During talking, our brains automatically generate predictions about the sound of our impending vocalizations in order to adjust ongoing speech to better match our intentions (Burnett et al., Reference Burnett, Freedland, Larson and Hain1998; Houde and Jordan, Reference Houde and Jordan1998; Sitek et al., Reference Sitek, Mathalon, Roach, Houde, Niziolek and Ford2013) and to mirror our social environment (Pardo, Reference Pardo2006). At a more fundamental level, our brains may use these predictions to distinguish auditory sensations resulting from our own actions, including overt actions (e.g. speech) and possibly covert actions (e.g. thoughts), from externally generated sounds (Crapse and Sommer, Reference Crapse and Sommer2008; Greenlee et al., Reference Greenlee, Jackson, Chen, Larson, Oya, Kawasaki, Chen and Howard2011). Individuals with schizophrenia, however, appear to have difficulties predicting the sensory consequences of their own actions (Ford and Mathalon, Reference Ford and Mathalon2012). Indeed, deficiencies in generating predictions about the sensations resulting from thoughts and inner speech, and a consequent failure to experience them as self-generated, have been posited to underlie psychotic symptoms including auditory hallucinations and the loss of a normal sense of agency (e.g. delusions of alien control) in schizophrenia (Feinberg, Reference Feinberg1978; Feinberg and Guazzelli, Reference Feinberg and Guazzelli1999).
An action-based predictive coding mechanism has been theorized to underlie our ability to anticipate the sensory consequences of our actions, detect mismatches between expected and observed sensations, and appropriately adjust future actions, in a largely unconscious and automatic fashion (Crapse and Sommer, Reference Crapse and Sommer2008; Houde and Nagarajan, Reference Houde and Nagarajan2011). This mechanism is posited to involve transmission of an ‘efference copy’ of a motor command to relevant regions of sensory cortex where it produces a ‘corollary discharge’ representing the anticipated sensory consequences of the motor action (Von Holst and Mittelstaedt, Reference Von Holst and Mittelstaedt1950). The efference copy/corollary discharge mechanism is ubiquitous and has been demonstrated in visual, sensorimotor and auditory systems across a range of species (Crapse and Sommer, Reference Crapse and Sommer2008) from crickets (Poulet and Hedwig, Reference Poulet and Hedwig2002), to primates (Eliades and Wang, Reference Eliades and Wang2003). In the auditory domain, regions subserving vocalization in the frontal lobes send motor commands via efferent motor pathways to muscle groups to produce the intended sound. Simultaneously, these frontal vocalization regions are posited to send an efference copy of the motor commands to auditory cortex, giving rise to a corollary discharge representing the predicted sound. When the corollary discharge matches the actual auditory consequence of the vocalization, the auditory cortical response to the generated speech sound is attenuated, and the match between intended and executed speech is unconsciously recognized (Houde and Jordan, Reference Houde and Jordan2002; Eliades and Wang, Reference Eliades and Wang2003, Reference Eliades and Wang2005). In this way, suppression of auditory cortex during speech may function not only to identify speech production errors, but also to tag vocalizations as self-generated, distinguishing them from externally generated sounds (Feinberg, Reference Feinberg1978; Seal et al., Reference Seal, Aleman and Mcguire2004).
