Clinical Implications
∙ Serotonin neurotransmission can modulate the behavior of GABA and glutamate cells.
∙ Serotonin’s effects on GABA and glutamate neurotransmission depend on regional and cellular receptor expression patterns, as well as the structure of local neuronal circuits.
∙ 5-HT, SSRI antidepressants, and the multimodal antidepressant vortioxetine have markedly different effects on excitatory and inhibitory neurotransmission in the prefrontal cortex and striatum.
Introduction
Cognitive dysfunction is a common but poorly understood aspect of major depressive disorder (MDD). MDD patients commonly experience impairments in a broad range of cognitive domains including attention, executive function, working memory, and psychomotor processing speed.Reference McIntyre, Cha and Soczynska1 Although there are a number of relatively effective treatments for the mood symptoms associated with MDD, empirical data suggest that many patients continue to experience high levels of functional disability despite improvements in depression symptoms.Reference McIntyre, Cha and Soczynska1 Furthermore, neurocognitive impairment predicts functional outcomes in MDD patients.Reference Jaeger, Berns, Uzelac and Davis-Conway2 Therefore, developing treatments capable of normalizing cognitive function in MDD patients is an important goal for restoring normal functional ability.
Vortioxetine is an antidepressant that recently has been approved for treatment of MDD. In 3 double-blind, randomized, placebo-controlled clinical studies, vortioxetine has shown positive effects on predefined cognition outcome measures in MDD patients with cognitive dysfunction, 2 of which included the active reference duloxetine.Reference Katona, Hansen and Olsen3–Reference Mahableshwarkar, Zajecka, Jacobson, Chen and Keefe5 Evidence from a large number of preclinical studies in rodents also supports a procognitive profile of vortioxetine and provides a mechanistic hypothesis for its pharmacological actions. Vortioxetine restored cognitive function across a range of rodent models of memory and cognitive flexibility applying cognitive disruptors such as time, serotonin (5-hydroxytryptamine; 5-HT) depletion, age, ovariectomy, N-methyl-D-aspartate (NMDA) receptor antagonism, and muscarinic cholinergic receptor antagonism.Reference du Jardin, Jensen, Sanchez and Pehrson6–Reference Pehrson and Sanchez12 Furthermore, in a quantitative electroencephalographic analysis in rats, vortioxetine dose-dependently increased cortical oscillatory power, suggesting that vortioxetine modulates cortical networks recruited during cognitive behaviors.Reference Leiser, Pehrson, Robichaud and Sanchez13
Vortioxetine is thought to exert its pharmacological effects through a multimodal mechanism of actionReference Zohar, Nutt and Kupfer14 that involves modulation of 5-HT receptors and inhibition of the serotonin transporter (SERT). In vitro studies in cellular assays show that vortioxetine is a 5-HT3, 5-HT7, and 5-HT1D receptor antagonist; a 5-HT1B receptor partial agonist; a 5-HT1A receptor agonist; and a 5-HT reuptake inhibitor.Reference Sanchez, Asin and Artigas15 However, although the pharmacological effects of this drug are relatively well understood, the precise mechanism underlying vortioxetine’s cognitive enhancing properties has yet to be fully elucidated. Although vortioxetine may indirectly modulate several neurotransmitter systems, previous work from this laboratory has advanced the hypothesis that vortioxetine enhances cognitive function by modulating gamma-aminobutyric acid (GABA) neurotransmission through its direct pharmacological actions on serotonergic receptors.Reference Pehrson and Sanchez16 These changes in GABA neurotransmission induce downstream increases in glutamate neurotransmission and neuroplasticity in key brain areas, such as the hippocampus and prefrontal cortex. In support of this hypothesis, electrophysiology studies have shown that vortioxetine through 5-HT3 receptor antagonism stimulated glutamatergic pyramidal neurons in rat hippocampus slices and in the medial prefrontal cortex of anesthetized rats.Reference Dale, Zhang and Leiser17, Reference Riga, Celada, Sanchez and Artigas18 The fact that 5-HT3 receptors in the rat brain are almost exclusively expressed on GABA interneurons that exert inhibitory control over pyramidal neurons further supports the hypothesis.Reference Morales, Battenberg, de Lecea and Bloom19 Work is currently ongoing to assess to what extent other portions of vortioxetine’s pharmacological profile play a role in its effects on GABA and glutamate neurotransmission.
An interesting corrollary to the idea that vortioxetine modulates GABA and glutamate neurotransmission through its pharmacological actions on 5-HT receptors is the idea that these effects may be regionally specific. Although serotonergic receptors are broadly expressed throughout the mammalian brain, the presence of individual receptor subtypes is regionally circumscribed. For example, simple visual inspection of autoradiographic maps of vortioxetine’s receptor targets demonstrates that although each of vortioxetine’s major pharmacological targets is expressed within the medial prefrontal cortex (mPFC; Figure 1), several of those receptor targets (notably 5-HT1A and 5-HT3 receptors) are not present or are present at very low levels within the striatal caudate and putamen subregions (CPu; Figure 2). Thus, the likelihood that vortioxetine will affect striatal neurotransmission in the same manner as it affects frontal cortex neurotransmission is small.

