INTRODUCTION
Although described over 200 years ago, the research and clinical literature characterizing the condition now known as Attention-Deficit/Hyperactivity Disorder (ADHD) has advanced substantially in the past 35 years. ADHD is now recognized as the most common behavioral disorder of childhood affecting nearly approximately 10% of U.S. children ages 4–17 years (Pastor, Reuben, Duran, & Hawkins, Reference Pastor, Reuben, Duran and Hawkins2015), although worldwide estimates are lower (around 5%) and differ as a function of varying diagnostic methods, practice guidelines, and access to specialized clinical care (Polanczyk, de Lima, Horta, Biederman, & Rohde, Reference Polanczyk, de Lima, Horta, Biederman and Rohde2007).
Symptoms of ADHD typically begin in early childhood, have a chronic course, and adversely affect the academics and social interactions of affected individuals (American Academy of Pediatrics, 2011). Despite advances in diagnostic processes, identification of biomarkers, and multimodal treatment, individuals with ADHD often struggle both socially and academically, even when treated. Approximately two-thirds of individuals have at least one co-existing developmental or psychiatric condition, such that “simple” ADHD is considered the exception rather than the rule (Larson, Russ, Kahn, & Halfon, Reference Larson, Russ, Kahn and Halfon2011).
ADHD is now recognized a major public health issue (Hinshaw & Ellison, Reference Hinshaw and Ellison2016). Recent estimates suggest that the annual incremental societal costs associated with ADHD total over 143 billion dollars in the United States alone (Doshi et al., Reference Doshi, Hodgkins, Kahle, Sikirica, Cangelosi, Setyawan and Neumann2012), with one-fourth of these costs borne by the U.S. education system. In school, children with ADHD are more likely to experience increased learning problems, school absences, troublesome relationships with peers, and a higher rate of ambulatory medical visits. As adults, these risks and associated problems result in 33% reduced earning and 15% increase in use of social assistance among those with ADHD (Fletcher, Reference Fletcher2014). The costly toll that ADHD takes on individual adjustment, family life, schools, health care, and social services underscores the importance of understanding its etiology, developmental course, and outcomes, with the ultimate goal of earlier (accurate) identification, treatment, and the reduction of individual and societal burden.
ADHD AS A DISORDER: HISTORICAL PERSPECTIVES
ADHD is now recognized as a persistent neurodevelopmental disorder with known structural and functional brain anomalies. The present conceptualization of ADHD has evolved in parallel with the field of psychiatry more generally in the past 35 years, acknowledging the shift from psychological and environmental explanations of behavior to biological (or biopsychosocial) framework, based on the exponential advances in neuroimaging and genetic research (Faraone et al., Reference Faraone, Asherson, Banaschewski, Biederman, Buitelaar, Ramos-Quiroga and Franke2015). The trend toward a biological explanation for ADHD was well established even before the term was coined in 1980, especially among psychiatrists who hypothesized a neurochemical basis (likely a dopamine deficiency) for the condition later described as Minimal Brain Dysfunction and ultimately ADHD.
Precursors to the formal adoption of the diagnosis of ADHD, going back to the nineteenth century, included the Hyperactive/Hyperkinetic Syndrome and ultimately the broad category of Minimal Brain Dysfunction. It is notable that the first use of a stimulant medication as a calming influence on hyperactivity was in children considered to have “brain damage,” rather than those with genetically based neurodevelopmental disorders such as Down syndrome (Bradley, Reference Bradley1937). The group of individuals defined as “brain damaged” was broadly defined by the combination of hyperactivity and motor impairments (but to a more minor degree falling short of the diagnosis of cerebral palsy).
The conference that introduced the term Minimal Brain Dysfunction built on the idea of “brain damage” as a combination of hyperactivity and minor motor dysfunction, and also added a heterogeneous set of learning and perceptual anomalies to the description (Clements, Reference Clements1966). Initially, dyslexia was set aside as a very special category, but later for educational classification was subsumed under the category of learning disabilities.
In the years between 1966 and the 1975, implementation of the U.S. federal special education law (PL 94–142), some states had classes for individuals with “brain damage,” while others had classes for those with “neurological impairments”; however, admission requirements for both included symptoms of hyperactivity, minor motor anomalies, and perceptual or learning impairments, but with IQ in the normal range. Thus, before 1980, the bond between minor motor anomalies, often referred to as soft signs, and the behavioral precursor to ADHD was very strong, such that one publication (Denckla & Rudel, Reference Denckla and Rudel1978) established that a quantitative motor examination could discriminate typically developing peers from hyperactive boys who were free of any learning disabilities.
