Introduction
Pseudosuccinea columella ( = Lymnaea columella) is a widely dispersed species originating from eastern USA and Central America (Cruz-Reyes & Malek, Reference Cruz-Reyes and Malek1987). This lymnaeid has been introduced successfully into other continents: Africa, Europe, Oceania and South America (Ponder, Reference Ponder1975; Boray, Reference Boray1978; Brown, Reference Brown1994; Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005; Bargues et al., Reference Bargues, González, Artigas and Mas-Coma2011). The large distribution of this snail throughout the world indicates that P. columella is an alien species (Taraschewski, 2006). This lymnaeid is also an intermediate host of Fasciola hepatica (Brown, Reference Brown1994). Its role as a suitable snail host was demonstrated in Australia (Boray, Reference Boray1978; Boray et al., Reference Boray, Fraser, Williams and Wilson1985), USA (McKown & Ridley, Reference McKown and Ridley1995) and Cuba (Gutiérrez et al., Reference Gutiérrez, Vázquez, Hevia, Sánchez, Correa, Hurtrez-Boussès, Pointier and Théron2011). Susceptible and resistant strains of this snail to experimental infection with local F. hepatica miracidia were also detected in Cuba (Gutiérrez et al., Reference Gutiérrez, Pointier, Fraga, Jobet, Modat, Pérez, Yong, Sánchez, Loker and Théron2003, Reference Gutiérrez, Hernandez and Sánchez2005). Compared with Galba cubensis, the main snail host in Cuba, the prevalence of F. hepatica in P. columella was lower: 71% and 49%, respectively (Vázquez et al., Reference Vázquez, Sánchez, Pointier, Théron and Hurtrez-Boussès2013). In France, a snail population originating from the Lot River was found to be susceptible to infection with French F. hepatica, with a prevalence of 100% (Pointier et al., Reference Pointier, Coustau, Rondelaud and Théron2007). The introduction of this lymnaeid in South Africa had increased the prevalence of fascioliasis in livestock (Brown, Reference Brown1994).
In Egypt, little information on the role of P. columella in fascioliasis transmission is available. This might be due to the fact that this species was found with another similar shell-shaped lymnaeid, Radix natalensis, in the same habitats. In a survey carried out over 2 years, P. columella was found in two water bodies from the Giza governorate, with high snail density in autumn (Ahmed & Ramzy, Reference Ahmed and Ramzy1999) and F. gigantica larvae were detected in 2% of collected specimens (shell height, >10 mm). In five water bodies of the Dakahlia governorate, no larval forms of Fasciola sp. were detected in collected P. columella (El-Shazly et al., Reference El-Shazly, Helmy, Haridy, El-Sharkawy and Morsy2002). As F. hepatica and F. gigantica are currently found in Egypt (Lofty et al., Reference Lofty, El-Morshedy, Abou El-Hoda, El-Tawila, Omar and Farag2002; Amer et al., Reference Amer, Dar, Ichikawa, Fukuda, Tada, Itagaki and Nakai2011; Dar et al., Reference Dar, Amer, Mercier, Courtioux and Dreyfuss2012), identification of Fasciola species using morphological criteria of larval stages was difficult. Several parameters, such as the diameter of the pharyngeal lumen, could be used to discriminate immature rediae of both Fasciola spp. in Galba truncatula and R. natalensis (Dar et al., Reference Dar, Vignoles, Rondelaud and Dreyfuss2003) but they were dependent on the snail species, its growth and experimental conditions.
In view of this conflicting situation in Egypt, the role of P. columella in local transmission of fascioliasis can be assessed via experimental infections of snails using molecularly identified isolates of F. hepatica or F. gigantica. The aim of this paper was to determine the susceptibility of three Egyptian P. columella populations as intermediate hosts of local F. hepatica and specify characteristics of these infections, taking into account the species of the definitive host (cattle or sheep) from which eggs were collected and the miracidial dose used for each snail at exposure. The results noted in the present study will be compared with those already reported for R. natalensis subjected to the same digenean (Dar et al., Reference Dar, Djuikwo Teukeng, Vignoles, Dreyfuss and Rondelaud2010).
