INTRODUCTION
The present study is an interdisciplinary contribution of marine biologists and chemists working on natural products derived from mollusc and echinoderm species in the southern Atlantic (Fontana et al., Reference Fontana, Muniain and Cimino1998; Gavagnin et al., Reference Gavagnin, Ungur, Castelluccio, Muniain and Cimino1999; Murray et al., Reference Murray, Muniain, Seldes and Maier2001; Chludil et al., Reference Chludil, Muniain, Seldes and Maier2002; Muniain et al., Reference Muniain, Giménez, Murray, Chludil and Maier2005, Reference Muniain, García and Fontana2007a,Reference Muniain, Centurión, Careaga, Espoz and Maierb; Maier et al., Reference Maier, Centurión, Muniain, Haddad and Eberlín2007).
The saponins derived from sea cucumbers, which are triterpene glycosides commonly known as holothurins, have drawn attention because of their wide spectrum of biological effects and antifungal, cytotoxic, virucidal and haemolytic activities (Shimada, Reference Shimada1969; Kalinin et al., Reference Kalinin, Anisimov, Prokofieva, Avilov, Afiyatullov, Stonik, Jangoux and Lawrence1996; Stonik et al., Reference Stonik, Kalinin and Avilov1999; Maier et al., Reference Maier, Roccatagliata, Kuriss, Chludil, Seldes, Pujol and Damonte2001; Chludil et al., Reference Chludil, Murray, Seldes, Maier and Atta-ur-Rahman2003; Maier & Murray, Reference Maier, Murray, Fingerman and Nagabhushanam2006).
Previous studies in the genus Psolus (Holothuroidea: Dendrochirotida: Psolidae) have been conducted only for the species P. fabricii (Dueben & Koren, 1846) and P. eximius Saveljeva (Kalinin et al., Reference Kalinin, Stepanov and Stonik1984, Reference Kalinin, Kalinovskii and Stonik1985, Reference Kalinin, Kalinovskii, Stonik, Dmitrenok and El'Kin1989, Reference Kalinin, Avilov, Kalinina, Korolkova, Kalinovsky, Stonik, Riguera and Jiménez1997).
The sea cucumber Psolus patagonicus Ekman, Reference Ekman1925 has been scarcely studied since its earliest description and subsequent taxonomic revisions (Ekman, Reference Ekman1925; Deichmann, Reference Deichmann1947; Pawson, Reference Pawson1964, Reference Pawson1969). The triterpene saponin Patagonicoside A was first isolated by our team work from complete specimens of P. patagonicus collected on the intertidal rocks in Ensenada Bay (Tierra del Fuego), exhibiting an important antifungal activity when tested against Cladosporium cucumerinum (Murray et al., Reference Murray, Muniain, Seldes and Maier2001; Muniain et al., Reference Muniain, Giménez, Murray, Chludil and Maier2005). In recent preliminary studies we found that this compound and its desulphated glycoside (ds-Patagonicoside A) also have antitumour activity against tumour cell lines (Careaga et al., Reference Careaga, Bueno, Muniain, Alché and Maier2007).
This research is part of a project on bioprospection of marine invertebrates of applied interest; it is a preliminary contribution to the knowledge of chemical–ecological relations occurring in these sea cucumbers, whose biological and ecological aspects are poorly understood. The present work examines chemical aspects of adult specimens of Psolus patagonicus collected from two different and distant localities, and compares results with previous data from studies of specimens from intertidal rocks. Antifungal activity was tested against a broad spectrum of fungi and cytotoxicity assays conducted in Artemia salina.
