Introduction
The minerals of the arctite supergroup belong to the hexagonal intercalated antiperovskites featured by the anion-centred octahedra units in its structure (Galuskin et al., Reference Galuskin, Galuskina, Krüger, KrügeR, Vapnik, Krzątała, Środek and Zieliński2021). Nowadays, this supergroup contains nine members, which, based on the antiperovskite and tetrahedral layers arrangement, are divided into the zadovite and arctite groups and one ungrouped member – aravaite, Ba2Ca18[(SiO4)6(PO4)3(CO3)]F3O (Krüger et al., Reference Krüger, Krüger, Galuskin, Galuskina, Vapnik, Olieric and Pauluhn2018; Galuskin et al., Reference Galuskin, Galuskina, Krüger, KrügeR, Vapnik, Krzątała, Środek and Zieliński2021). Zadovite, BaCa6[(SiO4)(PO4)](PO4)2F (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Włodyka, Dzierżanowski and Murashko2015b), aradite, BaCa6[(SiO4)(VO4)](VO4)2F (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Włodyka, Dzierżanowski and Murashko2015b), gazeevite, BaCa6(SiO4)2(SO4)2O (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Armbruster, Krzątała, Vapnik, Kusz, Dulski, Gardocki, Gurbanov and Dzierżanowski2017) and stracherite, BaCa6(SiO4)2[(PO4)(CO3)]F (Galuskin et al., Reference Galuskin, Krüger, Galuskina, Krüger, Vapnik, Pauluhn and Olieric2018b) represent the zadovite structural type (TA) characterised by intercalation of tetrahedral (T) and single antiperovskite layers (A). In turn, arctite, Ba(Ca7Na5)(PO4)4(PO4)2F3 (Khomyakov et al., Reference Khomyakov, Bykova and Kurova1983; Sokolova et al., Reference Sokolova, Yamnova, Egorov-Tismenko and Khomyakov1984), nabimusaite, KCa12(SiO4)4(SO4)2O2F (Galuskin et al., Reference Galuskin, Gfeller, Armbruster, Galuskina, Vapnik, Murashko, Włodyka and Dzierżanowski2015a), dargaite, BaCa12(SiO4)4(SO4)2O3 (Galuskina et al., Reference Galuskina, Gfeller, Galuskin, Armbruster, Vapnik, Dulski, Gardocki, Jeżak and Murashko2019) and ariegilatite, BaCa12(SiO4)4(PO4)2OF2 (Galuskin et al., Reference Galuskin, Krüger, Galuskina, Krüger, Vapnik, Wojdyla and Murashko2018a) belong to the arctite structural type (TA 3) described by intercalation of the tetrahedral (T) and triple antiperovskite layers (A 3). The ungrouped aravaite (TATA 3 structural type) is different and consists of blocks formed by the intercalation of zadovite and arctite structural modules (Krüger et al., Reference Krüger, Krüger, Galuskin, Galuskina, Vapnik, Olieric and Pauluhn2018; Galuskin et al., Reference Galuskin, Galuskina, Krüger, KrügeR, Vapnik, Krzątała, Środek and Zieliński2021).
With the exception of arctite, all supergroup members have been found in unique high-temperature pyrometamorphic rocks of the Hatrurim Complex distributed along the Dead Sea Rift of Israel, West Bank (Palestinian Autonomy), and Jordanian territories (Sokol et al., Reference Sokol, Kokh, Seryotkin, Deviatiiarova, Goryainov, Sharygin, Khoury, Karmanov, Danilovsky and Artemyev2020; Galuskin et al., Reference Galuskin, Galuskina, Krüger, KrügeR, Vapnik, Krzątała, Środek and Zieliński2021). Arctite was discovered in pegmatite veins in nepheline syenite at the Vuonnemiok River, Khibiny Massif, Kola Peninsula, Russia (Khomyakov et al., Reference Khomyakov, Bykova and Kurova1983). Some dargaite and gazeevite occurrences have been reported from the altered carbonate–silicate xenoliths within rhyodacites of the Shadil-Khoch volcano, Graeter Caucasus (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Armbruster, Krzątała, Vapnik, Kusz, Dulski, Gardocki, Gurbanov and Dzierżanowski2017).
In the present paper, we report a new occurrence for the three arctite-supergroup minerals, nabimusaite, gazeevite and zadovite: the Bellerberg volcano, Eifel district, Rhineland-Palatinate, Germany. We present chemical, spectroscopic, and structural data for these minerals and compare our results with the data reported for their holotype counterparts.
Occurrence, general appearance and mineral association
Geological settings
The Bellerberg volcano, worked as an active quarry, is characterised by the presence of various thermally metamorphosed carbonate–silicate and silicate xenoliths within basaltic lava. The locality belongs to the quaternary volcano region in the Eastern Eifel, Rhineland-Palatinate, Germany (Hentschel, Reference Hentschel1987; Mihajlovic et al., Reference Mihajlovic, Lengauer, Ntaflos, Kolitsch and Tillmanns2004). The wide variety of xenoliths occurring in the volcanic rocks of the Bellerberg volcano area is due to metamorphic transformation and a different protolith composition (Hentschel, Reference Hentschel1987; Mihajlovic et al., Reference Mihajlovic, Lengauer, Ntaflos, Kolitsch and Tillmanns2004; Juroszek et al., Reference Juroszek, Krüger, Galuskina, Krüger, Jeżak, Ternes, Wojdyla, Krzykawski, Pautov and Galuskin2018). The starting material could be from any of the host rocks that were penetrated and carried away by the erupting. These include: volcanic rocks of earlier eruptions of more or less different compositions; Devonian and younger sedimentary rocks, such as clays, slates, greywacke, sandstone, quartzite, and lime- to marlstones; rocks from the deeper areas of the Earth crust; or fragments of the Earth's mantle (Hentschel, Reference Hentschel1987). The presence of characteristic mineral assemblages indicate that, to a greater or lesser extent, xenoliths are transformed by surrounding magma, and mineral phases were formed due to metamorphism at very high-temperature conditions. The differences in the degree of conversion can be caused by many factors, such as: various temperatures of the magma; different cooling rates; different levels of water vapour and other volatile substances (fluorine, chlorine and sulfur); different sizes of xenoliths; and multiple heating of xenoliths and/or prolonged exposure to escaping hot gases or by lava entering the environment (Hentschel, Reference Hentschel1987). Moreover, the xenoliths from Bellerberg volcano are known for their diversity of secondary, low-temperature phases and the source of several new mineral species (Abraham et al., Reference Abraham, Gebert, Medenbach, Schreyer and Hentschel1983; Irran et al., Reference Irran, Tillmanns and Hentschel1997; Mihajlovic et al., Reference Mihajlovic, Lengauer, Ntaflos, Kolitsch and Tillmanns2004; Chukanov et al., Reference Chukanov, Britvin, Van, Möckel and Zadov2012, Reference Chukanov, Aksenov, Rastsvetaeva, Blass, Varlamov, Pekov, Belakovskiy and Gurzhiy2015; Galuskin et al., Reference Galuskin, Krüger, Krüger, Blass, Widmer and Galuskina2016; Juroszek et al., Reference Juroszek, Krüger, Galuskina, Krüger, Jeżak, Ternes, Wojdyla, Krzykawski, Pautov and Galuskin2018).