Figure 1 Expression pattern of serotonergic targets of the multimodal antidepressant vortioxetine within the rat prefrontal cortex. Autoradiographic images representing total (left panels) and nonspecific binding (right panels) for each of vortioxetine’s separate serotonergic targets in coronal rat brain sections at approximately 2.7 mm anterior from Bregma (according to Paxinos and WatsonReference Paxinos and Watson113). 5-HT1A receptors were mapped using 3nM [3H]8OHDPAT alone (A) or in combination with 1 µM of the 5-HT1A receptor selective antagonist WAY100635 to determine nonspecific binding (B). 5-HT1B/1D receptors were mapped using 1nM [3H] GR125743 alone (C) or in combination with 1 µM of the 5-HT1B receptor preferring antagonist SB216641 to determine nonspecific binding (D). 5-HT3 receptors were mapped using 3nM [3H] LY278584 alone (E) or in combination with 1 µM ondansetron to determine nonspecific binding (F). 5-HT7 receptors were mapped using 4.5nM [3H]SB269970 alone (G) or in combination with 1 µM of unlabeled SB269970 to determine nonspecific binding (H). Finally, SERT was mapped using 4.5 nM [3H] escitalopram alone (I) or in combination with 1 µM paroxetine to determine nonspecific binding (J). Scale bars represent 5 mm. Abbreviations: mPFC – medial prefrontal cortex.

Figure 2 Expression pattern of serotonergic targets of the multimodal antidepressant vortioxetine within the rat striatum. Autoradiographic images representing total (left panels) and nonspecific binding (right panels) for each of vortioxetine’s separate serotonergic targets in coronal rat brain sections at approximately 1.6 mm anterior from Bregma (according to Paxinos and WatsonReference Paxinos and Watson113). 5-HT1A receptors were mapped using 3nM [3H]8OHDPAT alone (A) or in combination with 1 µM of the 5-HT1A receptor selective antagonist WAY100635 to determine nonspecific binding (B). 5-HT1B/1D receptors were mapped using 1nM [3H] GR125743 alone (C) or in combination with 1 µM of the 5-HT1B receptor preferring antagonist SB216641 to determine nonspecific binding (D). 5-HT3 receptors were mapped using 3nM [3H] LY278584 alone (E) or in combination with 1 µM ondansetron to determine nonspecific binding (F). 5-HT7 receptors were mapped using 4.5nM [3H]SB269970 alone (G) or in combination with 1 µM of unlabeled SB269970 to determine nonspecific binding (H). Finally, SERT was mapped using 4.5 nM [3H] escitalopram alone (I) or in combination with 1 µM paroxetine to determine nonspecific binding (J). Scale bars represent 5 mm. Abbreviations: CPu – caudate/putamen.
The goal of this article is to derive specific hypotheses about how vortioxetine will affect neural activity, with a particular focus on principle output cells, within the rodent mPFC and striatum. These brain regions were chosen because they play a role in aspects of cognitive function and have markedly different profiles of serotonergic receptor expression. Toward this end, we will discuss the functional organization of the microcircuits comprising the prefrontal cortex (PFC) and striatum, including a discussion of important neuronal subtypes and their relationships to one another. We will discuss the regional distribution of serotonergic receptor targets and the cell types expressing each target before evaluating the way that 5-HT modulates the function of the various cellular players within the circuit in question. Finally we will make predictions on how an SSRI or the multimodal antidepressant vortioxetine might modulate the function of the circuit before evaluating the electrophysiological data on these effects, where available.
The Prefrontal Cortex
Functional roles and circuit organization
The neocortex is a large structure that serves as an association region, integrating and performing computations on information from numerous other brain regions. The PFC represents a specialized subset of the neocortex that mediates a variety of cognitive functions, including learning,Reference Pasupathy and Miller20 working memory,Reference Rosenkilde, Bauer and Fuster21 attention,Reference Totah, Kim, Homayoun and Moghaddam22 and behavioral flexibility.Reference Clarke, Dalley, Crofts, Robbins and Roberts23
The PFC is a complex structure where neurons are incorporated into a modular design featuring columns of neurons as a basic unit of information processing. Each column is organized into well-defined layers that feature distinct populations of cells or cellular features, which are depicted in Figure 3. As discussed in a recent review by Puig and Gulledege,Reference Puig and Gulledge24 layer I is primarily a neuropil layer, featuring afferent terminals from a variety of sources and the cell bodies of regular spiking GABAergic interneurons and glia. Layers II and III contain the cell bodies of small pyramidal neurons and GABAergic interneurons characterized by either regular- or fast-spiking behavior. Layers V and VI contain the cell bodies of large pyramidal neurons as well as GABAergic interneurons primarily characterized by fast-spiking behavior. While an in-depth discussion of each cell type is outside the scope of this review, we will briefly discuss the role of these different cell types and their relationships to one another in the following section.

Figure 3 Serotonergic influence on a theoretical prefrontal cortex microcircuit. The influence of serotonin on the behavior of cortical pyramidal neurons is mediated by several different factors. Non-parvalbumin-immunoreactive interneurons (depicted in red) in the shallow cortical layers receive serotonergic inputs that have mixed excitatory and inhibitory effects mediated via 5-HT1A, 5-HT2A, and 5-HT3 receptors (A) and have an inhibitory influence on pyramidal cell dendritic membrane polarization. Additionally, serotonin’s effects on parvalbumin-immunoreactive interneurons (depicted in purple) are also probably a mix of excitatory and inhibitory actions that are mediated by 5-HT1A, 5-HT2A, and perhaps 5-HT1B receptors (B). These cells exert a powerful inhibitory effect on pyramidal neuron activity via hyperpolarizing actions at the pyramidal neuron soma or axon hillock. Pyramidal neurons themselves (depicted in blue) also receive a mix of excitatory and inhibitory serotonergic signals, which are mediated by 5-HT1A, 5-HT2A/2C, 5-HT4, 5-HT5, 5-HT6, and 5-HT7 receptors (C). Finally, glutamate release from pyramidal neuron terminals in the prefrontal cortex may be inhibited by 5-HT1B receptor activation (D).
