namibia (Böhm, Reference Böhm, Beetze and Kaiser1926).
The turtle fauna includes at least four different species represented by shell elements. It constitutes the first turtle fauna from the upper Eocene strata of Morocco and is similar to that of the Fayum in the combination of marine littoral, deep-sea and terrestrial forms. It resembles that of the upper Eocene beds in the presence of the littoral Cordichelys cf. antiqua. The occurrence of terrestrial testudinids in Ad-Dakhla parallels the presence of this taxon in the lower Oligocene deposits at Fayum. Testudinid specimens from Ad-Dakhla represent the oldest record of the Testudinidae in Africa and extend the stratigraphic range of this family. Deep-sea forms belong to the cheloniids and dermochelyids and possibly include the Fayum dermochelyid genus Egyptemys.
The crocodilian material, restricted to incomplete procoelous vertebrae, osteoderms, teeth and an incomplete femur, is referred to one or several eusuchian species. It constitutes the first occurrence of crocodilians in upper Eocene deposits of Morocco. Crocodile remains are tentatively referred to Eusuchia indet.: Gavialidae indet. and/or of Crocodylidae indet., both families being represented in the Fayum (upper Eocene and lower Oligocene). One vertebra is similar to a vertebra attributed by Andrews (Reference Andrews1901) to the longisrostrine ‘Tomistoma’ africanum Andrews, Reference Andrews1901, from the Qasr El-Sagha beds, upper Eocene Fayum (Egypt). This taxon was later named Eogavialis africanus (Gavialidae) based only on the skull, but the Ad-Dakhla vertebrae could also correspond to ‘Crocodylus’ spp. from the Fayum. Teeth have a rather longirostrine morphotype such as that of Eogavialis. The crocodilian material from Ad-Dakhla indicates a Tertiary fauna at least partly comparable to that of the Fayum, and without the presence of mesosuchians.
Squamates are represented by two isolated vertebrae corresponding to a paleophiid snake tentatively referred to the genus Pterosphenus, even if affinities with Palaeophis are not excluded. In Africa, the genus Pterosphenus is represented by the species P. schweinfurthi retrieved from Qasr El-Sagha and Birket Qarun formations (Egypt) and Dur At-Talah (Libya). Inside the genus Palaeophis, the material from Ad-Dakhla seems closer to the derived P. nessovi from the Priabonian of Kazakhstan than to the African Palaeophis species retrieved from Nigeria, Morocco, Mali and Sudan (Andrew, Reference Andrews1924; Rage, Reference Rage1983a; Averianov, Reference Averianov1997; Rage & Werner, Reference Rage and Werner1999; Houssaye et al. Reference Houssaye, Rage, Bardet, Vincent, Amaghzaz and Meslouh2013).
Birds are represented in Ad-Dakhla by two mandibular fragments attributed to the pseudo-toothed birds (Pelagornithidae), based on the presence of pseudo-dentition along the tomia. The Ad-Dakhla material belonged to a fairly large pseudo-toothed bird (wingspan >3 m), but its fragmentary nature precludes assignment to previously described pelagornithid genera. All Palaeogene pelagornithids from Morocco occur in Thanetian/Ypresian deposits of the Ouled Abdoun Basin and belong to the genus Dasornis (Bourdon, Amaghzaz & Bouya, Reference Bourdon, Amaghzaz and Bouya2010), which is also recorded in Ypresian deposits of England (Harrison & Walker, Reference Harrison and Walker1976; Mayr, Reference Mayr2008). The genus Dasornis includes small, medium (albatross-sized) and large forms and is characterized by the plesiomorphic morphology of the wing bones compared to Neogene pelagornithids (Bourdon, Amaghzaz & Bouya, Reference Bourdon, Amaghzaz and Bouya2010). In contrast, the genus Pelagornis spans late Oligocene – late Pliocene time and only includes gigantic forms that were exceedingly specialized for soaring flight (Mourer-Chauviré & Geraads, Reference Mourer-Chauviré and Geraads2008; Mayr & Rubilar-Rogers, Reference Mayr and Rubilar-Rogers2010; Mayr, Goedert & McLeod, Reference Mayr, Goedert and Mcleod2013; Ksepka, Reference Ksepka2014; Solórzano & Rincón, Reference Solórzano and Rincón2015). A number of pelagornithid remains of uncertain affinities have been described between middle Eocene and middle Oligocene strata which exhibit some, but not all, derived features of Pelagornis (Mayr & Smith, Reference Mayr and Smith2010; Bourdon & Cappetta, Reference Bourdon and Cappetta2012; Mayr & Zvonok, Reference Mayr and Zvonok2012; Cenizo, Hospitaleche & Reguero, Reference Cenizo, Hospitaleche and Reguero2015). Further collecting in middle and late Eocene localities such as Ad-Dakhla can potentially shed light on the phylogenetic relationships between the earliest pseudo-toothed birds and the highly specialized gigantic forms of Neogene time.