Xenolith samples presented in this study were collected by Bernd Ternes from the Caspar and Scherer quarries in the Bellerberg volcano area.
Nabimusaite
Nabimusaite forms colourless subhedral crystals with a characteristic parting/cleavage along (001) (Fig. 1). The nabimusaite crystal size usually exceeds 100 μm, but the biggest ones reach ~500 μm. It was observed that some crystals are homogeneous (Fig. 1a,b), whereas others contain chlormayenite inclusions (Fig. 1c,d).
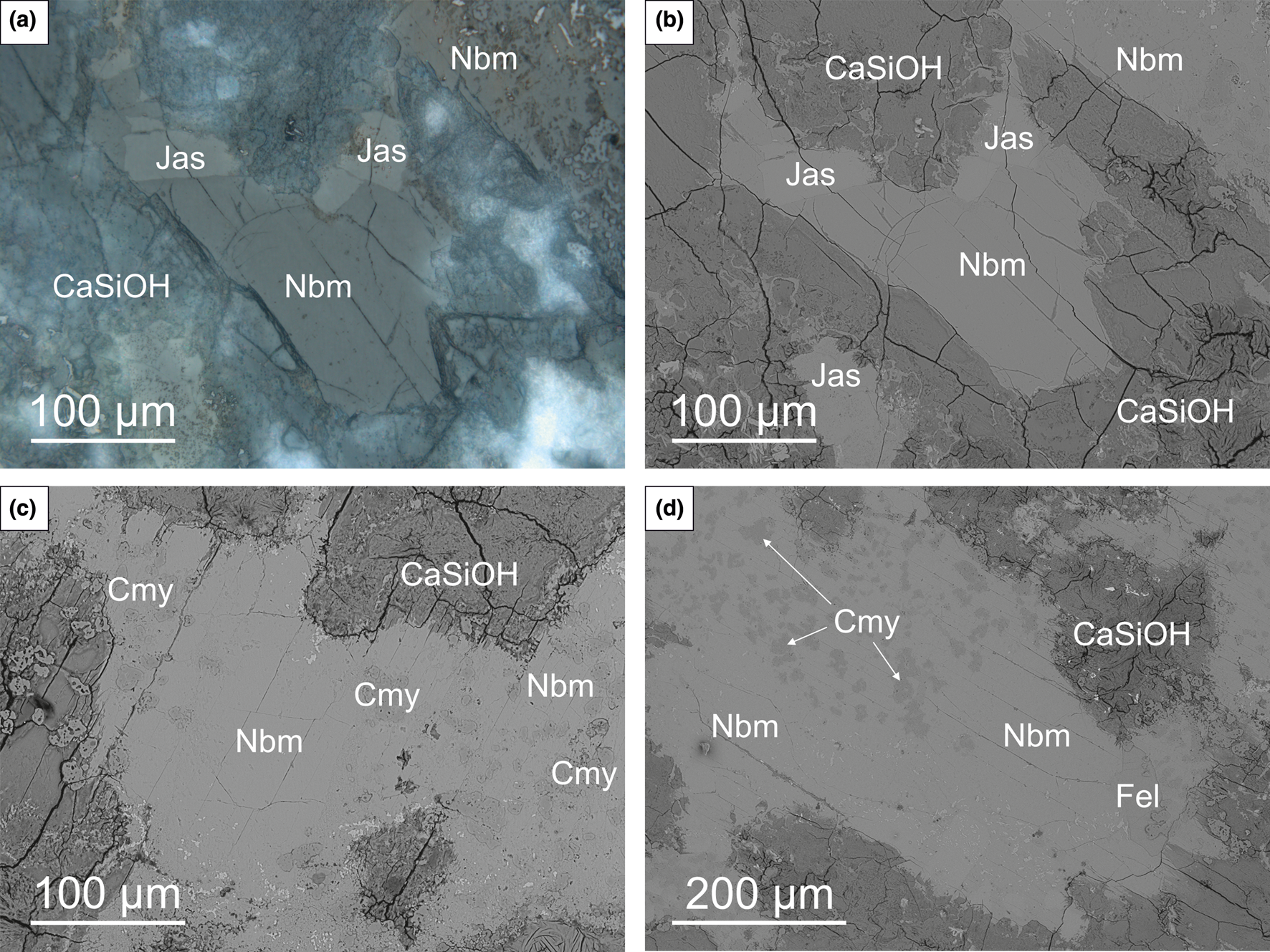
Fig. 1. Optical (a) and BSE (back-scattered electron) images (b–d) of nabimusaite and associated minerals in the carbonate–silicate xenoliths. Euhedral homogenous nabimusaite crystals with visible parting/cleavage (a–b) and nabimusaite crystals containing chlormayenite inclusions (c–d); CaSiOH – hydrated Ca-silicates; Cmy – chlormayenite; Fel – fluorellestadite; Jas – jasmundite; Nbm – nabimusaite. Sample #11.
In an analysed xenolith sample from Caspar quarry (50.35°N, 7.23°E), nabimusaite is a rock-forming mineral associated with the fluorapatite–fluorellestadite mineral series, jasmundite, larnite and ternesite (Fig. 1). Chlormayenite, occurring mainly as inclusions in nabimusaite crystals (Fig. 1c,d), minerals of the sharyginite–shulamitite series, brownmillerite, lakargiite and vapnikite are less common accessory phases. Most of the minerals mentioned are surrounded by secondary, low-temperature phases represented by various undiagnosed hydrated Ca-silicates, minerals of the ettringite–thaumasite series, and hydrocalumite. Periclase and Sr-bearing baryte are very rare.
Gazeevite
Gazeevite is an accessory mineral found in a xenolith sample from Scherer quarry (50.35°N, 7.24°E), which forms poikilitic anhedral and subhedral crystals ~50–150 μm in size (Fig. 2a–c). Usually, gazeevite crystals are embedded in the larnite matrix and overgrow and intergrow with fluorapatite–fluorellestadite individuals (Fig. 2a–c). Some gazeevite overgrown by magnesioferrite and srebrodolskite was also observed (Fig. 2d).