Important cortical cell types
Parvalbumin-immunoreactive (PV-IR) fast-spiking interneurons
One major category of cortical inhibitory interneuron can be identified by the expression of the calcium binding protein parvalbumin (PV). There are several subclasses of cortical inhibitory interneuron that express PV, which at least include a subset of basket cells and chandelier cells (for an excellent review, see KubotaReference Kubota25). PV-IR interneurons primarily have fast-spiking behavior, and thus for the purposes of this article, the terms “PV-IR interneuron” and “fast-spiking interneuron” will be used interchangeably. Importantly, each PV-IR interneuron subtype is thought to have a selective subcellular axonal target. PV-IR basket cells, which comprise approximately 40–50% of cortical non-pyramidal neurons, arborize at the soma and dendrites of pyramidal neurons, with each basket cell innervating between 200 and 1000 pyramidal cells.Reference Kubota25 PV-IR chandelier cells are a relatively small subset of PV-IR interneurons and synapse with pyramidal neurons almost exclusively at the axon hillock.Reference Kubota25 Given the placement of these axonal arborizations, cortical PV-IR interneurons can be expected to have a strong inhibitory effect on the output of pyramidal neurons. Interestingly, PV-IR interneuron subclasses such as fast-spiking basket cells are thought to be connected to one another via electrical gap junctions,Reference Kubota25 which may allow these cell types to easily form neural assemblies that could multiply the inhibitory signal they represent within the frontal cortex network.
Non-PV-IR interneurons
In addition to PV-IR interneurons, there are a number of other GABAergic interneuron classes in the frontal cortex. These subtypes can be identified by various neurochemical markers, including calbindin, calretinin, cholecystokinin, vasoactive intestinal peptide, somatostatin, and neuropeptide Y. Each interneuron subtype has a distinctive morphology, cellular target, and role in modulating the activity of frontal cortical networks.Reference Kubota25 Furthermore, the electrical activity of these cells can have a regular-spiking, late-spiking, or bursting character. However, information on the placement of serotonergic targets on these cortical interneuron subtypes has generally not reached a high level of detail. Therefore, we have chosen to consider these various classes together as one group for the purposes of this article. The most important group of non-PV-IR interneurons to consider from the perspective of understanding serotonergic neurotransmission’s effects on PFC activity is a set of interneurons in the shallow cortical layers (ie, layers I–III) that inhibits cortical pyramidal neuron activity.
Pyramidal neurons
Pyramidal neurons are excitatory glutamatergic cells that are characterized by a triangular-shaped soma, apical dendrites emanating from the soma’s vertex, and basal dendrites as well as a single branching axon originating from the soma’s base. Within the cortex, pyramidal neurons are thought to fill multiple functional roles. The small pyramidal neurons prevalent in layers II and III integrate subcortical and cortico-cortical afferent inputs and send axonal projections horizontally to layers II and III of neighboring cortical columns.Reference Adesnik and Scanziani26, Reference Gottlieb and Keller27 Additionally, layer II/III pyramidal neurons project axons vertically to layer V pyramidal neurons.Reference Adesnik and Scanziani26, Reference Gottlieb and Keller27 This combination of local projections drives lateral inhibitory effects on layer II/III pyramidal neurons from neighboring cortical columns, presumably by activating inhibitory interneurons, while also activating layer V pyramidal neurons both within the originating column and in neighboring columns.Reference Adesnik and Scanziani26 Thus, activation of a given layer II/III pyramidal neuron may suppress inputs from neighboring columns while at the same time magnifying the output of its own column.
The pyramidal neurons of the deep cortical layers (ie, layers V and VI) have a large soma in comparison to layer II/III pyramidal neurons, and layer V pyramidal neurons are responsible for sending excitatory glutamatergic projections to subcortical regions, such as the striatum. Thus, the pyramidal neuron is considered the principle cortical projection neuron.
In order to understand how 5-HT modulates PFC microcircuit activity, it is necessary to understand which cells within the network express each receptor and their effect on neural activity. Therefore, the following section will review information on 5-HT receptor expression within the PFC.
Serotonergic receptor expression in the prefrontal cortex
5-HT1A receptors
5-HT1A receptors are inhibitory G-protein coupled receptors. Autoradiographic and immunohistochemical studies have demonstrated strong 5-HT1A receptor expression in some portions of the hippocampal formation (see the detailed review by Dale et al Reference Dale, Pehrson and Jeyarajah28 elsewhere in this issue) and the brain stem raphe nuclei. 5-HT1A receptor expression within the PFC is moderate in strength (Figure 1, panels A and B), and has been demonstrated in all cortical layers with the exception of layer I.Reference Aznar, Qian, Shah, Rahbek and Knudsen29
Aznar et al Reference Aznar, Qian, Shah, Rahbek and Knudsen29 investigated the cellular subtypes that express the 5-HT1A receptor in the rodent cortex, and found that the majority of pyramidal neurons, PV-IR interneurons, and some non-PV-IR interneurons express 5-HT1A receptors (approximately 85% or more in each case). However, it should be noted that other research groups have suggested lower estimates in PFC subregions, with approximately 60% of pyramidal neurons in the prelimbic, and 40% of those in the infralimbic subregion, expressing 5-HT1A receptor messenger ribonucleic acid (mRNA).Reference Celada, Puig and Artigas30 Approximately 20% of GABAergic interneurons in these PFC subregions express 5-HT1A receptors.Reference Celada, Puig and Artigas30
On a subcellular level, 5-HT1A receptors are thought to be somatodendritically expressed, and in frontal cortex pyramidal neurons there is evidence supporting a placement on the axon hillock.Reference Cruz, Eggan, Azmitia and Lewis31
Based on this pattern of expression, 5-HT1A receptors can be expected to have complex effects on the behavior of the frontal cortex. Activation of 5-HT1A receptors expressed on PV-IR interneurons would inhibit these cells, and thereby allow pyramidal neuron firing to increase via disinhibition. However, 5-HT1A receptor expression on pyramidal neurons themselves would reduce their activation state. Therefore, 5-HT1A receptor activation should have mixed effects on the behavior of the PFC microcircuit. Moreover, electrophysiological evidence suggesting that selective activation of 5-HT1A receptors leads to an increase in firing followed by a reduction for most pyramidal neuronsReference Llado-Pelfort, Santana, Ghisi, Artigas and Celada32 is consistent with this conceptual framework.