MATERIALS AND METHODS
Collection of specimens
In October 1999, January 2000 and April 2003, a total of 183 specimens were collected from beds of the scallop Zigochlamys patagonica (King & Broderip, 1832) at a depth of 110 and 115 m in the South Atlantic Ocean (39° 27′10S 55° 56′7″W and 43° 47′84″ S 59° 56′80″W) by means of a non-selective dredge of 2.5 m mouth opening and 3 m in length (Lasta & Bremec, Reference Lasta and Bremec1997). Individuals of Psolus were removed manually from each of the scallop valves and frozen immediately on the ship (Coll. S. Campodónico and M. Lasta). They were transported to the Instituto Nacional de Investigaciones Pesqueras (INIDEP) and then to the Department of Organic Chemistry of the National University of Buenos Aires (UBA) for chemical analysis without breaking the cold chain. Taxonomic and biological studies were conducted on 12 individuals at the Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’ (MACN). In February 2007, 45 specimens were collected from rocky substrates of the Bridges Islands (Beagle Channel, Ushuaia, 54° 48′57″S 66° 25′00″W) at a depth of 4 to 10 m, by SCUBA diving (Coll. C. Muniain and H. Monsalve). Forty specimens were frozen immediately for further chemical analysis (UBA) and 5 specimens for taxonomic analysis (MACN). The voucher material is deposited in MACN-in: 34776 (4 specimens, 0–10 m) and MACN-in: 34777 (2 specimens, 110 m).
GENERAL PROCEDURE
1H and 13C NMR spectra were recorded in CD3OD on a Bruker AM 500 spectrometer. The fast atom bombardment mass spectroscopy (negative ion mode) was obtained on a VG-ZAB mass spectrometer, on a glycerol matrix. Preparative high-performance liquid chromatography (HPLC) was carried out on a SP liquid chromatograph equipped with a Spectra Series P100 solvent delivery system, a Rheodyne manual injector and a refractive index detector, using a Bondclone 10 µ column (30 cm × 7.8 mm i.d.). Thin layer chromatography (TCL) was performed on precoated Si gel F254 (n-BuOH-HOAc-H2O (12:3:5)) and C18 reversed-phase plates (60% MeOH-H2O) and detected by spraying with p-anisaldehyde (5% EtOH).
EXTRACTION AND ISOLATION OF TRITERPENE GLYCOSIDES
Sea cucumbers (45 g, 183 specimens; 10 g, 40 specimens) frozen prior to storage were homogenized in ethanol and centrifuged. After evaporation of the ethanol, the residue was partitioned between MeOH-H2O (90:10) and cyclohexane. The methanolic extract was evaporated and subjected to vacuum dry column chromatography on Davisil C-18 reversed-phase using MeOH-H2O mixtures. The fraction eluted with 80% MeOH contained Patagonicoside A (1) as well as minor triterpene glycosides. Fractions eluted with 60%, 50% and 40% MeOH contained Patagonicoside A as the main triterpene glycoside. These fractions were combined and purified by reversed-phase HPLC to obtain the pure glycoside 1 as the main component. Patagonicoside A (1) was desulphated as described elsewhere (Murray et al., Reference Murray, Muniain, Seldes and Maier2001) and the residue was subjected to reversed-phase HPLC to give the pure desulphated glycoside, ds-Patagonicoside A (2) (Figure 1).
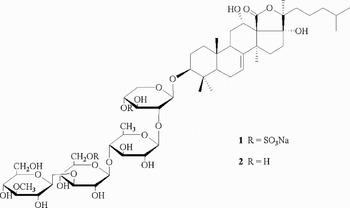
Fig. 1. Structure of the main component, Patagonicoside A (1), and its desulphated derivative ds-Patagonicoside A (2).
ANTIFUNGAL ASSAY
Geometric dilutions were obtained from freshly prepared stock solutions of Patagonicoside A (1), ds-Patagonicoside A (2) and reference compound, the commercial fungicide Benomyl (B) at concentrations of 1–10 mg ml−1 in an appropriate solvent. A volume of 10 µl of each solution was applied on TLC plates using graduated capillaries. The plates were then sprayed with a suspension of the fungi Cladosporium fulvum, Fusarium oxysporum and Monilia sp. in a nutritive medium and incubated in a glass box with a moist atmosphere for 2–3 d. Samplings were replicated three times; clear inhibition zones appeared against a dark grey background.
ARTEMIA SALINA BIOASSAY
Geometric dilutions of the combined vacuum dry C18 column chromatography fractions containing the triterpene glycosides from Psolus patagonicus were freshly prepared from a 10 mg/mL stock solution in distilled water. Aliquots of this solution (0.5 mL) were added to vials containing 10 shrimp/vial in marine water (3.8% (wt/vol) marine salts in distilled water), and the volume was adjusted to 5 ml/vial. The percentage of larval mortality was determined after exposure to the triterpene glycosides mixture at 25°C for 24 h and was calculated with data from three independent experiments by using the standard procedure of probit analysis (Meyer et al., Reference Meyer, Ferrigni, Putman, Jacobsen, Nichols and McLaughlin1982).