Fig. 2. The fragment of carbonate–silicate xenolith with gazeevite and associated minerals (a). The framed area magnified in b shows gazeevite crystals in the coarse-grained larnite matrix. Overgrows and intergrows of gazeevite crystals with fluorapatite–fluorellestadite (a–c), as well as with magnesioferrite and srebrodolskite grains (d); BSE images; CaMgSiOH – hydrated Ca-Mg-silicates; Ett – ettringite; Fap – fluorapatite; Fel – fluorellestadite; Gaz – gazeevite; Lrn – larnite; Mfr – magnesioferrite; Sre – srebrodolskite; Srä – strätlingite. Sample #EF15-3.
A group of gazeevite crystals was detected in the part of the xenolith near the contact zone with basaltic rock. The xenolith consists of high-temperature rock-forming minerals such as wollastonite, larnite, minerals of the gehlenite–alumoåkermanite–åkermanite, fluorapatite–fluorellestadite and brownmillerite–srebrodolskite series. In turn, the basaltic rock includes mainly K-feldspar, titanite, and minerals of the diopside–hedenbergite series. Occasionally, single quartz crystals were also noted. Garnets of the andradite–schorlomite–kerimasite series, perovskite, magnesioferrite, hematite, zircon, calzirtite and baddeleyite were recognised as accessory phases in the altered basaltic rock. Low-temperature secondary mineralisation is widespread in the sample studied and represented by ettringite, various hydrated Ca–Mg-silicates and Ca-aluminosilicates such as tobermorite, tacharanite, afwillite, strätlingite, hydrocalumite, and minerals of the zeolite group such as flörkeite and chabazite.
Zadovite
Zadovite is the rarest arctite-supergroup mineral and is described in xenoliths from the Caspar quarry for the first time. It forms tiny colourless subhedral and anhedral crystals reaching ~15–40 μm in size (Fig. 3). Usually, zadovite crystals are scattered in individual veinlets of an aluminosilicate matrix, composed of rock-forming phases, mainly wollastonite and gehlenite, which indicates signs of initial alteration, and some hydrated Ca-silicates (Fig. 3).
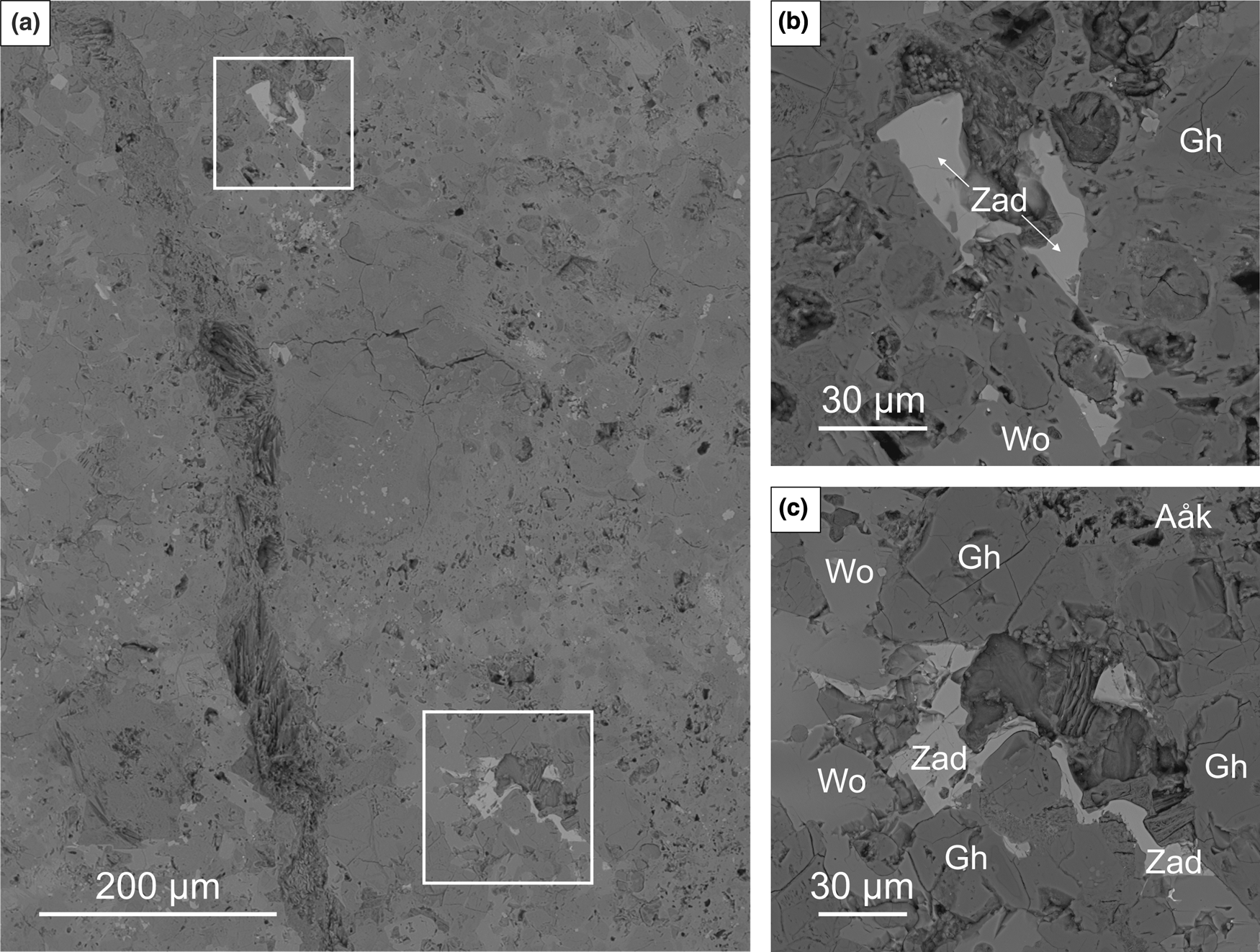
Fig. 3. Partially altered xenolith fragment composed mainly of wollastonite and gehlenite–alumoåkermanite series minerals (a). The framed sections are magnified in (b) and (c) and show zadovite crystals; BSE images; Aåk – alumoåkermanite; Gh – gehlenite; Wo – wollastonite; Zad – zadovite. Sample #15.
The xenolith fragment in which zadovite was identified is characterised by secondary alteration and the dominance of low-temperature mineral assemblages. The rock-forming minerals are mainly high-temperature phases such as wollastonite, larnite, gehlenite, nepheline and magnesioferrite. Kalsilite, bredigite, chlormayenite, fluorapatite, srebrodolskite, perovskite, hematite, minerals of the sharyginite–shulamitite series, alumoåkermanite and Sr-, Fe2+-bearing melilite-group minerals are less abundant than rock-forming phases. The xenolith specimen also contains various Zr minerals, such as zircon, wadeite, lakargiite, calzirtite and baddeleyite. Zadovite, together with walstromite, fresnoite, bennesherite, celsian and mazorite, form the unique Ba-mineralisation. Secondary phases are represented by various hydrated Ca-silicates and aluminosilicates such as tobermorite, jennite, afwillite and hydrocalumite, minerals of ettringite–thaumasite series, calcite, baryte, Sr-bearing baryte, and some zeolites.