5-HT1B receptors
Within the frontal cortex, 5-HT1B receptors are expressed at low to moderate levelsReference Bruinvels, Palacios and Hoyer33–Reference Sari, Miquel and Brisorgueil35 (Figure 1, panels C and D) and may exist with a combination of presynaptic and postsynaptic expression patterns. The observation that 5-HT1B receptor stimulation reduces extracellular 5-HT concentrations elicited by SSRIs in the frontal cortex suggests the presence of 5-HT1B receptors on serotonergic terminals in this region.Reference de Groote, Klompmakers, Olivier and Westenberg36 Additionally, Tanaka and NorthReference Tanaka and North37 have suggested that 5-HT1B receptors are present as postsynaptic terminal heteroreceptors on cortical glutamate terminals, as 5-HT1B receptors modulate cortical glutamate release. However, this should be viewed with caution, since there is no histological confirmation. Egeland et al Reference Egeland, Warner-Schmidt, Greengard and Svenningsson38 demonstrated 5-HT1B receptor immunoreactivity within a subpopulation of PV-IR interneurons of the cingulate gyrus, and this included some weak immunoreactivity in the soma of these cells. It is not known whether this expression pattern reflects somatodendritic insertion of 5-HT1B receptors on the neuronal membrane, as has been observed in the hippocampal formation,Reference Peddie, Davies, Colyer, Stewart and Rodriguez39, Reference Peddie, Davies, Colyer, Stewart and Rodriguez40 or simply 5-HT1B receptors that are early in the process of being trafficked to axon terminals.
Thus, activation of 5-HT1B receptors may negatively modulate the release of 5-HT and glutamate within the frontal cortex via their expression as terminal autoreceptors or heteroreceptors. Additionally, if 5-HT1B receptors have a somatodendritic expression in cortical PV-IR interneurons, then activation of these receptors may also serve to reduce the activity of these neurons. However, we have not been able to identify any electrophysiological studies confirming the presence of a 5-HT1B mediated current in cortical PV-IR interneurons.
Given that Bruinvels et al Reference Bruinvels, Palacios and Hoyer33 found that the closely related 5-HT1D receptor represents a negligible amount of binding in the frontal cortex, we will consider it to be absent in the PFC and will discuss it in more detail below, in the section dealing with the striatum.
5-HT2A/2C receptors
5-HT2 receptors are stimulatory G-protein coupled receptors that exist primarily as postsynaptic heteroreceptors within the CNS. 5-HT2A receptors are strongly expressed in the cortex. Within the frontal cortex, 5-HT2A receptor mRNA has been observed in pyramidal neurons and interneurons, with substantial variation in the proportion of these cellular populations expressing this receptor. In the prelimbic PFC, approximately one-half of pyramidal neurons and one-third of interneurons express 5-HT2A receptor mRNA, while in the infralimbic region only about 12% of pyramidal neurons and 22% of interneurons expressed 5-HT2A receptors.Reference Celada, Puig and Artigas30 Moreover, the presence of 5-HT2A receptor proteins has been confirmed in each of these cell populations.Reference Cornea-Hebert, Riad, Wu, Singh and Descarries41 Furthermore, the presence of 5-HT2A receptors on GABAergic interneurons has been extended, showing these receptors are present in cortical PV-IR and non-PV-IR interneurons.Reference Puig, Watakabe, Ushimaru, Yamamori and Kawaguchi42, Reference Weber and Andrade43
The structurally similar 5-HT2C receptor is present at moderate levels in the frontal cortex,Reference Abramowski, Rigo, Duc, Hoyer and Staufenbiel44 but has not been found in fast spiking interneurons.Reference Puig, Watakabe, Ushimaru, Yamamori and Kawaguchi42
Thus, within the frontal cortex, activation of 5-HT2A and 5-HT2C receptors is expected to have excitatory direct effects on pyramidal neurons, while 5-HT2A receptors should also excite GABAergic interneurons. However, given the tight interconnection between pyramidal and GABAergic interneurons in the frontal cortical microcircuitry, the effects of 5-HT2A/2C receptor stimulation are expected to be mixed. Moreover, the available electrophysiological evidence is consistent with this interpretation. In vivo recordings of the PFC after administration of the 5-HT2A/2C receptor agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) found that regular spiking cells had a mix of excitatory and inhibitory responses,Reference Puig, Celada, Diaz-Mataix and Artigas45 although the largest group of DOI-responsive neurons exhibited an excitatory response.