DIAGNOSTIC CRITERIA: HYPERKINETIC DISORDER, ATTENTION DEFICIT DISORDER, AND ADHD
Diagnostic and Statistical Manuals
By 1980, research by psychologists using continuous performance tests, most prominently Virginia Douglas (Douglas & Peters, Reference Douglas and Peters1979), became so influential that the mental health profession as a whole started to recognize the relationship between attention and the condition formerly known as the Hyperactive/Hyperkinetic Syndrome (or the broader version, Minimal Brain Dysfunction). The result was that attention became, and has remained, a central characterizing descriptor of the disorder. Eventually, the Hyperactive Reaction of Childhood diagnosis of the Diagnostic and Statistical Manual for Mental Disorders Second Edition (DSM-II) (American Psychiatric Association [APA], 1968) was renamed Attention Deficit Disorder (ADD) with publication of the DSM-III (APA, 1980). Subtypes of the disorder were also first introduced in the DSM-III, and included Attention Deficit Disorder, with or without Hyperactivity. Subsequent revision (DSM-III-R; APA, 1987) renamed the condition again to its present label (Attention-Deficit/Hyperactivity Disorder), but did away with subtypes in favor of a separate diagnosis, Undifferentiated ADHD, used for the individuals presenting without prominent hyperactivity. Subtypes were again introduced in the DSM-IV (APA, 1994), and included Inattentive, Hyperactive/Impulsive and Combined subtypes.
While there has been consistent support for the latent structure of symptom groups (inattention, hyperactivity/impulsivity), the validity of the three subtypes as was called into question in the research literature, given the temporal instability of the categories, inconsistent practices for symptom counts between home versus school informants (Valo & Tannock, Reference Valo and Tannock2010), and sexual dimorphism in symptom manifestation (Willcutt et al., Reference Willcutt, Nigg, Pennington, Solanto, Rohde, Tannock and Lahey2012). Also, the Hyperactive/Impulsive subtype was shown to be rare outside the preschool setting, and more appropriately considered a developmental precursor to the Combined subtype (Barkley, Reference Barkley1997). Concurrently, the DSM-IV age-of-onset criterion (requiring symptoms producing impairment by age 7 years) was also questioned, following observation of later symptom onset in girls (Todd, Huang, & Henderson, Reference Todd, Huang and Henderson2008), and the recognition of a potentially separate adult-onset manifestation (Barkley, Murphy, & Fischer, Reference Barkley, Murphy and Fischer2008).
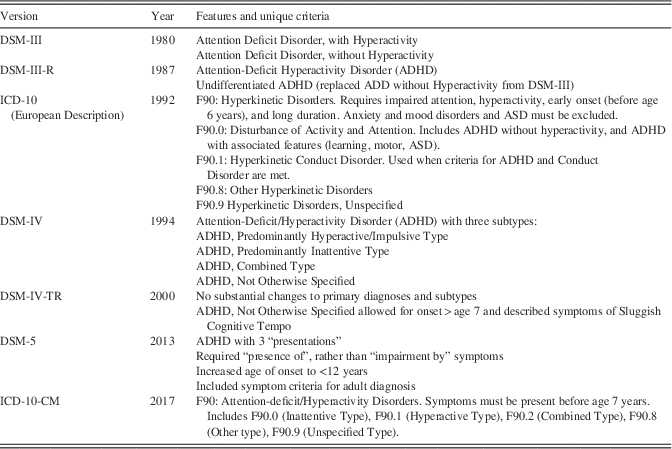
The diagnostic criteria changed in several important ways with the publication of the (APA, 2013). Prior editions of the DSM classified ADD and ADHD among the Disorders Usually Diagnosed in Infancy Childhood, or Adolescence. With publication of the DSM-5, however, ADHD was characterized as a Neurodevelopmental Disorder, more appropriately recognizing its onset and chronic developmental course (Shaw & Polanczyk, Reference Shaw and Polanczyk2017). While retaining most of the diagnostic criteria for ADHD that were previously included in the DSM-IV-TR (APA, 2000), three important changes were introduced: (1) age-of-onset criterion was increased from 7 to 12 years; (2) symptoms of inattention and/or hyperactivity/impulsivity were required to be merely present (as opposed to causing “impairment”); (3) there was provision for using a reduced symptom threshold for diagnosis in adults (ages 17 years and older), requiring five (rather than six) symptoms of inattention and/or hyperactivity/impulsivity. Highlights of diagnostic changes in the DSM are summarized in Table 1.
Table 1 Progression of diagnostic criteria for ADHD since 1980

Note. DSM=Diagnostic and Statistical Manual for Mental Disorders; ICD=International Classification of Diseases
International Classification Systems
The World Health Organization (WHO) has long recognized ADHD in its criteria for the International Classification of Diseases (ICD-9, ICD-10; WHO, 1978, 1993), but has, until recently, kept the earlier name of Hyperkinetic Disorder. The criteria for the ICD-10-CM (WHO, 2017) are more in line with the DSM-5, and are outlined in Table 1. Historically, the ICD criteria follow many of the same diagnostic criteria as the earlier versions of the DSM; however, the requirement for earlier onset (before age 7 years), and the exclusions of ADHD diagnosis when accompanied by other disorders (including mood disorders) has contributed to lower diagnostic rates in studies using the ICD criteria (Hinshaw et al., Reference Hinshaw, Scheffler, Fulton, Aase, Banaschewski, Cheng and Weiss2011).