Materials and methods of investigation
The preliminary chemical composition and crystal morphology of the arctite-supergroup minerals, as well as associated phases from the Bellerberg volcano xenoliths, were carried out using an analytical Phenom XL scanning electron microscope, which is equipped with an EDS (energy-dispersive X-ray spectroscopy) detector (Institute of Earth Sciences, Faculty of Natural Sciences, University of Silesia, Sosnowiec, Poland).
Electron microprobe analyses (EMPA) of nabimusaite, gazeevite, zadovite and associated minerals were performed on CAMECA SX100 apparatus at 15 kV acceleration voltage and 10–20 nA beam current (Faculty of Geology, University of Warsaw, Poland). The following lines (and standards) were used during the measurements: BaLα and SKα (baryte); PKα (YPO4 and Apat_BB); CaKα and SiKα (diopside); AlKα and KKα (orthoclase); TiKα (rutile); NaKα (albite), SrLα (celestine); FeKα (Fe2O3); VKα (V2O5); and FKα (synthetic fluorophlogopite). Due to the size and character of the crystals, the beam diameter differed for each arctite-supergroup member: 10 μm for nabimusaite, 3 μm for gazeevite and ~1 μm for zadovite.
The Raman spectra of nabimusaite, gazeevite and zadovite were recorded on a WITec alpha 300R Confocal Raman Microscope (Institute of Earth Sciences, Faculty of Natural Sciences, University of Silesia, Sosnowiec, Poland) equipped with an air-cooled solid laser 633 nm and a CCD (closed-circuit display) camera operating at –61°C. The laser radiation was coupled to a microscope through a single-mode optical fibre with a diameter of 50 μm. An air Zeiss (LD EC Epiplan-Neofluan DIC–100/0.75NA) objective was used. The scattered light was focused on multi-mode fibre (100 μm diameter) and a monochromator with a 600 mm–1 grating. Raman spectra were accumulated by 15 scans with an integration time of 10 s; resolution was 3 cm–1. The monochromator was calibrated using the Raman scattering line of a silicon plate (520.7 cm–1). All spectra were processed using the Spectracalc software package GRAMS. The Raman bands were fitted using a Gauss–Lorentz cross-product function with the minimum number of component bands used for the fitting process.
Single-crystal X-ray studies were carried out with synchrotron radiation. The diffraction measurements were performed at the X06DA beamline of the Swiss Light Source (Paul Scherrer Institute, Villigen, Switzerland). The experiments were done at ambient conditions with the wavelength tuned to 0.70848 Å, and a multi-axis PRIGo goniometer was used to position and rotate the crystals. The X-ray diffraction data were collected with a Pilatus-2M-F detector, which was placed at a distance of 90 mm from the crystals and vertically translated by 55 mm off-centre to increase the resolution of the data. A total of 3600 frames were recorded using a fine-sliced (0.1) ω-scan at 0.1 seconds per frame. Lattice parameters and data reduction were made using CrysAlisPro. The crystal structure refinement was performed with Jana2006 (Petřiček et al., Reference Petřiček, Dušek and Palatinus2014). The further details of the intensity data and crystal-structure refinements of nabimusaite, gazeevite and zadovite are summarised in Table 1. Information about final atomic coordinates, anisotropic displacement parameters, or selected bond lengths are listed in the crystallographic information files, deposited with the Principal Editor of Mineralogical Magazine and available as Supplementary material (see below).
Table 1. Parameters for X-ray data collection and crystal-structure refinement for nabimusaite, gazeevite and zadovite.

Results
Chemical data
Results of the electron microprobe analyses of nabimusaite, gazeevite and zadovite from the Caspar and Scherer quarries are presented in Table 2. The empirical formula of nabimusaite calculated on the basis of 27 (O+F) anions is as follows: (K0.79Sr0.10Ca0.04Ba0.02)Σ0.95(Ca11.94Na0.06)Σ12.00[(Si3.80Al0.10S0.06Ti0.02Fe0.02)Σ4.00O16.00][(S1.96P0.04)Σ2.00O8.00]O2(F0.93O0.07)Σ1.00. The latter empirical formula of nabimusaite is close to the end-member formula, KCa12(SiO4)4(SO4)2O2F, with the end-member content equal to 83%.
Table 2. The chemical composition of arctite-supergroup minerals from the Bellerberg volcano.*

* S.D. = 1σ standard deviation; n – number of analyses; n.d. – not detected
The empirical formula of gazeevite, calculated on 17 (O+F) anions, is as follows: (Ba0.86Sr0.05K0.05Ca0.04)Σ1.00(Ca6.00Na0.03)Σ6.03[(Si1.97Al0.02Fe0.01)2.00O8.00][(S1.71P0.17Si0.06V0.05)Σ1.96O8.00](O0.53F0.34)Σ0.87, with a simplified formula of BaCa6(SiO4)2(SO4)2(O,F).
The crystal-chemical formula of zadovite calculated on 17 (O+F) anions is as follows: (Ba0.99Sr0.03)Σ1.02(Ca5.96Na0.05)Σ6.01[(Si1.31P0.69)Σ2.00O8.00][(P1.53S0.36V0.10Fe0.01)Σ2.00O8.00]F1.01, and the end-member formula is BaCa6[(SiO4)(PO4)](PO4)2F (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Włodyka, Dzierżanowski and Murashko2015b). The increased Si and considerable S (0.36 atoms per formula unit (apfu)) contents with a simultaneous decrease of P content at both tetrahedral positions of the analysed sample (Table 2) confirms the Si4++S6+↔2P/V5+ substitution scheme characteristic for the zadovite-group minerals (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Włodyka, Dzierżanowski and Murashko2015b).
Crystal structure data
Nabimusaite belongs to the arctite structural type (TA 3), characterised by the intercalation of single tetrahedral (T) and triple antiperovskite layers (A 3) (Fig. 4a). The intercalated tetrahedral layers consist of large K cations and (SO4)2– tetrahedra (Fig. 4b). The K site in the nabimusaite structure is partly occupied by Ba, which was also reported in the previous description (Galuskin et al., Reference Galuskin, Gfeller, Armbruster, Galuskina, Vapnik, Murashko, Włodyka and Dzierżanowski2015a). Potassium cation is coordinated by twelve oxygen atoms (6 × O1 and 6 × O3) with the average bond distances K–O1 = 2.9111 Å and K–O3 = 3.3703 Å. In the SO4-tetrahedron, there are three shorter S–O1 = 1.4697(9) Å and one longer S–O4 = 1.4864(18) Å bond lengths, with <S–O> = 1.4739 Å. Each of the O1 atoms at the base of the SO4 tetrahedron bonds to K and Ca1 atoms in the antiperovskite layers. The apical O4 atom of a S-tetrahedron is also bonded to the Ca1 atom.