The function of efference copy/corollary discharge mechanisms has been inferred through studies demonstrating reduced auditory cortical responses to self-generated compared with externally generated sounds. For example, the N100 (N1) component of the auditory event-related potential (ERP) elicited by sounds, and its counterpart in magnetoencephalographic recordings (M100), are reduced in amplitude in response to vocalizations as they are produced relative to when they are played back (Curio et al., Reference Curio, Neuloh, Numminen, Jousmaki and Hari2000; Ford et al., Reference Ford, Mathalon, Heinks, Kalba and Roth2001; Houde et al., Reference Houde, Nagarajan, Sekihara and Merzenich2002; Heinks-Maldonado et al., Reference Heinks-Maldonado, Mathalon, Gray and Ford2005, Reference Heinks-Maldonado, Nagarajan and Houde2006, Reference Heinks-Maldonado, Mathalon, Houde, Gray, Faustman and Ford2007; Ford et al., Reference Ford, Gray, Faustman, Roach and Mathalon2007a, Reference Ford, Roach, Faustman and Mathalon2007b; Chen et al., Reference Chen, Mathalon, Roach, Cavus, Spencer and Ford2011; Greenlee et al., Reference Greenlee, Jackson, Chen, Larson, Oya, Kawasaki, Chen and Howard2011; Ford et al., Reference Ford, Mathalon, Roach, Keedy, Reilly, Gershon and Sweeney2013; Sitek et al., Reference Sitek, Mathalon, Roach, Houde, Niziolek and Ford2013; Wang et al., Reference Wang, Mathalon, Roach, Reilly, Keedy, Sweeney and Ford2014). This putative corollary discharge mechanism is present by adolescence, but it is unclear whether it is fully developed or continues to develop from adolescence through early adulthood as the brain matures (Perez et al., Reference Perez, Ford, Roach, Loewy, Stuart, Vinogradov and Mathalon2012).
In patients with schizophrenia, abnormal efference copy/corollary discharge mechanisms have been hypothesized to underlie impairments in the ability to make predictions about the sensory consequences of self-generated behaviors, including covert behaviors such as thinking/inner speech (Brebion et al., Reference Brebion, Amador, David, Malaspina, Sharif and Gorman2000; Frith et al., Reference Frith, Blakemore and Wolpert2000; Lindner et al., Reference Lindner, Thier, Kircher, Haarmeier and Leube2005), and have been proposed as potential mechanisms underlying delusional thinking and misperceptions associated with psychosis (Feinberg, Reference Feinberg1978; Feinberg and Guazzelli, Reference Feinberg and Guazzelli1999; Blakemore et al., Reference Blakemore, Smith, Steel, Johnstone and Frith2000; Ford and Mathalon, Reference Ford and Mathalon2005). Unusual thought content and disorganized communication differentiated between clinically high risk (CHR) individuals who transitioned to psychosis and those who did not (Addington et al., Reference Addington, Liu, Buchy, Cadenhead, Cannon, Cornblatt, Perkins, Seidman, Tsuang, Walker, Woods, Bearden, Mathalon and Mcglashan2015). ERP studies have demonstrated that patients with schizophrenia show less suppression of the auditory N1 in response to self-generated vocalization than healthy controls (HCs) (Ford et al., Reference Ford, Mathalon, Heinks, Kalba and Roth2001, Reference Ford, Roach, Faustman and Mathalon2007b; Mathalon and Ford, Reference Mathalon and Ford2008; Perez et al., Reference Perez, Ford, Roach, Loewy, Stuart, Vinogradov and Mathalon2012). Reduced suppression of auditory responses to vocalization extends to psychosis more generally and has been reported in patients with psychotic bipolar disorder and schizoaffective disease (Ford et al., Reference Ford, Mathalon, Roach, Keedy, Reilly, Gershon and Sweeney2013). We have also reported similar abnormalities in schizophrenia patients early in their disease course (Perez et al., Reference Perez, Ford, Roach, Loewy, Stuart, Vinogradov and Mathalon2012), suggesting they are not due to chronicity related clinical sequelae of the illness such as cumulative medication exposure and long-standing social and occupational dysfunction. Additionally, patients with schizophrenia do not display expected suppression of N1 in response to unaltered speech relative to real-time pitch-altered speech, as is seen in HCs (Heinks-Maldonado et al., Reference Heinks-Maldonado, Mathalon, Houde, Gray, Faustman and Ford2007). Together these findings suggest that patients with schizophrenia show attenuated or absent suppression of auditory cortex in response to self-generated sounds, possibly due to deficits in efference copy/corollary discharge mechanisms. These deficits, consequently, may underlie an inability to make predictions about the sensory consequences of self-generated actions and to utilize them to adjust behavior and tag experiences as self-generated.