CONSENSUS STATEMENTS AND PRACTICE GUIDELINES
The progression and refinement of diagnostic methods for ADHD from the 1960s through the 1990s led to advancements in both identification and treatment. Despite this progress, the disorder remained controversial, leading to conflicting opinions in the public sector, and confusion for clinicians and policymakers. One of the main controversies was the rapidly expanding use of stimulant medication. Also, by the late 1990s, there remained a belief by many that the disorder did not exist, and/or could not be reliably diagnosed. These controversies led professional researchers, clinicians, and other stakeholders to establish the first published consensus statements to address the controversies, and to outline considerations for care in children and adults. The NIH Consensus Statement on ADHD (NIH, 1998), and the International Consensus Statement on ADHD (Barkley, Reference Barkley2002), and the European Consensus Statement on Diagnosis and Treatment of Adult ADHD (Kooij et al., Reference Kooij, Bejerot, Blackwell, Caci, Casas-Brugué, Carpentier and Asherson2010), are summarized in Table 2.
Table 2 Published consensus statements on ADHD

During the same period, the leading professional practice organizations around the world also began to issue specific guidelines for the identification and treatment of ADHD in children and adolescents. A summary of the most recently published guidelines from the American Academy of Child and Adolescent Psychiatry (AACAP) (Pliskza & The AACAP Workgroup, Reference Pliszka2007), the National Institute for Clinical Excellence (NICE) guidelines from the United Kingdom (2009), based in part on the earlier European Clinical Guidelines for Hyperkinetic Disorder (Taylor et al., Reference Taylor, Dopfner, Sergeant, Asherson, Banaschewski, Buitelaar and Zuddas2004), the American Academy of Pediatrics (AAP, 2011), and the National Health and Medical Research Council (NHMRC) of Australia (2012) is outlined in Table 3. Of these, the NICE guidelines specifically include treatment recommendations for ADHD in adults.
Table 3 Current professional practice guidelines for ADHD

Note: AACAP=American Academy of Child and Adolescent Psychiatry; NICE=National Institute for Clinical Excellence; AAP=American Academy of Pediatrics; NHRMC=National Health and Medical Research Council of Australia.
The AACAP, NICE, and AAP practice guidelines specifically address treatment issues for preschoolers with ADHD, recognizing the validity of the diagnosis and appropriateness of treatment in that age range. The AAP and NICE guidelines state that for preschool children with ADHD (ages 4–5 years), clinicians should prescribe behavior therapy, rather than medication, as the first line of treatment (AAP, 2011); however, the NICE guidelines go one step beyond to virtually preclude pharmacotherapy in the preschool years, and to use medication only as a second line treatment in school-age children with mild to moderate symptoms of ADHD. Of relevance to neuropsychologists, the AACAP 2007 guidelines state that psychological assessment is not mandatory for ADHD diagnosis, but is recommended in patients with suspected low IQ or poor academic achievement.
INFLUENTIAL THEORIES OF ADHD
ADHD and Executive Functions
The prevailing neuropsychological theories of ADHD in the past 25 years have emphasized the behavioral and cognitive disruptions associated with executive functions, broadly defined, secondary to presumed anomalies in (pre)frontal-striatal brain circuitry, and modulated by expression of catecholamines. The most influential of these was put forth by Barkley (Reference Barkley1997) in an effort to extend prior explanations of behavioral dyscontrol more generally to those specific deficits observed in children with ADHD. Barkley’s theory built on the earlier writing of Quay (Reference Quay1988) and Gray (Reference Gray1982) who asserted that behavioral impulsiveness arises from the brain’s inhibitory control systems. The application of Barkley’s unifying theory was to interpret deficits in ADHD as a failure of self-control systems, with behavioral inhibition as the core. The theory specifically linked inhibition to four executive functions: working memory, self-regulation of affect-motivation-arousal, internalization of speech, and reconstruction (i.e., behavioral analysis and synthesis). With inhibition viewed as the primary deficit, these secondary executive function impairments ultimately led to deficits in motor control, including inefficient output (performance).