Materials and methods
Collection and examination of snails and flukes
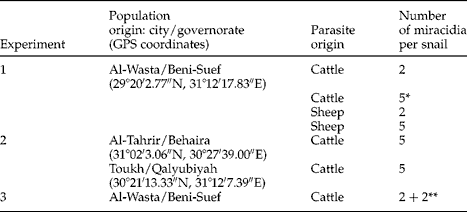
Pseudosuccinea columella populations from three different geographical origins were used in the present study (table 1). The characteristic spiral ridges of the periostracum (Brown, Reference Brown1994; Pointier et al., Reference Pointier, Coustau, Rondelaud and Théron2007), the succiniform slender shell with a small, pointed spire and the long ovate shell aperture (Hubendick, Reference Hubendick1951) allow the identification of this species in the field because this snail was often found in the same habitats with R. natalensis. Adult snails were collected from each population and transported to the laboratory to be placed in covered, aerated 5-litre aquaria with 50 individuals per recipient. These aquaria were subjected to constant laboratory conditions: temperature, 22 ± 1°C; light/dark period, 12 h/12 h. Dissolved calcium concentration in spring water was 35 mg/l. Snails were fed on pesticide-free fresh lettuce ad libitum, and spring water in aquaria was changed weekly. Egg masses laid by adult snails were collected using a fine forceps and were placed into small rearing aquaria. Newly hatched snails fed on finely powdered dry lettuce, and those that attained 3–4 mm in shell height were used for experimental infections.
Table 1 Geographical origin of Pseudosuccinea columella from three Egyptian water bodies and the characteristics of experimental groups, with 50 snails/group used in experiments 1 and 2, and 30 snails in experiment 3. *Snails also used in experiment 2, ** 4-hourly intervals between exposures.
Adult flukes were collected from the livers of naturally infected animals: 9 flukes from cattle and 17 from sheep, slaughtered at Al-Basateen abattoir, Cairo, Egypt. Flukes were washed with physiological saline solution (NaCl, 0.9%) and then incubated at 37°C for 4 h to allow egg-laying. Eggs were then washed several times with dechlorinated tap water and maintained in the dark at 22°C for 18 days to allow miracidial development (Ollerenshaw, Reference Ollerenshaw1971). On day 20 post-incubation, eggs were exposed to artificial light to stimulate miracidial hatching. Flukes were then fixed in 70% ethanol for molecular analysis in order to identify Egyptian Fasciola sp. French F. hepatica and Cameroonian F. gigantica previously conserved in the laboratory were used as reference samples.
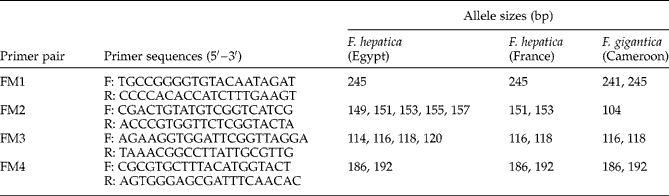
As F. hepatica and F. gigantica were found in Egypt (Lofty et al., Reference Lofty, El-Morshedy, Abou El-Hoda, El-Tawila, Omar and Farag2002; Amer et al., Reference Amer, Dar, Ichikawa, Fukuda, Tada, Itagaki and Nakai2011; Dar et al., Reference Dar, Amer, Mercier, Courtioux and Dreyfuss2012), species identification was performed using a DNA-based assay before carrying out experimental infections of snails. Flukes were identified using specific microsatellites according to the protocol by Dar et al. (Reference Dar, Amer, Courtioux and Dreyfuss2011) because these markers were able to separate closely related species (Gemmell et al., Reference Gemmell, Allen, Goodman and Reed1997). Four microsatellite primer pairs: FM1, FM2, FM3 and FM4, based on nucleotide sequences described by Hurtrez-Boussès et al. (Reference Hurtrez-Boussès, Durand, Jabbour-Zahab, Guegan, Meunier, Bargues, Mas-Coma and Renaud2004) for F. hepatica were used (table 2). Total DNA was extracted from each fluke using QIAamp® DNA Mini Kit (Qiagen, Germantown, Maryland, USA) following the manufacturer's recommendations. Microsatellite sequences were amplified using polymerase chain reaction (PCR) in a 25 μl reaction volume containing 1 μl fluke DNA, 10 μl of the primer mixture, 12.5 μl Qiagen Multiplex and 1.5 μl DNA- and RNA-free water. Amplifications were performed using a Perkin-Elmer Thermo Cycler (Applied Biosystems, Warrington, UK) with an initial denaturation at 95°C for 15 min, followed by 30 cycles, each comprising denaturation at 94°C for 30 s, annealing at 61°C for 3 min and extension at 72°C for 1 min, and ended by a final extension at 60°C for 30 min. One microlitre of PCR product was added to 23.5 μl formamide and 0.5 μl of internal size standard ROX 500 (Applied Biosystems), and the mixture was subjected to electrophoresis using an ABI PRISM® 310 genetic analyser (Applied Biosystems). Allele sizes were assigned using GeneMapper® software, version 4.0 (Applied Biosystems).