Fig. 4. The crystal structure of nabimusaite composed of single tetrahedral and triple antiperovskite layers (a). The K-octahedra (purple) are connected to the six SO4-tetrahedra (yellow) within the tetrahedral layers (b). The linear face-sharing triplets of anion-centred FCa6 (green) and OCa6-octahedra (red) coordinated by Ca atoms (dark yellow spheres) (a) and surrounded by SiO4-tetrahedra (blue) in an up and down sequence (c).
Along z, the antiperovskite layers in the nabimusaite structure form linear face-sharing triplets of anion-centred octahedra (Fig. 4a). Two SiO4 tetrahedra with the up and down sequence occur between these octahedral triplets (Fig. 4c). In the centre of antiperovskite layers, the (O8)2– anion is octahedrally coordinated by six Ca2 atoms, with the O8–Ca2 bond length of 2.3342(2) Å. Two adjacent octahedra of the triplets are occupied simultaneously by F– and O2– anions and coordinated by three Ca2 atoms, due to the shared face with O8Ca26 octahedra, and three Ca1 atoms. The bond length equals 2.4411(10) Å for O7–Ca2 and 2.3889(9) Å for O7–Ca1, respectively. According to the chemical composition, additional impurities at the Si sites are insignificant ~0.2 apfu, which should not affect the bond lengths. The average Si–O bond lengths for Si1 and Si2 tetrahedra are 1.639 Å and 1.630 Å, respectively. The Si1 tetrahedra share three O2 with the Ca2 atoms, whereas the apical O5 is linked only to Ca1. In turn, the apical O6 of Si2 tetrahedra is also bonded to the Ca2 atoms, while three O3 are joined to Ca1 and Ca2.
Gazeevite and zadovite represent the same structural type. Their structures are characterised by the alternation of single tetrahedral (T) and single antiperovskite layers (A) (Fig. 5). The main differences between these two related structures are observed in the diverse occupation at the T1 and T2 sites located at the three-fold inversion axis and different anion-centred octahedra in the antiperovskite layers (Fig. 5).
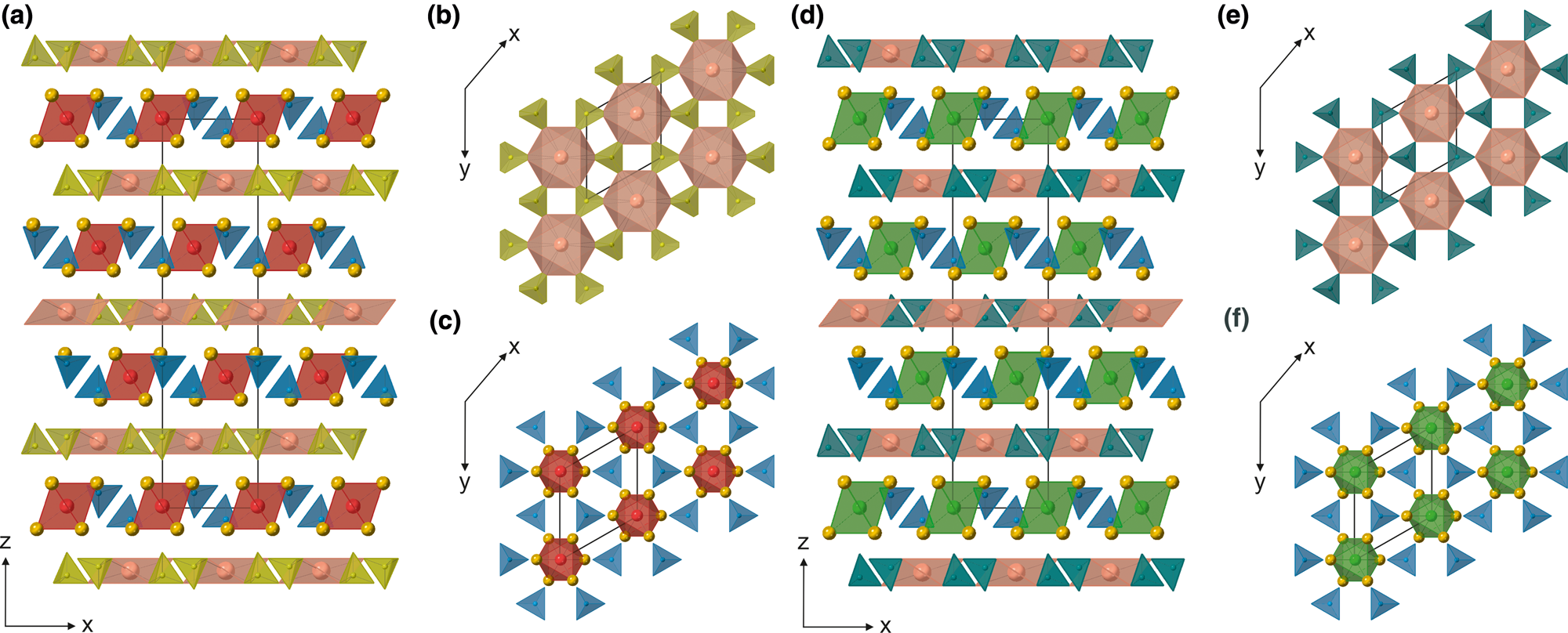
Fig. 5. The crystal structure of the zadovite type represented by gazeevite (a) and zadovite (d), characterised by the alternation of single tetrahedral (b,e) and single antiperovskite layers (c,f) along (001). The Ba-octahedra (pink) on T layers are surrounded by SO4-tetrahedra (yellow) and PO4-tetrahedra (sea-green) in the gazeevite (b) and zadovite (e) structure, respectively. The antiperovskite layers (A) are centred by O2– (red) in the gazeevite structure (c) and by F– (green) in the zadovite structure (f). Anion-centred octahedra are coordinated by Ca atoms (dark yellow spheres) and surrounded by six SiO4-tetrahedra in gazeevite and SiO4/PO4-tetrahedra in zadovite (both in blue).
The tetrahedral Ba-bearing layers in the gazeevite structure have the following composition Ba(SO4)2. Each large Ba atom coordinated by 6+6 oxygen atoms with 6 × 2.876(3) Å (Ba–O3) and 6 × 3.245(3) Å (Ba–O2) bonds is linked to six tetrahedra (Fig. 5b). The EMPA also confirmed a minor K amount (~8%) refined at this site. S/P occupies the T2 site in gazeevite. Due to the similar scattering factors, the refinement of S and P has not been performed. However, the substitution of S for P was revealed in chemical analyses (~0.2 apfu of P5+). In the T2 tetrahedron, there are three shorter T2–O3 = 1.476(4) Å and one longer T2–O4 = 1.499(4) Å bonds. Due to rotation, the BaO6 octahedron could be linked to six equivalent T2-tetrahedra with the triangular base O3–O3–O3 (Fig. 5b). However, the O3 atom is half occupied, implying that, on average, only three T2-tetrahedra are linked to the BaO6 octahedron. According to the EMPA, the emerged uncharged balance at the T2 site was balanced by F– at the O5 site in the gazeevite structure due to the S6+ + O2– ↔ P5+ + F– substitution scheme.