Beyond Executive Functions
Subsequent neuropsychological theories, including those based on growing awareness of frontostriatal functions as a result of brain mapping research, recognized that while deficient inhibitory control is a critical component of the ADHD phenotype, it is likely insufficient to characterize the range of functional behavioral problems observed. Heilman and colleagues (Heilman, Boeller, & Nadeau, Reference Heilman, Voeller and Nadeau1991) emphasized the importance of the striatum in executive control, as it acts to gate sensation into two parallel behavioral systems: how (organization and praxis) and when (response inhibition). Neuropsychological research over the past 25 years has highlighted the wide range of neuropsychological differences (beyond inhibitory and executive control deficits), emphasizing the dynamic (Nigg & Casey, Reference Nigg and Casey2005) and heterogeneous nature of ADHD (Reference Sonuga-BarkeSonuga-Barke, 2010 Sonuga-Barke 2010 to the ref list.-->). At the group level, children with ADHD show inconsistent and often small reductions in performance on performance-based tests of executive function, with mean effect sizes in the small to moderate range (Sergeant, Willcutt, & Nigg, Reference Sergeant, Willcutt and Nigg2007), and expression moderated by other more contextual variables, such as IQ (Mahone et al., Reference Mahone, Hagelthorn, Cutting, Schuerholz, Pelletier, Rawlins and Denckla2002). At the individual level, only a small proportion of children with ADHD manifest deficient performance across a range executive function tasks (Nigg, Willcutt, Doyle, & Sonuga-Barke, Reference Nigg, Willcutt, Doyle and Sonuga-Barke2005). Recognizing these limitations, recent theories have addressed a broader range of non-frontostriatal constructs, including state regulation, delay aversion, and performance variability.
Cognitive-Energetic Models
Based largely on the information processing models of Joseph Sergeant and colleagues, these state regulation models recognized the dynamic interplay between tonic versus phasic activation systems in the brain. The cognitive-energetic theory of ADHD (Sergeant, Reference Sergeant2000) characterized the behaviors observed in ADHD as a product of the dynamic interplay between energetic (bottom–up) systems involving arousal, activation and effort, and cognitive (top–down) executive control systems that limit the expression of the energetic drive states, often in an inverted U manner allowing for other executive functions (e.g., working memory) to also moderate output (Sergeant, Guerts, Huijbregts, Sheres, & Oosterlaan, Reference Sergeant, Guerts, Huijbregts, Sheres and Oosterlaan2003). In contrast to Barkley’s (Reference Barkley1997) executive function theory of ADHD, the cognitive-energetic model allowed for subcortical anomalies affecting arousal and activation to be primary in the manifestation of ADHD.
Motivation and Delay Aversion
The concept of delay aversion comes from the motivational model of ADHD, based on the influence of reward systems in the brain, and characterized behaviorally by the preference for “smaller sooner” (SS) over “larger later” (LL) rewards (Reference Sonuga-Barke, Sergeant, Nigg and WillcuttSonuga-Barke, Sergeant, Nigg, & Willcutt, 2008). Like the cognitive-energetic models, delay aversion theories incorporate executive control and dorsal-frontostriatal circuitry, represented behaviorally by inhibitory control problems. The dual pathway model, however, also recognizes the influence of a second (reward) circuit, represented by ventral frontostriatal dysfunction and reflected behaviorally in delay aversion (Sonuga-Barke, Reference Sonuga-Barke2003), and anomalous sensitivity to reward (Luman, Van Meel, Oosterlaan, Sergeant, & Guerts, Reference Luman, Van Meel, Oosterlaan, Sergeant and Guerts2009).
More recently, the model has been expanded to consider a third potential pathway influencing behavioral output (i.e., a triple-pathway model), involving temporal processing deficits, manifested in difficulties with timing, time discrimination, reproduction, and motor synchronization (Sonuga-Barke, Paraskevi, & Thompson, Reference Sonuga-Barke, Paraskevi and Thompson2010). The addition of these timing deficits to the model recognizes the influence of cerebellar functioning in the manifestation of cognitive and behavioral function in ADHD.
Response Variability
Intra-individual variability in response time (for both externally and self-paced tasks) is observed in groups of individuals with ADHD, both at the phenotypic and genetic levels, with explanations ranging from temporal processing deficits to arousal regulation and anomalies in the default-mode network (Kuntsi & Klein, Reference Kuntsi and Klein2012). Prior observation of slowed processing speed in ADHD, characterized by longer mean response times, is now recognized to be driven by significant skewing of the response-time distribution, and exacerbated by the presence of more frequent, very slow (“off task”) responses, measurable via the variable tau at the extreme tail of the distribution via ex-Gaussian analytic methods (Ghemlin et al., Reference Ghemlin, Fuermaier, Walther, Debelak, Rentrop, Westermann and Weisbrod2014).
Parallel to these findings, state regulation theories prompted investigations built on the notion that motor activation is facilitated by stimulus unpredictability. In a series of recent studies in children with ADHD, increased intra-individual variability (Ryan, Martin, Denckla, Mostofsky, & Mahone, Reference Ryan, Martin, Denckla, Mostofsky and Mahone2010) and lapses from performance (manifest by increased tau), have been shown to be normalized by a introducing a moderate degree of unpredictability in the stimulus presentation, operationalized by “jittering” the inter-stimulus interval (Lee et al., Reference Lee, Jacobson, Pritchard, Ryan, Yu, Denckla and Mahone2015).