Table 2 Allele sizes (bp) of amplified microsatellite sequences of Fasciola hepatica from Egypt and France and F. gigantica from Cameroon.
Three experiments were carried out using seven snail groups originating from three populations (table 1). In the first experiment, the susceptibility of P. columella to experimental infection with Fasciola sp. in relation to the species of definitive host from which Fasciola eggs were obtained, and miracidial dose used for snail infection were investigated. In this assay, four groups of 50 snails each, originating from the Beni-Suef population, were constituted. Snails from the first two groups were individually exposed to two and five cattle-derived miracidia, respectively. The same protocol was used for the other two groups, using two or five sheep-derived miracidia per snail.
In the second experiment, the interpopulation variability in the susceptibility of P. columella to Fasciola infection was studied. Snails from two groups, belonging to the Behaira and Qalyubiyah populations, were each exposed to five cattle-derived miracidia.
In the third experiment, the characteristics of Fasciola sp. infection and the dynamics of cercarial shedding from P. columella were investigated to compare the results with those reported by Dar et al. (Reference Dar, Djuikwo Teukeng, Vignoles, Dreyfuss and Rondelaud2010) in the F. hepatica/R. natalensis model. The protocol adopted was similar to that used by Dar et al. (Reference Dar, Djuikwo Teukeng, Vignoles, Dreyfuss and Rondelaud2010). Thirty snails originating from the Beni-Suef population were each subjected to two successive bi-miracidial infections with an interval of 4 h between both exposures. The time of exposure to miracidia was 4 h for each snail group. Snails were then raised in covered, aerated 5-litre aquaria at 22 ± 1°C as parent snails.
On day 30 post-exposure (pe), surviving snails from the seven groups were individually placed in 50-mm Petri dishes containing spring water and a small piece of fresh lettuce. The Petri dishes were also placed at 22 ± 1°C. Water and food were changed, if necessary, every day until snail death. When the first cercarial shedding occurred, metacercariae, either floating on the water surface or fixed on dish walls and/or bottom, were counted daily and removed from Petri dishes. At the death of infected snails of the first and second experiments, the cadaver of each snail was dissected under a stereomicroscope to count rediae and free cercariae present within its body. In the case of cercarial-shedding snails which died after day 80 pe, the shell height of 4–5 individuals in each group was measured using callipers just before snail dissection.
Data analysis
Snail survival on day 30 pe was determined for each experimental group. The frequency and life span of each snail category: snails shedding cercariae, infected snails without cercarial shedding and uninfected snails, were calculated. Prevalence of F. hepatica infection was determined by adding the frequencies of all infected snails. In the case of cercarial-shedding snails, the length of the pre-patent period between the date of exposure and the first cercarial shedding, the patent period, i.e. the duration of cercarial shedding, and the total number of metacercariae were also considered. Percentages of floating or fixed metacercariae were determined in relation to the total number of metacercariae. After the death of infected snails, the total numbers of rediae and free cercariae in snail cadavers were enumerated. A χ2 test, one- or multiple-way analysis of variance and Tukey honestly significant difference test were used to establish levels of significance. All analyses were performed using Statview 5.0 software (SAS Institute Inc., Cary, North Carolina, USA).
Minimum and maximum values of individual shell heights were included after day 80 pe, due to the low number of snails shedding cercariae in each group.
Results
Identification of Egyptian liver flukes
The four microsatellite sequences were successfully amplified for all the examined flukes (table 2). The separated band profile of Egyptian flukes was similar to that of French F. hepatica. FM1 microsatellite primer-pair amplified one band of 245 bp in the case of F. hepatica and two bands of 241 and 245 bp for F. gigantica from Cameroon. In addition, FM2 was composed of five polymorphic alleles of 149, 151, 153, 155 and 157 bp in the case of Egyptian F. hepatica and two alleles of 151 and 153 bp for French F. hepatica, whereas it amplified a monomorphic allele of 104 bp in F. gigantica. This result shows that Egyptian flukes belong to F. hepatica. The difference noted in FM2 and FM3 allele numbers indicates an intraspecific variation between Egyptian and French isolates of F. hepatica.