5-HT3 receptors
5-HT3 receptors are unique among the known 5-HT receptor subtypes in that they are the only stimulatory ligand-gated ion channel. On a regional level, 5-HT3 receptors are expressed at their strongest levels within the CNS in the nucleus of the solitary tract, and their level of expression in forebrain regions tends to be low to moderate by comparison.Reference Gehlert, Gackenheimer, Wong and Robertson46 Within the frontal cortex, cells expressing 5-HT3 receptor mRNA are segregated primarily into shallow cortical layers (ie, layers I–III), although some 5-HT3 receptor mRNA-positive cells have been observed in deeper layers.Reference Puig, Santana, Celada, Mengod and Artigas47 5-HT3 receptor protein expression in the frontal cortex tends to be present throughout the cortical layers, though again it is stronger in the shallow layersReference Gehlert, Gackenheimer, Wong and Robertson46, Reference Morales, Battenberg and Bloom48 (see Figure 1, panels E and F). On the level of cellular expression, 5-HT3 receptors are nearly exclusively expressed within GABAergic interneurons, more specifically in those expressing cholecystokinin, calretinin, vasoactive intestinal peptide, or neuropeptide Y, but not in those expressing somatostatin or parvalbumin.Reference Puig and Gulledge24, Reference Morales and Bloom49
Thus, 5-HT3 receptors represent a mechanism of 5-HT-mediated fast excitatory drive that is selective to non-PV-IR interneurons segregated into layers I–III. By extension, it can be expected that 5-HT3 receptor-mediated 5-HT neurotransmission will have an overall inhibitory effect on the output of cortical pyramidal cells, especially in the PFC, where there are somewhat more 5-HT3 receptors than in other cortical subregions. Electrophysiological data are consistent with this interpretation. Puig et al Reference Puig, Santana, Celada, Mengod and Artigas47 demonstrated that raphe stimulation increased the activity of a subset of GABAergic interneuron that could be blocked by selective 5-HT3 receptor antagonists, while Ashby et al Reference Ashby, Minabe, Edwards and Wang50 found that 5-HT3 receptor agonists reduced the activity of putative pyramidal neurons in the PFC. By extension, 5-HT3 receptor antagonists can be expected to increase pyramidal neuron output via disinhibition.
5-HT4 receptors
5-HT4 receptors are stimulatory G-protein coupled receptors for which there is relatively little accumulated knowledge. Autoradiographic evidence suggests that 5-HT4 receptors are expressed at very low levels in the frontal cortex.Reference Vilaro, Cortes and Mengod51 Furthermore, there is little and conflicting experimental data addressing their cellular expression in the frontal cortex.Reference Egeland, Warner-Schmidt, Greengard and Svenningsson38, Reference Peñas-Cazorla and Vilaró52
5-HT5 receptors
5-HT5 receptors are inhibitory G-protein coupled receptors that are broadly present within the rodent brain. Immunohistochemical evidence shows low to moderate 5-HT5 receptor expression levels within several cortical regions, highest within layers II and IV, where expression tended to be in the soma,Reference Oliver, Kinsey, Wainwright and Sirinathsinghji53 but the degree to which this information will translate to the PFC is questionable, since this region lacks a well-defined layer IV. Anatomical data on the cortical neuronal subtypes expressing 5-HT5 receptors is largely absent; however, Goodfellow et al Reference Goodfellow, Bailey and Lambe54 observed a 5-HT5 receptor-mediated inhibitory current in cortical layer V pyramidal neurons, suggesting that they are at least present in cortical principle cells. Based on these data alone, activating 5-HT5 receptors should be expected to reduce cortical output. However, given the relative lack of anatomical and electrophysiological data on this receptor subtype, 5-HT5 receptors may turn out to have more complex actions on the PFC network.
5-HT6 receptors
5-HT6 receptors are stimulatory G-protein coupled receptors that are present at low to moderate levels within the rodent frontal cortex, with stronger areas of expression in rostral compared to caudal cortical regions.Reference Gerard, Martres and Lefevre55 Similar results were found in human PFC, where 5-HT6 receptor expression was present at relatively low levels.Reference Marazziti, Baroni and Pirone56 The strongest 5-HT6 receptor expression in the human cortex was observed in layer I, where 5-HT6 receptors were primarily associated with glia. However, 5-HT6 receptor expression was associated with pyramidal neurons in deeper cortical layers,Reference Marazziti, Baroni and Pirone56 and expression in non-pyramidal neuronal types has not yet been reported. These data may suggest that 5-HT6 receptor activation will activate pyramidal neuron firing; however, we are not aware of any electrophysiology data that have investigated the consequences of 5-HT6 receptor stimulation in the PFC.
5-HT7 receptors
5-HT7 receptors are stimulatory G-protein coupled receptors that have varying levels of expression within the cortex, with strong expression in the shallow cortical layers and more moderate levels in deeper layersReference Neumaier, Sexton, Yracheta, Diaz and Brownfield57 (Figure 1, panels G and H). More specifically, 5-HT7 receptor expression has been observed in layer V pyramidal neurons, and in cells with relatively small oval-shaped soma that were not characterized further. Electrophysiological evidence indicates the presence of 5-HT7 receptors on hippocampal pyramidal neuronsReference Tokarski, Zahorodna, Bobula and Hess58 and GABAergic interneuronsReference Tokarski, Kusek and Hess59; thus it is possible that they will also be present in cortical interneurons, but this remains to be experimentally confirmed. A study by Tokarski et al Reference Tokarski, Zelek-Molik and Duszynska60 showed that acute intraperitoneal (i.p.) administration of the selective 5-HT7 receptor antagonist SB269970 at 1.25 mg/kg had no effect on the firing of frontal cortex pyramidal neurons, while repeated administration reduced firing. But Lundbeck internal data suggest that a 1 mg/kg dose of this drug via the subcutaneous (s.c.) route, which is in many respects similar to the i.p. route, results in negligible occupancy at 5-HT7 receptors. In fact, in our hands, a 30 mg/kg dose of SB269970 only engendered approximately 30% of 5-HT7 receptors 1 hour after administration, owing to difficulty penetrating the blood–brain barrier. Given these receptor occupancy data, it remains unclear how 5-HT7 receptor modulation will alter the activity of cells within the PFC.