Goals of this study
With the emergence of validated clinical criteria for identifying individuals at high risk for developing psychosis (Phillips et al., Reference Phillips, Yung and Mcgorry2000; Miller et al., Reference Miller, Mcglashan, Rosen, Somjee, Markovich, Stein and Woods2002, Reference Miller, Mcglashan, Rosen, Cadenhead, Cannon, Ventura, Mcfarlane, Perkins, Pearlson and Woods2003; Cannon et al., Reference Cannon, Cadenhead, Cornblatt, Woods, Addington, Walker, Seidman, Perkins, Tsuang, Mcglashan and Heinssen2008; Yung, Reference Yung2008; Woods et al., Reference Woods, Addington, Cadenhead, Cannon, Cornblatt, Heinssen, Perkins, Seidman, Tsuang, Walker and Mcglashan2009), research efforts have focused on examining whether neurobiological abnormalities present in schizophrenia are also evident during the clinical prodrome preceding the onset of psychosis. Previously, we reported that individuals at CHR for psychosis had N1 suppression values that were intermediate between healthy comparison (HC) subjects and patients with schizophrenia who were relatively early in their illness course (ESZ); however, the CHR group was not statistically distinguishable from either HC or ESZ (Perez et al., Reference Perez, Ford, Roach, Loewy, Stuart, Vinogradov and Mathalon2012). Our primary aim in this paper was to analyze the final sample collected in this project to achieve a better estimate of the true effect.
Based on our earlier findings, we predicted that CHR and ESZ would both have diminished speech-related N1 suppression, similar to our prior observations in chronic patients (Ford et al., Reference Ford, Mathalon, Heinks, Kalba and Roth2001, Reference Ford, Gray, Faustman, Roach and Mathalon2007a, Reference Ford, Roach, Faustman and Mathalon2007b, Reference Ford, Mathalon, Roach, Keedy, Reilly, Gershon and Sweeney2013; Heinks-Maldonado et al., Reference Heinks-Maldonado, Mathalon, Houde, Gray, Faustman and Ford2007). We further predicted that abnormalities would be greater in CHR subjects who later converted to a psychotic diagnosis. To test this, we compared CHR subjects who converted to psychosis with those who did not after 12 months of follow-up. A secondary aim, based on Feinberg's initial proposal (Feinberg, Reference Feinberg1978), was to examine whether abnormalities in the corollary discharge mechanism in ESZ and CHR individuals would be related to the severity of their unusual thought content. Thus, we predicted that ESZ and CHR would show a relationship between deficient N1 suppression during speech, a putative reflection of corollary discharge dysfunction, and the severity of unusual thought content. This was tested with clinical symptom ratings in the CHR and ESZ groups. Finally, because our sample of HC spanned a wide age range, we asked if age affected speech-related N1 suppression and whether the normal age relationship was altered in the ESZ and CHR samples.
Method
Participants
Study participants included 71 individuals at CHR for psychosis, 84 patients with ESZ, and 103 HC subjects. See Table 1 for demographic and clinical data.
Table 1. Group demographic dataa

U, unmedicated; A, atypical antipsychotic; T, typical antipsychotic; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; SOPS, Scale of Prodromal Symptoms.
a Values are given as number gender, handedness, CHR criteria, and antipsychotic type. Group means with the standard deviation for age, parental socioeconomic status, intelligence quotient, PANSS, and SOPS are reported. Gender and handedness were analyzed with Pearson χ2 tests. Age, parental socioeconomic status, and intelligence quotient were analyzed with one-way ANOVA.
b The Hollingshead (1975) four-factor index of parental socioeconomic status (SES) is based on a composite of maternal education, paternal education, maternal occupational status, and paternal occupational status. Lower scores represent higher SES. SES values are missing from one schizophrenia patient.