RESULTS
Psolus patagonicus: bioactivity and chemical ecology
CHEMICAL ANALYSIS
Most of the triterpenoid oligoglycosides isolated from sea cucumbers so far belong to two main series: glycosides based on a 3β-hydroxyholost-9(11)-ene aglycon and those containing a 3β-hydroxyholost-7-ene skeleton (Chludil et al., Reference Chludil, Murray, Seldes, Maier and Atta-ur-Rahman2003). Patagonicoside A (1) (molecular formula: C54H86O29S2Na2) is the first example of an holothurin with a 3β,12α,17α -trihydroxy-holost-7-ene type aglycon and a tetrasaccharide linear chain with the most common 3-O-Me-Glc-(1→3)-Glc-(1→4)-Qui-(1→2)-Xyl structure (Figure 1). It is the main saponin in P. patagonicus but other minor glycosides are also present in the mixture of triterpene oligoglycosides, as determined by 1H NMR of fraction eluted with 80% MeOH and by thin layer chromatographic analysis of Patagonicoside A (1), its desulphated derivative (2) and minor holothurins obtained from partially purified HPLC fractions of the oligoglicoside mixture (Figure 2). Patagonicoside A showed no absorption in the ultraviolet region. The saponin concentration estimated for a P. patagonicus individual is 2.4 mg/ind.

Fig. 2. Silica gel thin layer chromatography of 1, 2 and minority partially purified triterpene glycosides (Fr. 1–4) isolated from Psolus patagonicus, solvent: n-BuOH:HOAc:H2O (12:3:5).
ANTIFUNGAL AND CYTOTOXIC ACTIVITIES
The main compound in P. patagonicus, Patagonicoside A (1), and its semisynthetic desulphated analogue 2, were evaluated for antifungal activity against three phytopathogenic fungi Cladosporium fulvum, Fusarium oxysporum and Monilia sp. through a bioautographic technique (Homans & Fuchs, Reference Homans and Fuchs1970). Benomyl, a commercially available fungicide that is selectively toxic to microorganisms and invertebrates, was used as a reference compound. Comparing the antifungal activity against the three fungi, Patagonicoside A (1) was found to be more active than the desulphated analogue 2 against Cladosporium fulvum and Monilia sp., showing an antifungal activity comparable to Benomyl against Monilia sp. On the other hand, 1 was slightly more active than 2 against Fusarium oxysporum (Figure 3).
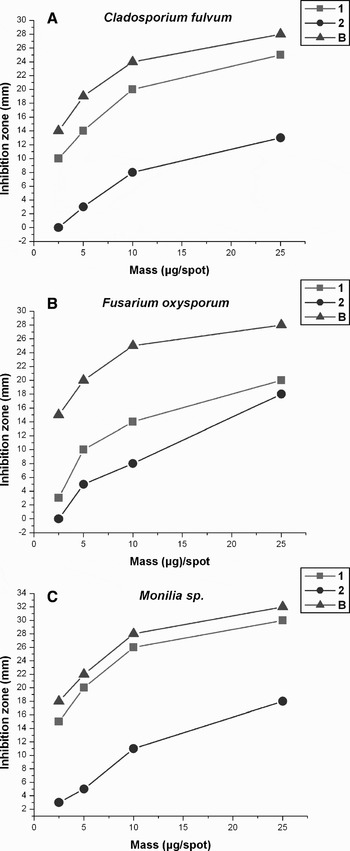
Fig. 3. Response curves for antifungal activity of Patagonicoside A (1), ds-Patagonicoside A (2) and Benomyl (B).
The cytotoxicity of the purified triterpene glycoside mixture of P. patagonicus was evaluated using a bioassay to determine brine shrimp (Artemia salina) larval mortality (Meyer et al., Reference Martínez, Giménez and Penchaszadeh1982), showing a significant toxicity in this assay (LC50 54.3 ppm).