Snail infection, parasite origin and miracidial dose used for infection
On day 30 pe, differences between snail survival rates in the infected Beni-Suef population were not significant (table 3). The frequency of cercarial-shedding snails in the five-miracidia groups was significantly higher (χ2= 16.27, P< 0.001) than those in the two-miracidia groups. Another significant difference (χ2= 22.46, P< 0.001) was noted in snails without cercarial shedding, with a greater percentage in the five-miracidia sheep group. The frequencies of uninfected snails were significantly higher (χ2= 13.82, P< 0.01) in the two-miracidia groups than in the five-miracidia groups, whatever parasite origin. If prevalence of infection is considered by adding the frequencies of snails shedding cercariae and those without cercarial shedding, the value of 75.5% noted in the five-miracidia sheep group was significantly greater (χ2= 18.58, P< 0.001) than those noted in the other three groups.
Table 3 Prevalence (%) of Fasciola hepatica in the freshwater snail Pseudosuccinea columella from Beni-Suef (experiment 1) together with snail survival (%) and life span (days) with or without cercarial shedding, lengths of the pre-patent and patent periods (days) and the occurrence of metacercarial cysts; each of two groups of 50 snails were examined on day 30 following exposure to either two or five miracidial doses of cattle or sheep origin, and all mean values show ± standard deviation (SD).

The life span of snails was significantly affected by the miracidial dose used for infection (F= 5.8, P< 0.05) and snail category (F= 40.08, P< 0.001), because snails without cercarial shedding had lower mean values than those found in cercarial-shedding and uninfected categories. In the case of non-cercarial-shedding snails, the life span in the two-miracidia groups was significantly longer than that noted in snails infected with five miracidia, whatever parasite origin. The length of the pre-patent period was significantly affected (F= 4.92, P< 0.05) by parasite origin, with higher values in cattle groups. The patent period noted in the five-miracidia sheep group was significantly longer (F= 9.4, P< 0.01) than that noted in the three others. Non-significant differences between the four groups were noted for the total number of metacercariae produced by cercarial-shedding snails and the percentages of floating and fixed cysts.
Snail infection and origin
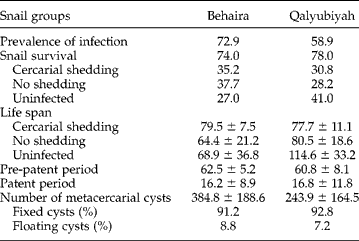
Non-significant differences between the Beni-Suef, Behaira and Qalyubiyah groups were noted for snail survival rate on day 30 pe, the frequency of each snail category, prevalence of infection, the lengths of pre-patent and patent periods, the total number of metacercariae and the percentages of floating and fixed cysts (tables 3 and 4). In contrast, snail population (F= 8.37, P< 0.001) and snail category (F= 17.08, P< 0.001) had significant effects on the P. columella life span. In the Beni-Suef group, snails without cercarial shedding had a shorter life span of 45.1 days compared with 78.8 and 99.9 days in shedding and uninfected snails, respectively. In the Behaira group, the life spans of snails were close to each other and non-significant differences were noted. In the Qalyubiyah group, uninfected snails had a longer life span of 114.6 days instead of 77.7 and 80.5 days in snails with or without cercarial shedding, respectively.
Table 4 Prevalence (%) of Fasciola hepatica in the freshwater snail Pseudosuccinea columella from Behaira and Qalyubiyah (experiment 2) together with snail survival (%) and life span (days) with or without cercarial shedding, lengths of the pre-patent and patent periods (days) and the occurrence of metacercarial cysts. Each group of 50 snails was examined on day 30 following exposure to five miracidial doses of cattle origin and all mean values show ± SD.
Larval burdens in snail cadavers
The shell heights of several cercarial-shedding snails after day 80 pe ranged from 10.1 to 15.0 mm, irrespective of snail group in the first and second experiments.