Even though numerous taxa from Ad-Dakhla are not accurately identified at the specific level, this fauna shows strong affinities with those of the upper Eocene beds of Egypt (Fayum Depression) and Libya (Dur At-Talah). In addition to the aforementioned taxa, several archaeocetes (e.g. Saghacetus sp., Stomerius sp., Dorudon atrox, Basilosaurus isis) and the dugondid Eosiren are also present in the Qasr El-Sagha Formation. These shared taxa support a close biogeographic relationship or connection between faunas from southeastern and southwestern coasts of the Mediterranean Sea. However, this conclusion is biased by the incomplete knowledge of the fossil record in North and East Africa. Indeed, if the Eocene marine fossil record has been relatively well documented in the eastern part of the Tethys (India and Pakistan Subcontinent) and Near East (Egypt) for several decades (e.g. Gingerich, Reference Gingerich1992; Bajpai & Gingerich, Reference Bajpai and Gingerich1998; Underwood et al. Reference Underwood, Ward, King, Antar, Zalmout and Gingerich2011; Zalmout & Gingerich, Reference Zalmout and Gingerich2012), the literature dedicated to the southwestern margin of the Mediterranean Sea, including the extant Maghreb (Tunisia, Algeria and Morocco) or the adjacent West African Atlantic margin, is quite poor. In Morocco, only the Ypresian–Lutetian deposits of the Phosphate basins (including the Maastrichtian–Lutetian Oulad Abdoun basin) and Palaeogene Ouarzazate Basin have been thoroughly studied (Bardet et al. Reference Bardet, Gheerbrant, Cappetta, Noubhani, Jouve, Bourdon, Pereda Suberbiola, Jalil, Vincent, Houssaye, Solé, El Houssaini Darif, Adnet, Rage, de Lapparent de Broin, Sudre, Bouya, Amaghzaz, Meslouh and Zouhri2017; Gheerbrant et al. Reference Gheerbrant, Cappetta, de Lapparent de Broin, Rage, Tabuce and Zouhri2017). For the Palaeogene Period this fauna shows similarities with that of Ad-Dakhla, with scombroid actinopterygians (Arambourg, Reference Arambourg1952; Khalloufi et al. Reference Khalloufi, Brito, Cavin, Dutheil and Zouhri2017), pelagornithid seabirds (Bourdon, Amaghzaz & Bouya, Reference Bourdon, Amaghzaz and Bouya2010) and palaeophiid snakes (Arambourg, Reference Arambourg1952; Houssaye et al. Reference Houssaye, Rage, Bardet, Vincent, Amaghzaz and Meslouh2013), but these taxa usually have a large palaeogeographic distribution. Similarly, cheloniids are also represented in the Palaeogene phosphate beds of Morocco, as well as bothremydid turtles unknown in Ad-Dakhla. However, because the cheloniid taxon from Ad-Dakhla is not defined, precise comparison is not possible. Likewise, undeterminate Eusuchian crocodiles occur in both Ad-Dakhla and the Oulad Abdoun and Ouarzazate basins, despite the absence of mesosuchian dyrosaurid crocodiles in Ad-Dakhla. The mammal fauna is also very different, since neither archaeocetes nor sirenians are known in the