Sluggish Cognitive Tempo
During the field trials for the DSM-IV, investigators identified a set of symptoms, later termed sluggish cognitive tempo (SCT), that were observed in children with ADHD, but were also in many ways distinct, including lethargy, daydreaming, confusion, and drowsiness. The characterization of SCT was ultimately included in the examples provided for the ADHD, Not Otherwise Specified diagnosis in DSM-IV-TR (APA, 2000). Initially, proposed revisions for DSM-5 included a separate Restrictive Inattentive ADHD presentation that overlapped with the SCT construct. Upon publication of DSM-5, however, neither the Restrictive Inattentive presentation, nor the diagnosis of SCT, was included. Most subsequent investigations of the SCT construct have identified three core symptoms, lethargy, underactivity, and slowness, that appear distinct from the inattention associated with ADHD and are hypothesized to be a function of earlier selective attention processes (Jacobson et al., Reference Jacobson, Murphy-Bowman, Pritchard, Tart-Zelvin, Zabel and Mahone2012).
LANDMARK TREATMENT STUDIES
Multimodal Treatment Study of Children With ADHD (MTA)
This multi-site, study represented the largest controlled trial to of treatment for ADHD to date (Arnold et al., Reference Arnold, Abikoff, Cantwell, Conners, Elliott, Greenhill and Wells1997), and enrolled 579 children with ADHD, ages 7–9, who were randomized to one of four treatment strategies: medication alone, psychosocial treatment alone, combined (medication + psychosocial treatment), and community control. Participants were treated actively for 14 months, with a follow-up 10 months after the conclusion of active treatment. At 14 months, all four groups showed sizeable reductions in symptoms, with those in medication-alone and combined strategies showing greatest improvement.
Behavioral therapy and community control strategy groups showed less improvement than the medication-alone and combined, but did not differ from one another (MTA Cooperative Group, 1999a). Medication management and behavioral therapies for this trial represented gold standard care, including careful titration of short-acting methylphenidate, dosed three times a day (for the mediation groups), and intensive behavioral therapy based on the summer program developed by Pelham and colleagues. In children with comorbid conditions, the medication groups showed less superiority to behavioral treatments (MTA Cooperative Group, 1999b), although admittedly, more intensive behavioral treatments (not offered in the MTA studies) may have been required to maintain greater effects.
At 24-month follow-up, the medication and combined groups continued to show persistence of improvement, but with notably diminished effects over time (MTA Cooperative Group, 2004). The initially superior results of the well-monitored medication condition were attenuated in long-term naturalistic follow-up, which showed not only the superiority of combination treatment, but also emergence of growth-related side effects in those treated with long-term medication, and the contribution of comorbidities (especially anxiety) and family-related processes (notably SES) that contributed significantly to functional outcome (Hinshaw, Arnold, & the MTA Cooperative Group, Reference Hinshaw and Arnold2015).
Preschool ADHD Treatment Study (PATS)
The PATS was a large multi-site, double-blind, crossover, placebo-controlled clinical trial to study the effects of methylphenidate in carefully-diagnosed preschoolers with ADHD, ages 3 to 5.5 years (Abikoff et al., Reference Abikoff, Vitiello, Riddle, Cunningham, Greenhill, Swanson and Wigal2007). Like the MTA study, methylphenidate immediate release was titrated and administered three times per day. The medication led to improvement in symptoms with effect sizes smaller than those observed in the MTA trials (Greenhill et al., Reference Greenhill, Kollins, Abikoff, McCracken, Riddle, Swanson and Cooper2006). Most receiving medication maintained improvement over 10 months; however, side effects were more common (including slowed growth rate) than observed in older children. A 6-year naturalistic follow-up of those enrolled in the PATS study showed a remarkable stability of the symptoms (and disorder) over time, with continued high severity, despite ongoing medication treatment (Riddle et al., Reference Riddle, Yershova, Lazzaretto, Paykina, Yenokyan, Greenhill and Posner2013).
Non-pharmacological Interventions
Long-term findings from the MTA and PATS studies highlight limitations of medication, and the importance of non-pharmacological treatments in overall care of individuals with ADHD. The types of non-pharmacological interventions that have been studied vary widely, and include, among others, behavioral treatments, dietary interventions, cognitive training, and neurofeedback, both in isolation and in combination with other treatments. A recent meta-analysis of these treatments identified challenges in study design around blinding of observers; however, among those well-blinded studies, interventions involving free fatty acid supplementation and exclusion of artificial food colors (among those with food sensitivities) showed small effects of unclear significance, with other treatments, including neurofeedback, cognitive training, and behavioral therapies produced inconsistent results with regard to core ADHD symptoms that require additional study using blinded assessments before conclusions can be drawn (Sonuga-Barke et al., Reference Sonuga-Barke, Brandeis, Cortese, Daley, Ferrin and Holtmann2013). Overall, these findings suggest that children need careful evaluations to identify comorbidities and that the most efficacious treatments continue to involve consideration of medication in conjunction with behavioral treatments and appropriate school interventions.