CHEMICAL ECOLOGY
The specimens of Psolus patagonicus were oval shaped and dorso-ventrally flattened; the external live coloration was intense pink in specimens from intertidal rocks and rocky substrates of the Bridges Islands, whereas specimens collected at 115 m in depth were pale cream to white. All living specimens were photographed during samplings (Figure 4A–D). The length of living specimens ranged from 5 to 40 mm, with an average of approximately 20 mm, those collected from depth reaching the largest sizes. They are well adapted to attach to these hard substrates, both to rocky walls and as part of the epibiont fauna of Z. patagonicus (Figure 4A & B).

Fig. 4. (A) Samples of Zygochlamys patagonica from scallop beds from 110 m; (B) detail of epibionts of Psolus patagonicus on the shell. Scale bar: 1 cm. Photographs: S. Campodónico; (C) living specimen of P. patagonicus from the intertidal rock of Bahía Ensenada (Tierra del Fuego). Scale bar: 0.25 mm. Photograph: C. Muniain; and (D) specimens of P. patagonicus (arrow), around one of Psolus cf. squamatus (Koren, 1844). (Photograph: H. Monsalve).
Psolus patagonicus is a suspension feeder. We collected a higher number of specimens in places with more evident currents and particle movement than in the intertidal. Externally, the sea cucumber appeared ‘hard and protected’, the dorsal surface was covered with imbricating granulated scales, showing distinct oral and anal valves (Figure 4C & D). The middle region, which corresponds to the internal organs, was very small. Ventrally, the soft sole, where the females incubated the eggs and embryos, was transparent and slightly concave and showed several rows of tube feet (podia) that help it stay well attached to the substrate, making its collection difficult.
Observations with scanning electron microscopy revealed the presence of calcareous ossicles on the body, and each tube foot had conspicuous end plate and submerged rounded plates in all the thin epidermis surfaces. The specimens studied showed a clean aspect, without evidence of fouling by invertebrates or algae, and up to the present, no predator of this species is known.
DISCUSSION
Echinoderms are an important resource with biotechnological potential and have been used for medicinal, agrochemical and feeding purposes since ancient times, especially in the Orient (China and Japan), as described in recent revisions on the topic (Kelly, Reference Kelly and Matranga2005; Petzelt, Reference Petzelt and Matranga2005; Yokota, Reference Yokota and Matranga2005).
Basically, ‘saponins’ are typical bioactive compounds of organisms belonging to the classes Asteroidea and Holothuroidea. The saponins derived from the asteroids are steroidal glycosides, whereas the holothurian saponins are based on triterpenoid aglycones.
In the present work we conducted a chemical study of specimens of Psolus patagonicus from two distant and different sampling environments, the Bridges Islands (Beagle Channel, Ushuaia) at a depth of 4 to 10 m, and the epibiont specimens of the scallop Z. patagonica, at about 100 m in depth (Figure 4A–D). The results of the chemical analysis indicated identical compounds in both samples (Figure 3), Patagonicoside A being the main one, originally described from specimens of the intertidal zone (Bahía Ensenada, Ushuaia) (Murray et al., Reference Murray, Muniain, Seldes and Maier2001; Muniain et al., 2005).
Regarding the bioactivity found, data on structure–activity correlations of saponins isolated from sea cucumbers by the work of our team (Murray et al., Reference Murray, Muniain, Seldes and Maier2001; Chludil et al., Reference Chludil, Murray, Seldes, Maier and Atta-ur-Rahman2003) and previous studies (Kalinin et al., Reference Kalinin, Anisimov, Prokofieva, Avilov, Afiyatullov, Stonik, Jangoux and Lawrence1996; Kalinin, Reference Kalinin2000) suggest that the aglycone structure and the presence of sulphate groups in the oligosaccharide chain might play an important role in the antifungal and cytotoxic activities of these triterpene glycosides. The presence of a linear tetrasaccharide fragment appears to be a determining structural feature for membranotropic action and antifungal activity. For example, the antifungal disulphated triterpene glycoside, Hemoiedemoside A, isolated from Hemioedema spectabilis (Ludwig, 1882) from the southern Atlantic coast, showed remarkable toxicity against Artemia salina (LC50 18.7 ppm), whereas the desulphated analogue was weakly active (LC50 424.5 ppm) (Chludil et al., Reference Chludil, Muniain, Seldes and Maier2002) and Patagonicoside A showed significant toxicity in this assay (LC50 54.3 ppm). Patagonicoside A (1) and Hemoiedemoside A present the same desulphated tetrasaccharide chain and differ in the triterpene aglycone.