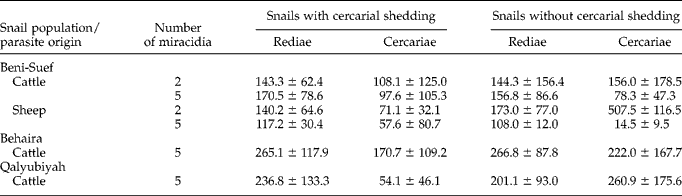
Redial burdens from the cadavers of snails with or without cercarial shedding showed no significant differences between the four groups of the first experiment (table 5). In contrast, the number of free cercariae was significantly affected (F= 4.37, P< 0.05) by the miracidial dose used for snail infection. Snails without cercarial shedding in the two-miracidia groups, whatever parasite origin, had higher numbers of free cercariae of 156 and 507.5 in their bodies compared with 78.3 and 14.5 cercariae in the five-miracidia groups. In the two-miracidia groups, the number of free cercariae was significantly greater (F= 5.20, P< 0.05) in snails without cercarial shedding than in those which shed cercariae. In the five-miracidia groups, the free cercariae contained in cercarial-shedding snails were more numerous than in the bodies of non-shedding snails, but the differences were not significant. In the second experiment (table 5), the number of free rediae found in the Behaira group was significantly higher (F= 5.20, P< 0.05) than redial burdens found in the other two groups, whatever snail category (with or without cercarial shedding). Significant differences in the numbers of free cercariae were noted between snail groups (F= 3.49, P< 0.05) and snail categories (F= 8.07, P< 0.001).
Table 5 Mean values (± SD) of Fasciola hepatica free rediae and cercariae in the cadavers of Pseudosuccinea columella from Beni-Suef (experiment 1), Behaira and Qalyubiyah (experiment 2).
Snail infection and dynamics of cercarial shedding
In the third experiment, snail survival on day 30 pe was 100% and prevalence of F. hepatica infection was 63.3%, with 17 cercarial-shedding snails and 2 without shedding among the 30 individuals surviving on day 30 pe. The life spans were 91.2 ± 16.9 days for snails shedding cercariae, 55.5 ± 85 days for those without cercarial shedding and 97.2 ± 28.5 days for uninfected snails. In the case of cercarial-shedding snails, the lengths of pre-patent and patent periods were 53.8 ± 5.7 days and 37.5 ± 18.4 days, respectively. The number of shed cercariae was 311.7 ± 189.2. The number of cercariae peaked on day 5 of the patent period with a mean of 30.8 (fig. 1). Three other peaks were also observed on days 10, 16 and 32, with mean numbers of 39.5, 50.5 and 103 cercariae, respectively. Later, shedding of cercariae progressively decreased with small peaks, and was discontinuous over time until the end of patent period.

Fig. 1 Mean number of Fasciola hepatica cercariae ( ± SD) shed from Pseudosuccinea columella per day during the patent period (experiment 3).
Discussion
The present study demonstrates that P. columella could play a role in F. hepatica transmission in Egypt and is an efficient intermediate host in which this fluke can accomplish its asexual cycle and produce viable metacercariae. Indeed, high prevalences ranging from 58.9 to 75.5% were observed in the five-miracidia group at 22°C, whatever the snail origin (tables 3 and 4). In the literature, prevalence of F. hepatica infection in P. columella greatly varied under laboratory conditions. For example, prevalence values in P. columella of height 3.2–7.5 mm, of North American origin and individually exposed to 1, 5 or 10 miracidia were 50 and 51.8% at 22–26°C (Cruz-Reyes & Malek, Reference Cruz-Reyes and Malek1987). In a French population of this lymnaeid, 100% prevalence was noted using 3–4-mm-high snails individually exposed to five miracidia and raised at 26°C (Pointier et al., Reference Pointier, Coustau, Rondelaud and Théron2007). Susceptibility of one-week-old P. columella, originating from eight Cuban populations, to different isolates of local F. hepatica ranged from 17 to 56% in five-miracidia infections (Vázquez et al., Reference Vázquez, Sánchez, Pointier, Théron and Hurtrez-Boussès2013). These differences might be due to the snail size at the time of exposure. According to Coelho et al. (Reference Coelho, Guimarães and Lima2008), prevalence of F. hepatica infection in snails measuring 15 mm at the time of exposure was lower, at 2%, compared with 79% in snails measuring 5–6 mm. In contrast, Vázquez et al. (Reference Vázquez, Sánchez, Pointier, Théron and Hurtrez-Boussès2013) explained these differences by variability in miracidial infectivity.