c The Crovitz-Zener (1962) questionnaire was used to measure handedness and categorize as right (R), left (L), or ambidextrous (A).
d The Wechsler Adult Intelligence Scale (WAIS-III) full-scale intelligence quotient (FSIQ) was estimated based on the Wechsler Test of Adult Reading (WTAR) for native English-speaking subjects who were 16 years of age or older at testing (N = 219) or Wechsler Abbreviated Scale of Intelligence (WAIS-II) two-subtest (Vocabulary and Matrix Reasoning) T scores for all other subjects (N = 32). Estimated IQ values are missing from four HC subjects and three CHR patients.
CHR participants were recruited from the University of California, San Francisco's (UCSF) Prodromal Assessment, Research, and Treatment Clinic. CHR patients met Criteria of Prodromal Syndromes (COPS) based on the Structured Interview for Prodromal Syndromes (SIPS) (Miller et al., Reference Miller, Mcglashan, Rosen, Somjee, Markovich, Stein and Woods2002, Reference Miller, Mcglashan, Rosen, Cadenhead, Cannon, Ventura, Mcfarlane, Perkins, Pearlson and Woods2003). COPS criteria comprise three non-mutually exclusive sub-syndromes: (i) attenuated psychotic symptoms (n = 69/71), (ii) brief intermittent psychotic states (n = 0/71), and (iii) genetic risk with deterioration in social/occupational functioning (n = 7/71).
ESZ patients within 5 years of illness onset (1.85 ± 1.43 years) were recruited from the Early Psychosis Clinic at UCSF and the community. Diagnosis of schizophrenia or schizoaffective disorder was confirmed using the Structured Clinical Interview for DSM-IV (SCID) (First et al., Reference First, Spitzer, Gibbon and Williams2002). ESZ had no DSM-IV substance dependence in the past year.
HC participants were recruited from the community and did not meet criteria for any Axis I diagnosis based on the SCID, or for participants 16 years of age, the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime Version (Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao, Flynn, Moreci, Williamson and Ryan1997). HC had no history of substance abuse within the past year based on a SCID interview and no first-degree relative with a psychotic disorder.
Exclusion criteria for all groups included estimated intelligence quotient <70, a history of significant medical or neurological illness, or a history of head injury resulting in loss of consciousness. The study was approved by the institutional review board of UCSF, and adult participants provided written informed consent. In the case of minors, parents provided written informed consent and minors provided written informed assent. All interviews were conducted by trained interviewers, including a clinical psychologist, clinical psychology pre-doctoral intern, clinical social worker, or research assistant.
Clinical ratings
For the ESZ sample, a clinically trained research assistant, psychiatrist, or clinical psychologist rated symptoms using the SAPS (Andreasen, Reference Andreasen1984). Symptom interviews were typically done within 1 week of ERP testing, ranging from 64 days to the same day (M = 8.1, s.d. = 8.7 days). For the CHR sample, prodromal symptoms were rated using the Scale of Prodromal Symptoms (SOPS) administered as part of the SIPS interview (Miller et al., Reference Miller, Mcglashan, Rosen, Somjee, Markovich, Stein and Woods2002, Reference Miller, Mcglashan, Rosen, Cadenhead, Cannon, Ventura, Mcfarlane, Perkins, Pearlson and Woods2003). Symptom ratings were less proximal to ERP testing in the CHR sample, ranging from 170 days to the same day (M = 23.6, s.d. = 25.5 days).
Procedure
Participants completed the Talk–Listen paradigm, as described previously (Ford et al., Reference Ford, Roach and Mathalon2010), using Presentation software (http://www.neurobs.com/presentation). In the Talk condition, participants were trained to pronounce short (<300 ms), sharp vocalizations of the phoneme ‘ah’ repeatedly in a self-paced manner, about every 1–2 s, for 187 s. The speech was recorded using a microphone connected to the stimulus presentation computer and transmitted back to subjects through Etymotic ER3-A insert earphones in real-time (zero delay). In the Listen condition, the recording from the Talk condition was played back, and participants were instructed simply to listen. The number of ahs generated for both Talk and Listen conditions by ESZ, CHR, and HC groups was not significantly different.