BIOMARKERS OF ADHD AND THE EVOLUTION OF HUMAN BRAIN MAPPING RESEARCH
Genes, Environment, and ADHD
The recognition that ADHD runs in families prompted extensive research into the genetic etiology of the disorder. Based on observations from twin studies, the heritability of ADHD is as high as 70–80%, and first-degree relatives of probands with ADHD have a 5- to 10-fold increased risk in developing the disorder themselves (Faraone et al., Reference Faraone, Asherson, Banaschewski, Biederman, Buitelaar, Ramos-Quiroga and Franke2015). Studies of candidate genes emphasized those associated with dopamine or other neurotransmitters (e.g., DAT1, DRD4, SNAP25; Wu, Yang, & Wang, Reference Wu, Yang and Wang2014), although effect sizes have been small, and results have often not been replicated. The COMT gene has also been explored, given its role in degradation of prefrontal dopamine (Sun, Yuan, Shen, Ziong, & Wu, Reference Sun, Yuan, Shen, Xiong and Wu2014). The heterogeneity of the phenotypic presentation, and variable patterns of lifetime symptom development, suggest that ADHD is associated with multiple genes of small effect, and gene–environment interactions, such that identification of a single genetic etiology is unlikely.
Neuroimaging and ADHD
A remarkable accumulation of evidence converging on the neurobiological nature of ADHD began with the availability of magnetic resonance imaging (MRI). Anatomic (aMRI) measurements, not clinical readings, focused on the basal ganglia, showing volume reductions (Aylward et al., Reference Aylward, Reiss, Reader, Singer, Brown and Denckla1996) and shape deformations (Qiu et al., Reference Qiu, Crocetti, Adler, Mahone, Denckla, Miller and Mostofsky2009) in children with ADHD, with evidence that stimulant medication treatment may normalize morphological features of the basal ganglia (Sobel et al., Reference Sobel, Bansal, Maia, Sanchez, Mazzone, Durkin and Peterson2010). Somewhat later, appreciation of the other regions participating in control circuits led to the finding of volume reductions in the cerebellum (Wyciszkiewicz, Pawlak, & Krawiec, Reference Wyciszkiewicz, Pawlak and Krawiec2017) and functionally defined frontal lobe sub-regions (Mahone, Ranta, et al., Reference Mahone, Ranta, Crocetti, O’Brien, Kaufmann, Denckla and Mostofsky2011).
Shaw and colleagues used longitudinal analyses of aMRI data to examine trajectories of development in children with ADHD (Shaw et al., Reference Shaw, Eckstrand, Sharp, Blumenthal, Lerch, Greenstein and Rapoport2007). Their landmark study demonstrated that in most regions of the cortex, children with ADHD showed a developmental lag, up to 3 years, in attaining peak cortical thickness, leading to the conclusion that ADHD may be best conceptualized as a delay in brain maturation, rather than as a discrete categorical entity. More recent longitudinal aMRI studies demonstrated that this rate of “thinning” is actually proportional to the level of symptoms, such that those with the most rapid thinning have the fewest ADHD symptoms, while those with the slowest (most delayed) cortical thinning have the most severe ADHD symptoms (Shaw et al., Reference Shaw, Gilliam, Liverpool, Weddle, Malek, Sharp and Giedd2011), lending support to the dimensional (spectrum) view of ADHD.
In the past 15 years, multimodal neuroimaging methods (beyond aMRI) have added to the growing understanding of the neurobiology of ADHD. Using task-activated functional MRI (fMRI), a recent review of 55 studies of children with ADHD in terms of their task-responsive activations recorded a pattern of decreases in the executive/frontoparietal and, visual-attentional/ventral attentional networks, contrasted with increases in the default mode and somatomotor networks (Cortese et al., Reference Cortese, Kelly, Chabernaud, Proal, Di Martino, Milham and Castellanos2012). Resting state (non–task-activated/baseline) fMRI measurements of connectivity in children and adults with ADHD show atypicality of the default mode network, sometimes described as reduced coherence or decreased integrity thereof (Fair et al., Reference Fair, Posner, Nagel, Bathula, Costa Dias, Mills and Nigg2010).
The burgeoning group of publications assessing functional connectivity in children with ADHD has added data showing interplay of the default mode, cognitive control, reward, attention, and amygdala-seeded networks (Castellanos & Aoki, Reference Castellanos and Aoki2016). At the same time, using of diffusion tensor/weighted imaging, a recent review revealed anomalies in frontostriatal white matter pathways, albeit not always in the same direction, in children and adolescents with ADHD (Tamm, Barnea-Goraly, & Reiss, Reference Tamm, Barnea-Goraly and Reiss2012).