Summary of serotonergic receptor mechanisms in frontal cortex neuronal subtypes
This section summarizes the 5-HT receptor subtypes that have been demonstrated on each of the important neuron types within the PFC microcircuit.
PV-IR interneurons
In cortical PV-IR fast-spiking interneurons, there is evidence supporting the expression of inhibitory 5-HT1A as well as excitatory 5-HT2A receptors, although 5-HT1A and 5-HT2A receptors appear to be largely expressed by separate populations of PV-IR interneurons.Reference Puig, Watakabe, Ushimaru, Yamamori and Kawaguchi42 There is also some evidence suggesting that 5-HT1B receptors are expressed on cortical PV-IR interneurons, but the subcellular localization of 5-HT1B receptors on cortical PV-IR cells is not clear at this time. Based on this evidence, cortical PV-IR cells will likely respond to 5-HT with either an excitatory or inhibitory response on the single-cell level, while on the population scale there will likely be a mixed excitatory and inhibitory response. Whether this population level mix of excitatory and inhibitory responses skews to one side or the other may depend on a number of issues, including (1) how commonly 5-HT1A and 5-HT2A receptors are expressed within frontal cortical PV-IR cells, (2) the affinity of 5-HT for each of these targets (Table 1), and (3) the concentration of 5-HT applied to the neural system.
Table 1 5-HT receptor subtypes in the rat prefrontal cortex and striatum: expression patterns, affinities for 5-HT, and effects on neural activity.
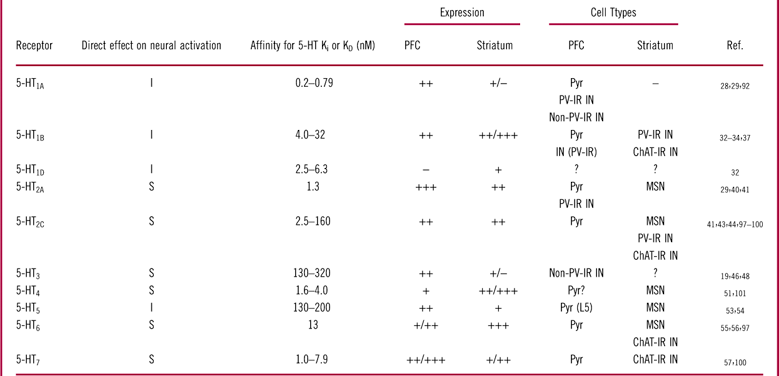
Affinities for 5-HT were calculated based on pKi/pKD data from the IUPHAR database (www.iuphar-db.org).−: absent; +: low; ++: moderate; +++: strong; ?: unknown; ChAT-IR: choline acetyltransferase immunoreactive; I: inhibitory; IN: interneuron; MSN: medium spiny neuron; PFC: prefrontal cortex; PV-IR: parvalbumin immunoreactive; Pyr: pyramidal neuron; S: stimulatory.
Puig et al Reference Puig, Watakabe, Ushimaru, Yamamori and Kawaguchi42 have suggested that about half of PV-IR interneurons in layers II/III express 5-HT1A receptor mRNA, while about 20% express 5-HT2A receptor mRNA. However, in layer V, approximately one-third of PV-IR neurons express 5-HT1A receptors, while a separate third of the population express 5-HT2A receptors. Thus, these data may suggest that PV-IR interneurons in shallow cortical layers are more likely to be inhibited by 5-HT than those in deep cortical layers, where there is more likelihood for an even split in excitatory and inhibitory responses within this cell population. However, given that 5-HT’s affinity for the 5-HT1A receptor is anywhere from 1.6- to 6.5-fold higher than for the 5-HT2A receptor (Table 1), it is possible that the effect of 5-HT on cortical fast-spiking interneuron behavior will skew more heavily toward inhibition at lower levels of 5-HT concentrations, with excitation becoming more prominent as 5-HT concentrations increase.
Non PV-IR interneurons
In non-PV-IR cortical GABAergic interneurons, there is evidence to support 5-HT1A receptor expression on calbindin-IR interneurons, and 5-HT3 receptors have been found on CR-IR and CCK-IR interneurons located in shallow cortical layers. Additionally, electrophysiological evidence has suggested that 5-HT2A receptors are also present in cortical non-PV-IR interneurons, although the specific subtype remains unclear at this time. When considering the overall effects of 5-HT in this subpopulation of cells, inhibitory 5-HT1A receptors may again tend to predominate in low concentration ranges of 5-HT, given its higher affinity for the endogenous ligand by comparison to 5-HT2A and especially 5-HT3 receptors (Table 1). However, it is important to note that it is essentially unknown at this time whether these receptors are expressed in serotonergic synapses. Although SERT expression in the frontal cortex is relatively strong (see Figure 1, panels I and J), it is currently believed that only about one-quarter of serotonergic terminals in this brain region have synaptic specializations.Reference Seguela, Watkins and Descarries61 However, the serotonergic terminals that do form synapses are thought to be primarily on GABAergic interneurons residing in the shallow cortical layers,Reference Smiley and Goldman-Rakic62 which may include 5-HT3 receptor-expressing interneurons. If true, this pattern may make it easier to achieve sufficiently high 5-HT concentrations to stimulate 5-HT3 receptor activity than for those serotonergic receptor targets that are primarily modulated by extracellular serotonin concentrations. Additionally, 5-HT3 receptors are a fast-desensitizing serotonergic target (for example, see Dale et al Reference Dale, Zhang and Leiser17); thus at high concentrations, it may be the case that a larger proportion of this receptor population is non-functional than at lower concentrations. Taken together, it is reasonable to suggest that serotonergic neurotransmission is more likely to activate non-PV-IR interneurons in the shallow cortical regions.