Data acquisition and pre-processing
Electroencephalogram (EEG) data were recorded from 64 channels using a BioSemi ActiveTwo system (http://www.bi osemi.com). Electrodes placed at the outer canthi of both eyes, and above and below the right eye, were used to record vertical and horizontal electro-oculogram data. EEG data were continuously digitized at 1024 Hz and referenced offline to averaged earlobe electrodes before applying a 1 Hz high-pass filter using EEGlab (Delorme and Makeig, Reference Delorme and Makeig2004). Data were next subjected to Fully Automated Statistical Thresholding for EEG artifact Rejection (FASTER) using a freely distributed toolbox (Nolan et al., Reference Nolan, Whelan and Reilly2010). The method employs multiple descriptive measures to search for statistical outliers (>±3 s.d. from mean). This process included five steps: (1) outlier channels were identified and replaced with interpolated values in continuous data, (2) outlier epochs were removed from participants’ single trial set, (3) spatial independent components analysis was applied to remaining trials, outlier components were identified [including components that correlated with electrooculography (EOG) activity], and data were back-projected without these components, (4) within an epoch, outlier channels were removed and interpolated, and (5) ERP averages for the Talk and Listen conditions were subtracted and difference waveforms were separately assessed in each subject group to identify outlier subjects. Unlike our previous report (Perez et al., Reference Perez, Ford, Roach, Loewy, Stuart, Vinogradov and Mathalon2012), the FASTER processing approach was modified here between steps 2 and 3 to include canonical correlation analysis (CCA). CCA was used as a blind source separation technique to remove broadband or electromyographic noise from single trial EEG data, generating de-noised EEG epochs. Our approach is similar to the CCA method described by others (De Clercq et al., Reference De Clercq, Vergult, Vanrumste, Van Paesschen and Van Huffel2006; Ries et al., Reference Ries, Janssen, Burle and Alario2013), with some important differences (see online Supplementary Methods).
Epochs were time-locked to the onset of each ‘ah’ and baseline corrected using the −100 to 0 ms baseline preceding vocalization. ERP averages were generated using a trimmed means approach, excluding the top and bottom 5% of single trial values at every data sample in the epoch before averaging to produce a more robust mean estimation (Leonowicz et al., Reference Leonowicz, Karvanen and Shishkin2005).
To remove any remaining baseline contamination by speech-related artifacts, a temporal pro-max-rotated principal components analysis (PCA) was performed on the ERP data (Sinai and Pratt, Reference Sinai and Pratt2002; Kayser and Tenke, Reference Kayser and Tenke2003). ERPs were reconstructed after excluding factors that had a maximum loading during the temporal baseline window preceding ‘ah’ onset or that accounted for <0.3% of the variance. N1 was identified in the ERP as the most negative peak between 60 and 140 ms ‘ah’ onset. The N1 Talk–Listen suppression effect was estimated using the N1 peak amplitude Talk–Listen difference score at Cz, following the method we used in our prior report (Perez et al., Reference Perez, Ford, Roach, Loewy, Stuart, Vinogradov and Mathalon2012).
Statistical correction for normal aging effects
To control for the effects of normal brain maturation and aging, N1 amplitude Talk–Listen difference scores at Cz were regressed on age in the HC group, and the resulting regression equation was used to calculate age-corrected N1 Talk–Listen difference z-scores for all groups, including CHR and ESZ groups. This was done by subtracting the predicted N1 Talk–Listen difference score based on a subject's age from his/her observed difference score, and then dividing by the standard error of regression associated with the age-regression model run in HC. The resulting age-corrected z-scores reflect deviations from the value expected for a healthy individual at a specific age. This method has been used previously (Perez et al., Reference Perez, Ford, Roach, Loewy, Stuart, Vinogradov and Mathalon2012), and it is preferable to using age as a covariate in an analysis of covariance (ANCOVA) model because it only removes normal aging effects whereas ANCOVA tends to also remove pathological aging effects from the patient data. We also assessed N1 to vocalizations from the Talk and Listen conditions separately, after removing any effects of normal aging using the method just described.