Phosphates of Morocco. The three proboscidean genera described from the Oulad Abdoun basin (Eritherium, Phosphatherium and Daouitherium) have no representatives in Ad-Dakhla, which only yielded a proboscidean tooth referred to Numidotherium by Adnet, Cappetta & Tabuce (Reference Adnet, Cappetta and Tabuce2010). These four genera belong to the ‘barytherioids’, a possible paraphyletic taxon that is also known from the Fayum Depression and Dur At-Talah by the genus Barytherium. Numidotherium is also reported from the lower Eocene deposits of El Kohol in Algeria (Mahboubi et al. Reference Mahboubi, Ameur, Crocher and Jaeger1986), where a mesosuchian ziphodont crocodile is known (Buffetaut, Reference Buffetaut1989). Close to Ad-Dakhla the Bartonian fauna of the Aridal Formation is composed of archaeocetes, including protocetids and basilosaurids (Gingerich & Zouhri, Reference Gingerich and Zouhri2015). In Tunisia, the late Ypresian – early Lutetian locality of Djebel Chambi has yielded mammal remains including the oldest African sirenian (Benoit et al. Reference Benoit, Adnet, El Mabrouk, Khayati, Ben Haj Ali, Marivaux, Merzeraud, Merigeaud, Vianey-Liaud and Tabuce2013). Nevertheless, this sirenian is only known from a petrosal of uncertain affinities, precluding comparison with Eosiren from Ad-Dakhla. In the Ypresian Phosphates of Tunisia, eusuchian crocodiles, cheloniids and dermochelyids are also represented (Jouve et al. Reference Jouve, Iarochène, Bouya and Amaghzaz2005; Bardet et al. Reference Bardet, Gheerbrant, Cappetta, Noubhani, Jouve, Bourdon, Pereda Suberbiola, Jalil, Vincent, Houssaye, Solé, El Houssaini Darif, Adnet, Rage, de Lapparent de Broin, Sudre, Bouya, Amaghzaz, Meslouh and Zouhri2017), but comparison is limited to this taxonomic level (other taxa from the Tunisian Phosphates include mesosuchian dyrosaurid crocodiles and bothremydid turtles, which are not found in Ad-Dakhla). Nigeria has yielded a middle Eocene marine vertebrate fauna comprising the protocetid Pappocetus lugardi, a dermochelyid (Cosmochelys dolloi, distinct from that of Ad-Dakhla), the pelagornithid Gigantornis eaglesomei and some siluriform remains (Andrews, Reference Andrews1916, Reference Andrews1919; Halstead & Middleton, Reference Halstead and Middleton1974, Reference Halstead and Middleton1976). In Togo, the middle Eocene locality of Kpogamé-Hahotoé has yielded protocetid archaeocetes, sirenians, pelagornithid seabirds and selachians (Cappetta & Traverse, Reference Cappetta and Traverse1988; Bourdon & Cappetta, Reference Bourdon and Cappetta2012; Gingerich & Cappetta, Reference Gingerich and Cappetta2014). Hautier et al. (Reference Hautier, Sarr, Tabuce, Lihoreau, Adnet, Domning, Samb and Hameh2012, Reference Hautier, Sarr, Lihoreau, Tabuce and Hameh2014) have recently described prorastomid sirenians and protocetids from the Lutetian deposits of the Taïba Formation in Senegal.