In the antiperovskite layers of gazeevite, the octahedron is centred by an (O5)2– anion coordinated by six Ca1 atoms, with the Ca1–O5 bond length equal to 2.4472(7) Å (Fig. 5c). The T1 (SiO4)4– tetrahedra are placed between them. The Si atom is surrounded by three O2 in the base and one apical O1 atom with distances of 1.618(2) Å and 1.634(4) Å Si–O, respectively.
In the zadovite structure, similar to that of gazeevite, the polyhedral sites in the tetrahedral layers are occupied by Ba, enclosed by six PO4 tetrahedra ascribed to the T2 site (Fig. 5e). The average Ba–O bond lengths (6 × Ba–O2 and 6 × Ba–O3) equal 3.3239 Å and 2.8012 Å, respectively. The average 1.539 Å P–O distance is nearly ideal for PO4-tetrahedra, which does not confirm the chemical calculation and partial S substitution at this site. The antiperovskite layers in zadovite consist of regular FCa6 octahedra with a F–Ca bond length of 2.5848(4) Å and tetrahedra at the T1 sites occupied by Si and P (Fig. 5f). The Si/P atoms at the T1 site bond three times to O2 = 1.588(12) Å and one slightly longer O1 = 1.604(3) Å, yielding the average 1.592 Å T1–O bond length.
Raman spectroscopy data
The Raman spectra of the arctite-supergroup minerals from xenoliths of the Bellerberg volcano are characterised by several fundamental bands in the 800–1200 cm–1 spectral range (Fig. 6). These bands are assigned to the symmetric (ν1) and asymmetric (ν3) stretching modes related to the (TO4) tetrahedral group vibrations. The EMPA results confirm that the vibrations of the (SiO4)4–, (SO4)2– and (PO4)3– groups should be dominant in the Raman spectra of analysed mineral phases.

Fig. 6. The Raman spectra of nabimusaite, gazeevite and zadovite from the xenoliths of the Bellerberg volcano.
In the Raman spectrum of nabimusaite and gazeevite (Fig. 6), the most intense bands at 993 cm–1 and 1002 cm–1 are ascribed to the ν1 symmetric stretching vibrations of the sulfate group, which occupy the T2 tetrahedral sites in the structure. The asymmetric ν3 stretching modes of (SO4)2– are observed in spectral ranges 1110–1145 cm–1 and 1075–1140 cm–1 for nabimusaite and gazeevite, respectively. In the Raman spectrum of zadovite, due to the substitution of S6+ at the T2 site confirmed by EMPA (Table 2), the band related to ν1 (SO4)2– appears at 993 cm–1, with intensity relatively lower in comparison to this band intensity in two other spectra (Fig. 6). The zadovite spectrum is characterised by splitting the most intense band in the range ~950–970 cm–1 (Fig. 6). These two bands are related to the symmetric stretching vibrations of a (PO4)3– group and indicated that the phosphorus occupied two different T sites in the crystal structure. This fact was also reported in a previous publication and proved that the band at 949 cm–1 is assigned to the T2 site, whereas the band at 967 cm–1 is assigned to the T1 site, according to the site occupation and Raman spectra of the zadovite–aradite mineral series (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Włodyka, Dzierżanowski and Murashko2015b). Moreover, bands attributed to the ν3 of (SiO4)4– group, not observed in the zadovite spectrum, may overlap with the ν1 (PO4)3– vibrations in this spectral region. The band near ~1030 cm–1 with the relatively large full width at half maximum (FWHM) is ascribed to the asymmetric stretching vibrations of (PO4)3–. A more detailed fitting analysis allows dividing this band into two components placed at 1025 and 1037 cm–1, indicating they assign the (PO4)3– vibrations to two different tetrahedra. Low intense Raman bands ascribed to the symmetric stretching (PO4)3– vibrations placed at 957 cm–1 in the gazeevite spectrum may be related to a noticeable P (0.17 apfu) content in its chemical composition.
The remaining Raman bands in this spectral range correspond to the symmetric stretching vibrations of the (SiO4)4– group detected in each of the three presented spectra (Fig. 6). In the case of zadovite and gazeevite, in which the structure is featured by a single antiperovskite layer, the Raman band related to ν1 (SiO4)4– appears at ~870 cm–1 with shoulders at 850 and 865 cm–1, respectively. Due to the presence of two symmetry-independent Si sites within the triple antiperovskite layer in the nabimusaite structure, the doubling of the Raman lines assigned to the ν1 (SiO4)4– vibrations are observed (Fig. 6). Bands related to this mode type occur at 830 cm–1 with low intense shoulders at 843 cm–1 and ~880 cm–1.
The spectral range between 350 and 650 cm–1 is attributed to the symmetric (ν2) and asymmetric (ν4) bending modes associated with the vibrations of (TO4) groups. The Raman spectra of nabimusaite and gazeevite in this region are similar and characterised by single bands at ~465 and 640 cm–1 assigned to the symmetric and asymmetric bending modes of (SO4)2–, respectively. Moreover, the Raman band at ~405 cm–1 with two low intense bands in the range ~525–560 cm–1 are related to the bending vibrations of (SiO4)4– groups. In zadovite, bands associated with the modes of sulfate and silicate bending vibrations are also in similar spectral ranges. The Raman bands with wavenumber 467 cm–1 and at 625–636 cm–1 correspond to the ν2 and ν4 bending modes of (SO4)2– group, whereas bands at 383 and 515 cm–1 correspond to the same vibrations of the (SiO4)4– group. In addition, the zadovite spectrum is featured by a broad and intense band at 432 cm–1 with a shoulder at 416 cm–1, which is connected to the symmetric bending vibrations of the (PO4)3– groups, while asymmetric bending modes are observed as a few low intense bands in the range ~575–605 cm–1 (Fig. 6).
In the Raman spectra (Fig. 6), several bands in the range 280–330 cm–1 are ascribed to the rotational and translational modes of (TO4) groups. The single Raman bands at 246 cm–1 in nabimusaite, 256 cm–1 in gazeevite, and 220 cm–1 in zadovite are related to the K–O, Ba–O and Ca–O stretching vibrations, respectively. Mentioned bands belong to the lattice acoustic modes and may also occur in the low wavenumber region below 200 cm–1.