Despite the high prevalences noted in the five-miracidia groups, the number of metacercariae in the six snail groups of the first and second experiments did not significantly differ (tables 3 and 4). This result indicates that snail origin, parasite origin and the miracidial dose used for each snail did not have an effect on metacercarial production of F. hepatica in these experimentally infected P. columella. The absence of differences between metacercarial production in the two- and five-miracidia groups might be explained by the development of one or two F. hepatica miracidia in each snail. An argument supporting this assumption was the report by Dreyfuss et al. (Reference Dreyfuss, Vignoles, Rondelaud and Vareille-Morel1999). According to these authors, multimiracidial infections in the snail G. truncatula were less effective because the mean number of metacercariae recorded was the same as those found in single-miracidium and bi-miracidial groups.
Contrary to metacercarial production, parasite origin had a significant effect on the lengths of pre-patent and patent periods, as sheep-derived miracidia developed more rapidly in their snail hosts, whatever the dose of infection, and produced cercariae during longer patent periods only in the five-miracidia sheep group. These findings suggest a more compatible relationship between sheep-derived miracidia and their snail hosts. Such differences in the compatibility between miracidial isolates and their snails may be explained by a more or less complete adaptation between both partners, as demonstrated by Rondelaud & Dreyfuss (Reference Rondelaud and Dreyfuss1995) and Dar et al. (Reference Dar, Lounnas, Djuikwo Teukeng, Mouzet, Courtioux, Hurtrez-Boussès, Vignoles, Dreyfuss and Rondelaud2013) in the model G. truncatula/F. hepatica.
Non-significant differences between the mean numbers of rediae found in the cadavers of the four Beni-Suef groups (table 5) indicate that redial production was not affected by parasite origin and the miracidial dose used for snail exposure. This finding confirms the above hypothesis according to which only one or two miracidia could develop in snails and produce the different successive generations of rediae (Dreyfuss et al., Reference Dreyfuss, Vignoles, Rondelaud and Vareille-Morel1999). In addition, the redial burdens noted in the cattle and sheep groups ranged within the same scale of values, thus reflecting their efficiency in F. hepatica larval development. Significantly higher numbers of rediae found in the bodies of infected snails from the Behaira population (table 5) might be due to a variance in the shell growth of cercarial-shedding snails during the experiment rather than to an interpopulation variation, as snail infection with F. hepatica reduced the growth rate of P. columella (Salazar et al., Reference Salazar, Estrada and Velásquez2006).
Comparison of findings noted for P. columella in the third experiment with those reported by Dar et al. (Reference Dar, Djuikwo Teukeng, Vignoles, Dreyfuss and Rondelaud2010) for R. natalensis experimentally infected with a French isolate of F. hepatica according to the same protocol demonstrates that the former snail species had higher performance as the intermediate host for F. hepatica. Indeed, P. columella had higher prevalence for F. hepatica infection (63.3% instead of 58.5% in R. natalensis), longer cercarial-shedding periods (37.5 instead of 24.3 days), and a greater number of shed cercariae (311.7 instead of 90.7). The daily cercarial shedding during the patent period showed four peaks in P. columella, compared with two peaks in the case of R. natalensis. These results indicate that the population of P. columella used for this experiment was more compatible than R. natalensis with F. hepatica. However, the report by Dar et al. (Reference Dar, Djuikwo Teukeng, Vignoles, Dreyfuss and Rondelaud2010) was performed on experimental infections of Egyptian populations of R. natalensis with a French isolate of F. hepatica. So the suitability of this lymnaeid to sustain larval development of F. hepatica needs to be verified using local isolates of the parasite.
In conclusion, P. columella seems currently to be an important intermediate host in Egypt for larval development of local F. hepatica under experimental conditions. This lymnaeid, in addition to G. truncatula and R. natalensis (Dar et al., Reference Dar, Djuikwo Teukeng, Vignoles, Dreyfuss and Rondelaud2010, Reference Dar, Lounnas, Djuikwo Teukeng, Mouzet, Courtioux, Hurtrez-Boussès, Vignoles, Dreyfuss and Rondelaud2013), could contribute to F. hepatica transmission in Egypt. However, field studies would be useful to determine prevalence of F. hepatica infection in different natural populations of P. columella.
Acknowledgements
The authors gratefully thank Dr J. Cook-Moreau for revising the English text.
Financial support
Y.D. was supported by a grant from the Ministry of Higher Education and Scientific Research, Egypt.
Conflict of interest
None.