ADHD PHENOTYPES AND SPECIAL SUB-POPULATIONS
ADHD and Learning Disabilities
The history of learning disabilities (LD), first published as such in 1962, and the pre-1980 conceptualization of ADHD is one of over-inclusiveness under the Minimal Brain Dysfunction label (Clements, Reference Clements1966). Two of the most common neurodevelopmental disorders, ADHD and dyslexia, co-occur more often than would be expected by chance (Cuoto et al., Reference Couto, Gomex, Wigg, Ickowicz, Pathare, Malone and Barr2009), suggesting shared genetic risks. When starting with a sample of young children with ADHD, the observed emergence of LD is striking, such that one third struggle to learn basic reading. At later ages, when demands for “learning to read” give way the need for “reading to learn,” difficulties with both written expression and mathematics (associated with increased demands for executive control) are observed, raising the co-occurrence of between LD (all types) and ADHD to approximately two-thirds (Denckla, Reference Denckla2005; Hooper & Williams, Reference Hooper and Williams2005).
ADHD and Motor Control
Whether regarded as an associated or core feature of ADHD, anomalies of motor development have a long history of parallel description alongside attentional and executive attainments, suggesting a shared neural circuitry (Diamond, Reference Diamond2000). Overflow movements involve unconsciously produced concomitant movements of limbs or parts thereof (e.g., fingers) that are not the primarily requested movements, including feet-to-hands (with heel or toe walking) and mirroring of patterned hand or finger movements during repetitive and sequenced movements. Overflow movements are especially sensitive developmental biomarkers, diminishing to a point of disappearance during the first decade of life in typically developing children the functional outcome of maturation in the brain’s collateral inhibition (Gidley Larson et al., Reference Gidley Larson, Mostofsky, Goldberg, Cutting, Denckla and Mahone2007).
Overflow movements persist, however, in children with neurological impairments, including ADHD (Cole et al., Reference Cole, Mostofsky, Gidley Larson, Denckla and Mahone2008). Mirror movements, an important subtype of overflow, have been long recognized as the most diagnostically discriminating motor sign for hyperactive and typically developing children (Denckla & Rudel, Reference Denckla and Rudel1978), the physiology for which has been revealed recently by transcranial magnetic stimulation (Gilbert, Isaacs, Augusta, MacNeil, & Mostofsky, Reference Gilbert, Isaacs, Augusta, MacNeil and Mostofsky2011).
Other aspects of anomalous motor development, including meeting balance and gait milestones and speed, rhythm, and patterning of foot and hand/finger movements, have also been found to be characteristic of children with ADHD, especially before adolescence, and more dramatically in young girls with ADHD (Cole et al., Reference Cole, Mostofsky, Gidley Larson, Denckla and Mahone2008). The motor endophenotype designation is meaningful in ADHD, as motor control matures earlier than cognitive or social-emotional control. As such, motor development may serve as a type of “canary in the coal mine” in diagnosis or prediction of ADHD. For example, the ability to hop on one foot at age 4 years was reported long ago to be the best predictor of hyperactivity at age 7 years (Nichols & Chen, Reference Nichols and Chen1981).
Sexual Dimorphism and Girls with ADHD
Available neuroimaging research examining brain development highlights a striking pattern in which girls reach maturation (i.e., peak cortical thickness) 2–5 years earlier than boys in most functional regions (Lenroot et al., Reference Lenroot, Gogtay, Greenstein, Wells, Wallace and Clasen2007). At birth, it has been estimated that girls are 3 weeks ahead of boys in physical maturation, and by the time they enter school, girls are approximately 1 year ahead (Eme, Reference Eme1992). These patterns of development in girls set the stage for sexually dimorphic manifestation of motor and executive dysfunction in children with ADHD, including risk genes for ADHD with different effects in males and females (Davies, Reference Davies2014). While initial studies suggested that girls with ADHD had better adolescent and adult outcomes than boys with ADHD, more recent findings, most notably from the large-scale longitudinal studies, highlight considerable functional impairment in girls/women with ADHD, including persistent executive dysfunction (Miller, Loya, & Hinshaw, Reference Miller, Loya and Hinshaw2013), mood and externalizing disorders (Tung et al., Reference Tung, Li, Meza, Jezior, Kianmahd, Hentschel, O’Neil and Lee2016), and severe behavioral problems (i.e., suicide attempts, self-injury) into adulthood (Hinshaw et al., Reference Hinshaw, Owens, Zalecki, Huggins, Montenegro-Nevado, Schrodek and Swanson2012).
Anatomic MRI studies of ADHD in girls also highlight patterns that are differently (rather than less) deviant in girls with ADHD, consistent with the observed later onset and greater social (vs. academic) impairment. Specifically, the medial and orbitofrontal regions (sometimes called the limbic frontal regions) are smaller to a greater degree than the dorsolateral and premotor regions, in contrast to the male ADHD frontal profile (Dirlikov et al., Reference Dirlikov, Rosch, Crocetti, Denckla, Mahone and Mostofsky2014). The breakdown of sub-domains of executive functions is also differently distributed among girls versus boys with ADHD (O’Brien, Dowell, Mostofsky, Denckla, & Mahone, Reference O’Brien, Dowell, Mostofsky, Denckla and Mahone2010).