Underwater observations and examination of specimens of P. patagonicus prior to chemical processing revealed the absence of epibiont organisms, which may indicate antifouling activity provided by the presence of Patagonicoside A on the body wall. Through in vitro assays we recently found that both Patagonicoside A(1) and its desulphated analogue (2) inhibit the proliferation of three different types of tumour cells (Careaga et al., Reference Careaga, Bueno, Muniain, Alché and Maier2007). This possible antifouling activity of the compound is currently under investigation by our research group.
The Cuvierian tubules, peculiar organs that play a defensive role, are present only in some species of Aspidochirotida. Cooper (Reference Cooper1880) reported that some holothuroids discharged saponin concentrate in Cuvierian tubules. After expulsion and autotomy, tubules are readily regenerated as an efficient defensive mechanism (Hamel & Mercier, Reference Hamel and Mercier2000; Vandenspiegel et al., Reference Vandenspiegel, Jangoux and Flammang2000; Flammang et al., Reference Flammang, Ribesse and Jangoux2002, Reference Flammang, Santos, Haesaerts and Matranga2005). Kerr & Chen (Reference Kerr and Chen1995) found that the triterpene precursor of saponins in the sea cucumbers, Holothuria floridea and Actinopyga agassize, have been identified as parkeol, and demonstrated that biosynthesis occurs exclusively in the holothurian Cuvier gland. On the other hand, in holothurian species without Cuvierian tubules, such as the family Psolidae, saponins are distributed in the body wall (Yokota, Reference Yokota and Matranga2005; present work), and they could be originated in the synthesis by modifying precursors obtained through feeding (Harper et al., Reference Harper, Bugni, Copp, James, Lindsay, Richardson, Schnabel, Tasdemir, Van Wagoner, Verbitski, Ireland, McClintock and Baker2001).
Up to the present, no likely predators of P. patagonicus are known; we only found the reference of Bingham & Braithwaite (Reference Bingham and Braithwaite1986) stating that the asteroid Dermasterias imbricate (Grube) is the only main predator of Psolus chitonoides H. L. Clark 1901. In P. patagonicus, the presence of a dorsal surface covered with imbricating scales and calcareous ossicles, and the tube feet (podia) involved in the attachment to the substratum through adhesive secretions play an important role in the defence mechanism of this organism. The presence of P. patagonicus as an epibiont species associated with the scallop Zygochlamys patagonicus has been mentioned for different areas in the Argentine Sea (Bremec & Lasta, Reference Bremec and Lasta2002). Based on the concentrations of Patagonicoside A located in the body wall, we may preliminarily suggest a defensive role of this compound; further experimental in situ studies with fish, crustaceans and sea stars would allow us to test this hypothesis. On the other hand, further investigations on the presence of Patagonicoside A during the reproductive behaviour of incubating eggs and embryos under the sole (Hernández, Reference Hernández1981; Martínez et al., 2006), will permit more precise conclusions regarding the chemical ecology in this species. Previous studies have demonstrated that eggs, larva and juveniles of many holothurian species possess chemical deterrents to predation that persist through early life stages (McEuen, Reference McEuen1984; Iyengar & Harvell, Reference Iyengar and Harvell2001).
ACKNOWLEDGEMENTS
This work was partially supported by Fundación Antorchas, GEF/PNUD-ARG. 02/018, the University of Buenos Aires (Grant X314), CONICET (PIP 5509) and the Agencia Nacional de Promoción Científica y Tecnológica (PICT 34111). We thank G. Cimino and A. Fontana from ICMIB (Naples, Italy), and anonymous referees for their helpful comments to improve the manuscript. We are grateful to M. Lasta and S. Campodónico from INIDEP (Mar del Plata, Argentina) for their valuable assistance during sampling of P. patagonicus on the banks of the scallop Zigoclamys patagonica. We are grateful to G. Lovrich and J. Calvo for providing logistic support and laboratory facilities to C.M. in CADIC (Ushuaia). To C. Lizarralde (DCyT. Tierra del Fuego) and E. López (D.P. Ushuaia), for helping to obtain the permits to conduct marine samplings in Tierra del Fuego. C.M. and M.S.M. are Research Members of the National Research Council of Argentina (CONICET).