Statistical analysis
Group differences for age-adjusted z-scores representing Talk–Listen N1 amplitude suppression, Talk N1 amplitude, and Listen N1 amplitude were assessed using analysis of variance (ANOVA). Pair-wise group differences were assessed using least squares differences (LSD) post-hoc tests, which controls for type I errors in the special case of three groups (Howell, Reference Howell2017). To assess for differences in the relationship between N1 suppression and age among the three groups, we used a general linear model with age, group, and group × age as regressors. In this model, the group × age interaction tests for group differences in the slopes of the age relationships.
Although our focus was on Unusual Thought Content and Delusions, we assessed the relationship between symptom severity and the age-adjusted z-scores representing Talk–Listen N1 suppression for all five positive symptom items from the SOPS in the CHR sample (P1: Unusual Thought Content; P2: Suspiciousness; P3: Grandiose Ideas; P4: Perceptual Abnormalities/Hallucinations; P5: Disorganized Communication) and all four global items from the SAPS in the ESZ sample (Hallucinations, Delusions, Thought Disorder, and Bizarre Behavior). The significance levels were Bonferroni corrected to p = 0.01 for the CHR sample, and p = 0.0125 for the ESZ sample.
Results
Group differences in speech-related N1 suppression
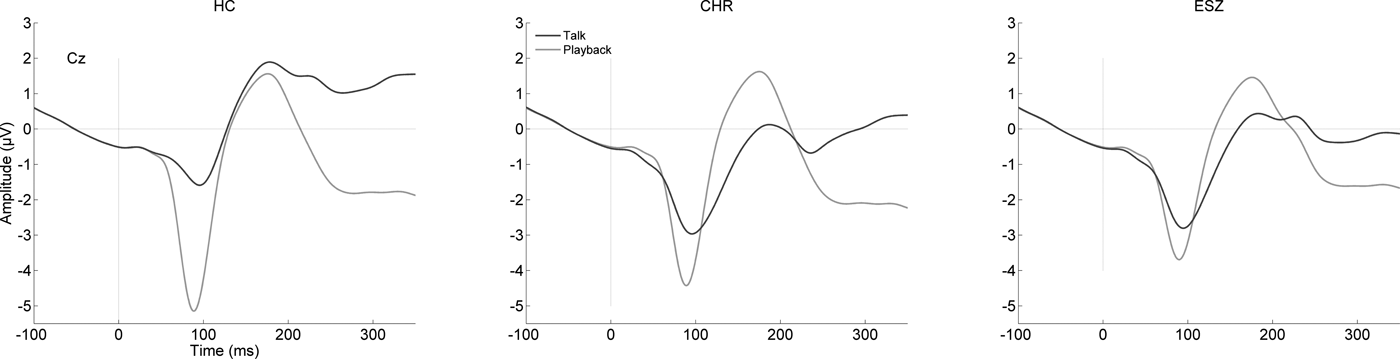
The grand average ERP waveforms at Cz, showing N1 for Talk and Listen conditions in each group, are presented in Fig. 1. Inspection of these waveforms reveals the expected N1 suppression during Talk compared with Listen. Mean N1 amplitudes at Cz for the Talk and Listen conditions are plotted in Fig. 2, where we also show the N1 suppression effect after z-scoring to remove the effects of normal aging. In Table 2, we show the results of the one-way ANOVA of the z-scored N1 suppression values, and the follow-up tests. There was a significant main effect of group due to HC having greater N1 suppression than CHR and ESZ, who did not differ from each other.