Discussion
The individual analysis of holotype nabimusaite discovered in the Jabel Harmun locality (Hatrurim Complex, West Bank) indicated enrichment of Ba, up to as much as ~5.0 wt.% BaO (Galuskin et al., Reference Galuskin, Gfeller, Armbruster, Galuskina, Vapnik, Murashko, Włodyka and Dzierżanowski2015a). In turn, the highest K2O content is equal to 3.65 wt.%. For the average chemical composition of the holotype nabimusaite, the ratio of these two components is ~27% to 63% for Ba2+ and K+, respectively. These results indicated the significant involvement of the BaCa12(SiO4)4(SO4)2O3 end-member, known as dargaite (Galuskina et al., Reference Galuskina, Gfeller, Galuskin, Armbruster, Vapnik, Dulski, Gardocki, Jeżak and Murashko2019), in a complex solid-solution represented by the holotype nabimusaite. In the case of the German counterpart, the polyhedral site is 83% occupied by K+, whereas the Ba2+ contents are a negligible amount, ~2% in accordance with chemical results. In addition, the notable contribution of ~11% Sr2+ is also observed (Table 2). For the tetrahedral sites, the presence of additional impurities does not exceed 0.2 and 0.4 apfu in nabimusaite from Bellerberg and Jabel Harmun localities, respectively.
The holotype gazeevite described from the Shadil-Khokh volcano and a few outcrops of pyrometamorphic rocks of the Hatrurim Complex is characterised by similar compositions (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Armbruster, Krzątała, Vapnik, Kusz, Dulski, Gardocki, Gurbanov and Dzierżanowski2017). Approximated results were also obtained for the gazeevite detected in the carbonate–silicate xenolith from Bellerberg volcano, Germany. In both cases, the differentiated substitution of S6+ for P5+ at the T2 position in tetrahedral layers is observed. The presence of P5+ at this site implies the occurrence of F– in the OCa6 anion-centred octahedron, which compensates charge balance due to the S6+ + O2– ↔ P/V5+ + F– scheme.
The main difference between the data obtained and the holotype zadovite was observed primarily in the V5+ content that is six-times higher (0.65 apfu) in the holotype sample than in the zadovite from Germany (0.10 apfu). The authors of the holotype material description confirm that the significant variations in the chemical composition are caused by the occurrence of the zadovite–aradite series in the pyrometamorphic rocks of the Hatrurim Complex (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Włodyka, Dzierżanowski and Murashko2015b). Based on their results, the V2O5 content in zadovite was never lower than 2–3 wt.%. However, the chemical analyses performed for zadovite, detected in paralava from Zuk Tamrur locality (Hatrurim Complex, Israel), confirm lower V2O5 contents ~1.15 wt.% (Krzątała et al., Reference Krzątała, Krüger, Galuskina, Vapnik and Galuskin2020), which is approximate to the values obtained for zadovite reported in Caspar quarry (Table 2). Moreover, in both specimens, due to the Si4++S6+↔2P5+ substitution scheme at the tetrahedral sites, variations of these element content are observed.
The results obtained with the single-crystal X-ray diffraction in this work are in good agreement with the structural data for the holotype nabimusaite, gazeevite and zadovite (Galuskin et al., Reference Galuskin, Gfeller, Armbruster, Galuskina, Vapnik, Murashko, Włodyka and Dzierżanowski2015a, 2015b, 2017). The minor differences appear in the site population and bond lengths between atoms, mostly in tetrahedra, caused by the difference in composition. However, in the previous description of the nabimusaite structure, fluorine (corresponding to the O8 site) was incorporated into the central antiperovskite layer, and the refinement was performed in analogy to the synthetic KCa12(SiO4)4(SO4)2O2F phase (Fayos et al., Reference Fayos, Glasser, Howie, Lachowski and Perez-Mendez1985; Galuskin et al., Reference Galuskin, Gfeller, Armbruster, Galuskina, Vapnik, Murashko, Włodyka and Dzierżanowski2015a). The subsequent BVS (bond-valence sums) calculation revealed that instead of being internal, F occupied the external antiperovskite layers related to the O7Ca6 octahedra (Galuskina et al., Reference Galuskina, Gfeller, Galuskin, Armbruster, Vapnik, Dulski, Gardocki, Jeżak and Murashko2019). Therefore, the original model for natural and synthetic nabimusaite is not entirely correct and should be revised. The present paper has demonstrated the correct refinement for nabimusaite from the Bellerberg volcano. The anion-centred octahedra in the internal antiperovskite layer are occupied by O2– (O8), whereas F– (O7) is located in the external layers together with O2– in a ratio of 50/50.
Unlike the holotype specimen, gazeevite described here does not show the splitting of oxygen atoms on O3 and O3A and the rotational disorder of SO4-tetrahedra of ca. ± 6° around [001] within the tetrahedral layers. In the previous description, this effect was explained by the various occupancies of the Ba site by Ba2+ (81%) and a considerable amount of K+ (19%) and their differences in charges and ionic radii (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Armbruster, Krzątała, Vapnik, Kusz, Dulski, Gardocki, Gurbanov and Dzierżanowski2017). Nevertheless, gazeevite from the Caspar quarry xenolith revealed a much lower K occupation (8%) at the Ba site, which did not cause deformations in the structure.
The Raman spectra for arctite-supergroup minerals from the Bellerberg volcano are very similar to the spectra of the holotype species (Galuskin et al., Reference Galuskin, Gfeller, Armbruster, Galuskina, Vapnik, Murashko, Włodyka and Dzierżanowski2015a; Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Włodyka, Dzierżanowski and Murashko2015b, Reference Galuskin, Gfeller, Galuskina, Armbruster, Krzątała, Vapnik, Kusz, Dulski, Gardocki, Gurbanov and Dzierżanowski2017) and are compared in Table 3. Minor differences in the number of bands and their intensity are related to the random crystal orientation during the measurements and some variability in the chemical composition. In the case of nabimusaite spectra from both localities, differences are indistinguishable (Table 3). The same observations were made for gazeevite spectra. However, we consider that band at 560 cm–1 in the holotype gazeevite is more probably related to the symmetric bending vibrations of (SiO4)4– than (VO4)3–, as was proposed before (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Armbruster, Krzątała, Vapnik, Kusz, Dulski, Gardocki, Gurbanov and Dzierżanowski2017). The higher SO3 content observed in German zadovite made it possible to also distinguish, in addition to the bands attributed to stretching vibrations, bands related to the (ν2) symmetric bending vibration of (SO4)2– at 467 cm–1, which was not detected in the Raman spectrum of the holotype zadovite. In contrast, due to the higher V2O5 content in the holotype zadovite, Raman bands at 839 and 342 cm–1 are assigned to the symmetric stretching and bending vibrations of (VO4)3–, respectively (Table 3). The results suggest that minerals belonging to the arctite supergroup might be easily identified by Raman spectroscopy.