Pyramidal neurons
In cortical pyramidal neurons, there is evidence for the expression of a variety of serotonergic receptors. Inhibitory 5-HT1A and 5-HT5 receptors have been found in somatodendritic subcellular regions of pyramidal neurons, 5-HT1B receptors may be expressed in the terminals of cortical pyramidal neurons. Additionally, several excitatory serotonergic receptors are present on these cells, including 5-HT2A, 5-HT2C, 5-HT4, 5-HT6, and 5-HT7 receptors.
Given the wide range of excitatory and inhibitory serotonergic receptors that have been demonstrated in cortical pyramidal neurons, along with the differences in affinity these receptors have for 5-HT, it is not possible to accurately determine 5-HT’s overall effects on cortical pyramidal neurons. However, within the native frontal cortex microcircuit, pyramidal neurons are under powerful inhibitory control from a variety of inhibitory interneurons, and thus some of the effects 5-HT will have on pyramidal neurons will depend on its effects on these inhibitory interneurons.
Electrophysiological effects of 5-HT in the frontal cortex
In vivo electrophysiology
Studies that have investigated the effects of 5-HT on cortical fast-spiking (presumably PV-IR) interneuron behavior in vivo using anesthetized animals have generally found that 5-HT has inhibitory effects. For example, Puig et al Reference Puig, Watakabe, Ushimaru, Yamamori and Kawaguchi42 found that stimulating the midbrain raphe nuclei at low levels and recording in vivo from the medial PFC induced mixed effects in fast-spiking interneurons, with the majority of these cells exhibiting inhibition (61%), and small populations exhibiting excitation (approximately 10%) or excitation followed by inhibition (approximately 6%). Moreover, the inhibitory actions of raphe nucleus stimulation were antagonized by administration of the selective 5-HT1A receptor antagonist WAY100635, whereas the few excitations that were observed were antagonized by the 5-HT2 receptor antagonist ritanserin.
Superficially, these data appear to be at odds with data from the same lab demonstrating that separate but equal proportions of layer V PV-IR interneurons within the cortex express inhibitory 5-HT1A receptors and excitatory 5-HT2A receptorsReference Puig, Watakabe, Ushimaru, Yamamori and Kawaguchi42. A corollary to these data would seem to be that approximately equal proportions of layer V fast-spiking interneurons would exhibit an inhibitory or excitatory response to physiological 5-HT levels. However, during periods of light anesthesia that more closely resembled an “awake” cortical network, Puig et al.Reference Puig, Watakabe, Ushimaru, Yamamori and Kawaguchi42 also found a higher proportion of cells exhibiting an excitatory response to raphe stimulation (approximately 31% compared to 10%). These data fit with an interpretation that in vivo electrophysiology in anesthetized rats is biased towards 5-HT1A receptor mediated inhibition.
Although inhibition of PV-IR interneurons would be expected to have a disinhibitory effect on pyramidal neurons, it seems that pyramidal neurons also have an overwhelmingly inhibitory response to raphe nucleus stimulation in anesthetized in vivo electrophysiological experiments. Puig et al Reference Puig, Artigas and Celada63 demonstrated that low-level stimulation of the raphe nucleus inhibited activity in the majority of recorded putative pyramidal neurons (66%), while 13% exhibited an excitatory response and another 20% had mixed effects characterized by inhibition followed by excitation. 5-HT1A receptor antagonism attenuated the inhibitory responses (but did not entirely block them), while 5-HT2A receptor antagonism blocked the excitatory responses. Similar results were found by several other research groups after raphe nucleus stimulation in anesthetized in vivo electrophysiological preparations.Reference Amargos-Bosch, Bortolozzi and Puig64–Reference Hajos, Gartside, Varga and Sharp66
While the results of these in vivo anesthetized electrophysiology experiments consistently suggest that 5-HT has an overwhelmingly inhibitory effect on not only pyramidal neurons but also PV-IR interneurons, there are some important limitations to consider when evaluating these data. The presence of anesthesia may cause a number of deviations from normal cortical network states, for example a reduction in tonic or phasic 5-HT concentrations resulting from reduced raphe nucleus activity. This may bias the effects of 5-HT stimulation toward receptors with high affinity for 5-HT, such as the inhibitory 5-HT1A receptor, which is present on pyramidal neurons, as well as multiple subtypes of non-pyramidal neurons in this region. It may be the case that in an awake and behaving animal, stimulation of the raphe nucleus would be more likely to induce excitatory responses mediated by lower-affinity receptors, such as the 5-HT2A receptor (Table 1).
In addition to this distal effect of anesthesia, local cortical networks may also be abnormally regulated by these unusually high GABAA receptor mediated currents. This may have the effect of partially divorcing the activity of pyramidal neurons and some types of interneurons from the activity of the inhibitory cells governing their behavior, therefore abrogating the downstream effects of serotonergic modulation. This, along with the apparent bias toward 5-HT1A receptor mechanisms, may help to explain why pyramidal neuron firing is overwhelmingly reduced by raphe stimulation or SSRI treatments within anesthetized cortical networks in the face of reduced fast-spiking interneuron firing.