Fig. 1. ERP waveforms for Talk and Listen conditions show the N1 component during the Talk (blue) and Listen (red) conditions recorded at Cz. The N1 amplitude during Talk is reduced relative to Listen in HC (left). This effect is attenuated in the CHR patients (middle) and ESZ patients (right).

Fig. 2. (Left) Bar graphs show group means and standard errors for N1 amplitude at Cz assessed during Talk and Listen conditions. Normal speech-related N1 suppression is shown in HC (left; Talk: M = −2.14, s.e. = 0.393; Listen: M = −5.42, s.e. = 0.379), while there is reduced N1 suppression in ESZ patients (left; Talk: M = −3.38, s.e. = 0.497; Listen: M = −3.97, s.e. = 0.285). CHR patients (middle; Talk: M = −3.71, s.e. = 0.446; Listen: M = −4.59, s.e. = 0.312) show an effect that is similar to the ESZ. All amplitude values are given in microvolts (μV). (Right) Line graph shows mean N1 Talk–Listen difference scores at Cz (in μV) for the HC group, CHR, and ESZ patients. Age-correction was done using the HC group, causing their group average to be equal to zero, while negative suppression z-scores in CHR and ESZ reflect reduced suppression in these groups, accounting for normal aging effects in N1 suppression.
Table 2. Group analyses for speech-related N1 amplitudes at Cz for suppression (top), during Talk (middle), and Listen (bottom)

*Significance based on α = 0.05, two-tailed.
Group differences in N1 during talk and listen conditions
The means and waveforms suggest that the attenuated suppression effects in ESZ compared with HC was due to larger N1s during Listen in the HC. This was confirmed by ANOVA of the N1 values during the Listen condition (Table 2). The data shown in Figs 1 and 2 also suggest that the attenuation in the CHR group was due to both larger N1s during Talk and smaller N1s during Listen than seen in the HC; however, the group (CHR v. HC) comparison was not significant for either single condition (Table 2).
Converter v. non-converter differences in N1 suppression
CHR individuals who converted to a psychotic disorder (converters n = 8) were compared with CHR non-converters (n = 37) who had been followed clinically for at least 12 months. The converters did not have significantly less N1 suppression than the non-converters followed for 12 months (p = 0.73). Converters had larger N1s during Talk than non-converters, but this was not significant (p = 0.158). Finally, N1 during Listen was not affected by converter status (p = 0.364).
Correlational analyses with clinical ratings
In the CHR group, unusual thought content was correlated with age-corrected suppression of N1 during Talk compared with Listen (r = −0.404, p < 0.001) such that subjects with more unusual thought content showed less N1 suppression. This is shown in the scatterplots of Fig. 3. This was not true for the other symptoms (p = 0.18–0.94). There were no significant correlations between N1 suppression and the four SAPS global scores (p = 0.24–0.78) in the ESZ group.

Fig. 3. Scores from SOPS Unusual Thought Content item are plotted against N1 amplitude suppression z-scores at Cz (left) and N1 amplitude at Cz (in μV) during Talk (right) for the CHR sample.
Heterogeneity of suppression-age relationship among groups
The main HC model used for age correction revealed statistically significant evidence of a relationship between N1 suppression and age (r = 0.3095, p = 0.0015) with N1 suppression increasing 0.25394 µV with each year of age. To test for group difference in the N1 suppression relationship with age, N1 amplitude suppression was regressed on age, group, and age × group interaction terms. The F-test for homogeneity of slopes (test of the group × age interaction term) showed a marginally significant group difference in slopes (F 2,252 = 2.8753, p = 0.05825). Because there was evidence of a significant age-suppression relationship in the HC, we followed up this marginal effect by testing for slope differences between the HC and CHR (t (252) = −0.319, p = 0.75) as well as between HC and ESZ (t (252) = −2.378, p = 0.0182). The latter test indicates that relative to the age-related increase in HC, ESZ showed no such age relationship. Accordingly, when expressed as age-adjusted z-scores, ESZ showed increasingly deficient N1 suppression with age (r = −0.2797, p = 0.0099; online Supplementary Figure, bottom right). Scatterplots of these N1 suppression relationships with age are shown for both raw amplitudes and age-adjusted z-scores to demonstrate how the age-adjustment procedure removes the normal aging effect (online Supplementary Figure, top right) but retains pathological aging effects in the clinical groups (online Supplementary Figure, middle and bottom right).