The palaeoenvironment of Ad-Dakhla was tentatively reconstructed by Adnet, Cappetta & Tabuce (Reference Adnet, Cappetta and Tabuce2010) and Zouhri et al. (Reference Zouhri, Gingerich, Elboudali, Sebti, Noubhani, Rahali and Meslouh2014) as a near-shore continental shelf close to an emerged land. Taxa described here agree with this reconstruction, with the co-occurrence of terrestrial and marine forms. In addition to the proboscidean described by Adnet, Cappetta & Tabuce (Reference Adnet, Cappetta and Tabuce2010), the only fully terrestrial taxon consists of chelonian shell remains attributed to a Testudinidae; the presence of a continental Crocodylidae is however not excluded if all the specimens are not from a littoral longirostrine form. Other chelonian remains correspond to marine Dermochelyidae and Chelonidae, and to littoral Podocnemididae. The material referred to Chelonidae consists of neural plates sharing similarities with the genera Lepidochelys and Caretta, both represented today by marine species. Podocnemididae are mostly known by continental taxa but littoral forms have been described, such as those of the Shweboemys subgroup which includes the material from Ad-Dakhla. Pelagornithids were large-range pelagic seabirds that were specialized for gliding flight, such as extant albatrosses (Diomedeidae). They were equipped with huge beaks bearing bony tooth-like processes, which they used for grasping large fish and squid near the water surface while in flight (Olson, Reference Olson, Farner, King and Parkes1985; Zusi & Warheit, Reference Zusi, Warheit and Campbell1992; Ksepka, Reference Ksepka2014). The occurrence of pelagornithids in Ad-Dakhla is consistent with the fact that remains of these birds are usually found in near-shore marine environments (e.g. Mourer-Chauviré & Geraads, Reference Mourer-Chauviré and Geraads2008; Bourdon, Amaghzaz & Bouya, Reference Bourdon, Amaghzaz and Bouya2010; Mayr & Rubilar-Rogers, Reference Mayr and Rubilar-Rogers2010; Boessenecker & Smith, Reference Boessenecker and Smith2011; Bourdon & Cappetta, Reference Bourdon and Cappetta2012; Mayr & Zvonok, Reference Mayr and Zvonok2012; Cenizo, Hospitaleche & Reguero, Reference Cenizo, Hospitaleche and Reguero2015; Solórzano & Rincón, Reference Solórzano and Rincón2015). The ophidian Pterosphenus has a wide palaeogeographic distribution; it is preferentially found in Mangrove areas but the genus also occurs in open marine, brackish and freshwater environments, usually close to the coasts (Rage et al. Reference Rage, Bajpal, Thewissen and Tiwari2003). Among actinopterygians the scombroid hypural plate shows an extensive hypurostegy, indicating an adaptation to fast swimming, possibly related to an open-water environment. The siluriform spine probably belongs to the family Ariidae, which is one of the few extant families of marine catfishes (even if some ariids are freshwater species, e.g. Arius gigas), usually occurring in littoral or neritic environments (Nelson, Grande & Wilson, Reference Nelson, Grande and Wilson2016).
Based on the selachian assemblage and the presence of basilosaurids, Adnet, Cappetta & Tabuce (Reference Adnet, Cappetta and Tabuce2010) assigned a Bartonian–Priabonian age to the fossiliferous levels B1 and B2 of Unit 2. However, they considered a Priabonian age more probable due to the faunal composition and the presence of some ‘modern’ selachian taxa. Zouhri et al. (Reference Zouhri, Gingerich, Elboudali, Sebti, Noubhani, Rahali and Meslouh2014) correlated bed B1 with the Pr-2 low sea stand in Egypt, which separates the lower and middle Priabonian deposits (Peters et al. Reference Peters, Antar, Zalmout and Gingerich2009). They also indicated that bed B2, due to a significantly different composition of the mammal fauna, probably represents a second low sea stand, either a later phase of Pr-2 during middle Priabonian time or possibly Pr-3 between middle and late Priabonian time. Most actinopterygians and reptiles described here are not identified at the specific or generic level and cannot be used as precise biostratigraphic indicators. However, they are largely known in the Eocene Epoch and do not invalidate a Priabonian age for the fossiliferous beds of the Samlat Formation.
Acknowledgements
We are grateful to Salvador Bailon (UMR 7194, MNHN, Paris), Philippe Béarez (UMR 7209, MNHN), Gaël Clément (UMR 7207, MNHN), Ronald Böttcher and Rupert Wild (SMNS) for access to collections. Pictures were taken by Philippe Loubry (UMR 7207, MNHN). This research was supported by grant 9202–12 from the National Geographic Society and by a grant from the Hassan II Academy of Science and Technology. Annelise Folie (Royal Belgian Institute of Natural Sciences) and an anymous reviewer provided useful comments on the manuscript.