ADHD in Preschoolers
ADHD has become the most commonly diagnosed form of psychopathology in the preschool years, with CDC prevalence estimates at 2.7% of children ages 4–5 years. The increase in identification and treatment of preschool ADHD occurs in the context of parallel increases in enrollment in preprimary academic programs in the preschool years (Bosco & Bona, Reference Bosco and Bona2016), which has led some researchers to caution of an epidemic of preschoolers being wrongfully diagnosed (Hinshaw & Scheffler, Reference Hinshaw and Scheffler2014). Nevertheless, neuroanatomical changes in preschoolers with ADHD as young as 4 years are now identified, with rate of anatomic “delay” associated with symptom severity (Mahone, Crocetti, et al., Reference Mahone, Crocetti, Ranta, Gaddis, Cataldo, Slifer and Mostofsky2011). The picture is concerning, because even when treated in preschool years, children with ADHD continue to have symptoms and significant functional impairment in elementary school (Riddle et al., Reference Riddle, Yershova, Lazzaretto, Paykina, Yenokyan, Greenhill and Posner2013).
ADHD in Adults
The primary symptoms of ADHD most often begin in early childhood, and persist (in some form) into adulthood for as many of 85% of individuals with the disorder (van Lieshout et al., Reference Van Lieshout, Luman, Twisk, van Ewijk, Groenman, Thissen and Oosterlaan2016). In the past 20 years, adult onset ADHD has also been recognized, and (Faraone et al., Reference Faraone, Asherson, Banaschewski, Biederman, Buitelaar, Ramos-Quiroga and Franke2015), and is now acknowledged as valid condition with genetic associations that are potentially unique from the child-onset variation (Agnew-Blais et al., Reference Agnew-Blais, Polanczyk, Danese, Wertz, Moffitt and Arseneault2016). The DSM-5 criteria for ADHD also allow for an adjusted diagnostic threshold in individuals ages 17 years and older, such that only five of nine symptoms of hyperactivity-impulsivity and/or inattention are required (APA, 2013). Adult diagnosis is acknowledged to be more difficult than in the early years due to multiple comorbidities accruing by adult life and variable access to childhood records or histories.
SUMMARY AND FUTURE DIRECTIONS
In the past 35 years, ADHD has emerged as one of the most diagnosed, treated, and researched neurologically based behavioral conditions. Nevertheless, outcomes remain poor. The preponderance of research involving behavioral and phenotypic expression of ADHD has suffered from the population bias, such that most samples are biased toward a population of boys aged 6–12 years. Moreover, diagnostic methods and societal gender role beliefs have contributed to a male bias in ADHD diagnosis, where boys are at risk for over-diagnosis, and girls are at risk for under-diagnosis (Bruchmuller, Margraf, & Schneider, Reference Bruchmuller, Margraf and Schneider2012). Neuropsychologists working with individuals with ADHD (whether or not it is the primary reason for referral) are encouraged to recognize that the developmental course is often lifelong, necessitating a “family practice” approach to consultation and intervention, in which caregivers provide assistance in different contexts and in different formats throughout the life of the individual with the condition.
At the time of this writing, the psychiatric conceptualization of ADHD remains categorical, while neuropsychological theories of ADHD emphasize functions as dynamic, dimensional constructs. In the next 35 years, research using dimensional frameworks such as Research Domain Criteria (RDoC; Insel et al., Reference Insel, Cuthbert, Garvey, Heinssen, Pine, Quinn and Wang2010), which also call for integration of multiple units of analysis (physiology, behavior), may be better suited to study the individual with ADHD, without the constraints of categorical diagnoses, especially for those individuals whose behaviors and cognitive skill patterns fall sub-threshold for diagnosis.
In conclusion, several prominent clinical and research questions remain, and will likely be the focus of the next generation of investigation into ADHD. Our (admittedly incomplete) list of suggested future investigations would include: (i) Links between multimodal brain mapping and phenotypic dimensions associated with ADHD, with emphasis on accessing international large datasets of biomarkers (e.g., enigma.io). (ii) Clarification of the neurobiological and phenotypic presentation of adult onset ADHD as a disorder potentially distinct from the more common developmental onset variety. (iii) Tracking the epidemiology of ADHD following implementation of DSM-5, with its more liberal diagnostic requirements compared to its predecessors. (iv) Investigations into the Sluggish Cognitive Tempo (SCT) construct, and its relationship with ADHD, emphasizing objectively measurable signs of the condition. (v) Continued neurobiological investigation of those individuals with attention deficits, who have never manifested developmentally inappropriate symptoms of hyperactivity/impulsivity. Do such individuals exist, and if so, do they represent a novel condition separable from ADHD or other neurodevelopmental disorders?
ACKNOWLEDGMENTS
Supported by U54 HD079123, R01 HD068425.