Discussion
The primary aim of this study was to assess speech-related N1 suppression in CHR patients, using a larger sample than was available previously. We now show, for the first time, that suppression of N1 during talking compared with listening is significantly altered in CHR patients. That is, abnormal N1 suppression is not progressive and is evident before many of the sequelae of chronic illness emerge (e.g. chronic disability and medication exposure, long standing social, and occupational dysfunction).
Surprisingly, the ESZ and CHR groups showed equivalent amounts of suppression, in spite of the fact that very few of the CHR sample had converted to a diagnosis of psychosis. Details of N1 suppression are worth considering in light of this. Suppression is calculated by subtracting N1 during Listen from N1 during Talk, and a small suppression value can result from a small N1 during Listen or a large N1 during Talk, or both. ESZ had significantly smaller N1s during Listen, as reported in the literature [reviewed in Rosburg et al. (Reference Rosburg, Boutros and Ford2008)], but did not have larger N1s during Talk. The CHR patients did not have significantly reduced N1s during Listen, but tended to have larger N1s during Talk. So, while their suppression values are not different, the components of the suppression score are.
Feinberg initially proposed that disruptions in the corollary discharge mechanism in schizophrenia blurred the distinction between mental events that occur from endogenous neural activity, from the neural activity generated from external stimuli (Feinberg, Reference Feinberg1978). In the current study, attenuated speech-related N1 suppression was related to unusual thought content in the CHR patients but not in the ESZ patients.
With this expanded sample, we were also able to show the effects of age on N1 suppression. With increasing age, N1 suppression increased, approaching an increasingly normal pattern. Interestingly, ESZ patients showed the opposite pattern, with N1 suppression slightly decreasing with age, with older patients showing a greater abnormality. This is difficult to understand in light of other ERP data showing schizophrenia accelerates the normal effects of aging (Pfefferbaum et al., Reference Pfefferbaum, Wenegrat, Ford, Roth and Kopell1984). Caution is warranted in interpreting this weak relationship. It is also worth noting that this relationship was not seen when we included HC up to age 59 years to match chronic schizophrenia patients (Perez et al., Reference Perez, Ford, Roach, Loewy, Stuart, Vinogradov and Mathalon2012). Perhaps the correlation between age and N1 suppression in young HCs is compromised by the accumulation of age-associated illnesses.
This study demonstrates that putative corollary discharge dysfunction during speech occurs in people at high clinical risk for schizophrenia, before the inevitable sequelae of chronic illness, and remains reduced in patients early in the course of their illness, consistent with what we have shown in chronic patients (Ford et al., Reference Ford, Mathalon, Heinks, Kalba and Roth2001, Reference Ford, Gray, Faustman, Roach and Mathalon2007a, Reference Ford, Roach, Faustman and Mathalon2007b, Reference Ford, Mathalon, Roach, Keedy, Reilly, Gershon and Sweeney2013; Heinks-Maldonado et al., Reference Heinks-Maldonado, Mathalon, Houde, Gray, Faustman and Ford2007). Importantly, the dysfunction we see in the high-risk individuals is especially prominent in individuals with unusual thought content.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718002659.
Acknowledgements
This study was supported by grants from the VA Merit I01CX000497 program, VA Senior Research Career Award to JMF, and the National Institutes of Health (NIH) grants (R01MH076989; R01 MH058262).
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.