Table 3. A comparison of Raman band assignments for nabimusaite, gazeevite and zadovite from the Bellerberg volcano in Germany and the Hatrurim Complex in Israel.

The Hatrurim Complex located in Israel, Jordan and some territories of Palestine (West Bank), is a large geological unit composed of high-temperature low-pressure pyrometamorphic rocks (Bentor et al., Reference Bentor, Gross and Heller1963; Gross, Reference Gross1977; Burg et al., Reference Burg, Starinsky, Bartov and Kolodny1991; Geller et al., Reference Geller, Burg, Halicz and Kolodny2012; Novikov et al., Reference Novikov, Vapnik and Safonova2013). The most common are clinker-like pyrometamorphic rocks (spurrite marbles, larnite pseudoconglomerates, gehlenite hornfelses) and various paralavas (Bentor et al., Reference Bentor, Gross and Heller1963; Sokol et al., Reference Sokol, Novikov, Vapnik and Sharygin2007; Vapnik et al., Reference Vapnik, Sharygin, Sokol and Shagam2007; Grapes, Reference Grapes2010; Galuskina et al., Reference Galuskina, Vapnik, Lazic, Armbruster, Murashko and Galuskin2014). Previous research focusing on minerals of the arctite supergroup has shown that nabimusaite and gazeevite were found in larnite–ye'elimite nodules of pyrometamorphic rocks known as pseudoconglomerates, featured by the presence of visible microfractures and increased porosity (Galuskin et al., Reference Galuskin, Gfeller, Armbruster, Galuskina, Vapnik, Murashko, Włodyka and Dzierżanowski2015a, Reference Galuskin, Gfeller, Galuskina, Armbruster, Krzątała, Vapnik, Kusz, Dulski, Gardocki, Gurbanov and Dzierżanowski2017), whereas zadovite was usually detected within the rankinite paralava hosted by gehlenite hornfelses (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Włodyka, Dzierżanowski and Murashko2015b; Krzątała et al., Reference Krzątała, Krüger, Galuskina, Vapnik and Galuskin2020). Gazeevite was also recognised in an altered carbonate–silicate xenolith embedded in rhyodacite lava on the northwest slope of the Shadil-Khokh volcano (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Armbruster, Krzątała, Vapnik, Kusz, Dulski, Gardocki, Gurbanov and Dzierżanowski2017). According to mineral paragenesis and temperature conditions, it was reported that the formation of nabimusaite and gazeevite in the Hatrurim Complex is combined with high-temperature alteration of primary mineral assemblages, mainly fluorellestadite and larnite, affected by K- and Ba-enriched sulfate melt generated probably by the combustion processes (Galuskin et al., Reference Galuskin, Gfeller, Armbruster, Galuskina, Vapnik, Murashko, Włodyka and Dzierżanowski2015a, Reference Galuskin, Gfeller, Galuskina, Armbruster, Krzątała, Vapnik, Kusz, Dulski, Gardocki, Gurbanov and Dzierżanowski2017). In addition, the occurrence of gazeevite in xenoliths of the Shadil-Khokh volcano might be connected with the presence of sulfate-enriched intergranular melt (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Armbruster, Krzątała, Vapnik, Kusz, Dulski, Gardocki, Gurbanov and Dzierżanowski2017). In turn, the formation of zadovite in small enclaves within the paralava of the Hatrurim Complex is an effect of residual melt crystallisation enriched in incompatible elements such as Ba, V, P, U, S, Ti and Nb, formed after relatively fast crystallisation of the main constituents of paralava (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Włodyka, Dzierżanowski and Murashko2015b; Galuskina et al., Reference Galuskina, Galuskin, Vapnik, Prusik, Stasiak, Dzierżanowski and Murashko2017; Krzątała et al., Reference Krzątała, Krüger, Galuskina, Vapnik and Galuskin2020).
We conclude that nabimusaite and gazeevite from Germany probably formed during a similar crystallisation scheme described above. High-temperature clinker-like phases such as larnite, ternesite and fluorellestadite detected in xenoliths were altered and reacted later with sulfate-bearing melt, leading to nabimusaite and gazeevite crystallisation. Moreover, the presence of jasmundite in association with nabimusaite may indicate that the reaction of altered primary phases occurs not only in the presence of melt but also with some volatile components transported by different gases generated by volcanic activity. In turn, xenomorphic zadovite grains identified in a xenolith sample from the Bellerberg volcano between wollastonite, gehlenite and alumoåkermanite crystals, together with other Ba-minerals (bennesherite, fresnoite, walstromite and celsian) formed after them, due to reactions of Ba concentrated in the intergranular spaces filled with a liquid phase, most probably melt with silica composition enriched in Na, as well as Al, Ti and Al.
Minerals of the arctite supergroup are very rare and have been described from only a few localities to date, most of which belong to the Hatrurim Complex (Galuskin et al., Reference Galuskin, Galuskina, Krüger, KrügeR, Vapnik, Krzątała, Środek and Zieliński2021). In addition to pyrometamorphic rocks and xenoliths from the Shadil-Khokh volcano, the Bellerberg volcano is now another locality where more than one member of this supergroup was detected. As was noted, usually, these mineral phases occur as an accessory, however, some might be distinguished as rock-forming minerals, such as the nabimusaite described herein or the aravaite identified in some spurrite rock of the Hatrurim Complex (Galuskin et al., Reference Galuskin, Galuskina, Krüger, KrügeR, Vapnik, Krzątała, Środek and Zieliński2021). The difference between the analysed samples and previous reports is related to the co-occurrence of the arctite-supergroup minerals within each rock sample. In three analysed xenolith samples from the Bellerberg volcano, nabimusaite, gazeevite and zadovite occur separately, whereas, in rocks of the pyrometamorphic Hatrurim Complex, the solid solution of zadovite–aradite (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Włodyka, Dzierżanowski and Murashko2015b), nabimusaite–dargaite (Galuskina et al., Reference Galuskina, Gfeller, Galuskin, Armbruster, Vapnik, Dulski, Gardocki, Jeżak and Murashko2019), or nabimusaite, dargaite, gazeevite (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Armbruster, Krzątała, Vapnik, Kusz, Dulski, Gardocki, Gurbanov and Dzierżanowski2017) and ariegilatite–stracherite (Galuskin et al., Reference Galuskin, Krüger, Galuskina, Krüger, Vapnik, Wojdyla and Murashko2018a) associations within one rock specimen were reported. Further investigation of carbonate–silicate xenoliths from the Bellerberg volcano area in Germany could potentially allow for similar observations and identification of other members of the arctite supergroup.
Acknowledgements
We thank Hannes Krüger (University of Innsbruck) for significant technical assistance during the diffraction experiments at X06DA and three anonymous reviewers for their helpful and constructive comments, which allowed us to improve a previous version of the manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2022.103
Competing interests
The authors declare none.