In vitro electrophysiology
In stark contrast to the in vivo electrophysiological techniques in anesthetized rodents, research groups investigating the effects of 5-HT on fast-spiking interneuron behavior in brain slices have generally found that 5-HT has an activating effect on fast-spiking interneurons. For example, Zhong and YanReference Zhong and Yan67 found that 5-HT (20-40 µM) led to an increased firing rate of fast spiking interneurons, with relatively little effect of 5-HT at lower concentrations (1–2 µM). The idea that 5-HT activates fast-spiking interneuron behavior in slices is further supported by observations that 5-HT depolarized fast-spiking interneuronsReference Weber and Andrade43 and increased spontaneous inhibitory postsynaptic currents (sIPSCs) recorded from pyramidal neurons.Reference Weber and Andrade43, Reference Zhong and Yan68, Reference Zhou and Hablitz69
Additionally, in the concentration ranges that drove increases in fast-spiking interneuron activity, Zhong and YanReference Zhong and Yan67 observed concomitant reductions in the firing rate of cortical pyramidal neurons. But the inhibitory effects of 5-HT on pyramidal neuron firing were not limited to downstream actions on GABAergic interneurons. Araneda and AndradeReference Araneda and Andrade70 found that 5-HT hyperpolarized layer V cortical pyramidal neuronal membranes, which could be blocked by 5-HT1A receptor antagonists but not a 5-HT2 receptor antagonist.
As expected by the fact that both inhibitory 5-HT1A and stimulatory 5-HT2A receptors are expressed in cortical pyramidal neurons, 5-HT’s effects on pyramidal neuron firing in slice electrophysiological preparations are not universally inhibitory. Several groups have found that 5-HT application increases spontaneous excitatory postsynaptic currentsReference Zhou and Hablitz69, Reference Beique, Chapin-Penick, Mladenovic and Andrade71 (sEPSCs), induces membrane depolarizations,Reference Araneda and Andrade70 and modulates after hyperpolarization currents in a manner that supports increased activity.Reference Araneda and Andrade70, Reference Villalobos, Beique, Gingrich and Andrade72
Taken together, in contrast to in vivo recordings in anesthetized animals, these data from brain slices suggest that 5-HT has a primarily excitatory effect on cortical fast-spiking interneurons, as well as mixed excitatory and inhibitory effects on pyramidal neuron activation. Slice electrophysiology has a number of advantages, including a stronger ability to identify the cell type from which each recording is taken and the absence of anesthesia. However, there are also limitations to consider. First, although it can be argued that local neuronal circuits are largely intact, the process of sectioning tissue does attenuate some aspects of the neural network, most obviously from distal regions such as the raphe nucleus. Additionally, it should be noted that slice electrophysiological studies are often conducted in brain slices collected from adolescent rodents, and thus the degree to which the results translate to the adult cortical network are difficult to know. But perhaps the most important limitation of slice electrophysiology in the context of this article is that it is difficult to ascertain what the effects of physiological 5-HT concentrations would be. It is more or less not possible to determine whether the 5-HT concentrations used to elicit a primarily excitatory response via bath application are physiologically relevant, and in fact the concentrations commonly used to elicit these responses are in a very high range. Although it is common for slice electrophysiologists to use relatively high concentrations of a given ligand to ensure that it penetrates to the recorded cells (typically in the range of 100–1000 times Ki), concentrations of 5-HT of 20–40 µM seem disproportionately high from the perspective that the Ki for 5-HT at 5-HT2A receptors is1.3 nM (Table 1).
Thus, it seems likely that in vivo investigations of 5-HT’s effects in anesthetized PFC networks primarily represent the actions of this neurotransmitter at low concentrations, while those from the slice represent 5-HT’s actions at high concentrations. In order to further advance our understanding of how 5-HT and serotonergic antidepressants modulate prefrontal cortical networks, it would be advantageous to conduct investigations in awake and behaving rodents.
Hypothesized effects of 5-HT, SSRIs, and vortioxetine on the overall frontal cortex circuit
5-HT and SSRIs
Based on the receptor profiles that are present in each of the major cell types we have considered in the frontal cortex microcircuitry, as well as electrophysiological evidence of 5-HT’s effects, the emerging theme seems to be that 5-HT has largely inhibitory actions at low concentrations dominated by 5-HT1A receptor mediated actions at some PV-IR and non-PV-IR interneurons. Under normal circumstances, this would be expected to disinhibit pyramidal neuron activity, although pyramidal neurons themselves would also experience direct inhibitory effects of 5-HT1A receptor activation. At higher levels of 5-HT tone, excitatory actions would start coming into play at inhibitory interneurons, primarily mediated by 5-HT2A receptors at PV-IR fast-spiking interneurons and by 5-HT2A and 5-HT3 receptors in non-PV-IR interneurons. These actions on cortical interneurons would be expected to drive a GABA-mediated inhibitory influence on pyramidal neuron firing, particularly given the powerful inhibitory action PV-IR interneurons have on pyramidal neuron behavior. However, pyramidal neurons would also experience some direct excitatory actions of 5-HT mediated through a variety of serotonergic receptor targets. Therefore, at multiple levels of organization, 5-HT has opposite directed effects that overall do not appear to clearly influence the output of frontal cortical output cells toward excitation or inhibition. On a conceptual level, 5-HT may therefore play a role in fine-tuning the output of frontal cortical networks, acting in a sense as a buffer that will tend to normalize the activity of pyramidal output neurons. Given this concept and the idea that SSRI antidepressants increase synaptic and extrasynaptic serotonin concentrations without directly modulating serotonergic receptors, we hypothesize that, under acute conditions, these treatments will have mixed effects that will not clearly bias frontal cortex output in either direction. Moreover, this idea is supported by electrophysiological studies that have demonstrated that acute and short-term repeated SSRI administration have little effect on PFC firing in anesthetized rats.Reference Gronier and Rasmussen73, Reference Ceci, Fodritto and Borsini74 However, long-term SSRI administration seems to have an inhibitory effect on PFC firing,Reference Gronier and Rasmussen73, Reference Ceci, Fodritto and Borsini74 which may imply that desensitization of some serotonergic receptor systems are important for the long-term effects of SSRI administration on frontal cortical network behavior. It is not clear based on available anatomical and electrophysiological data precisely which serotonergic receptor mechanisms might be involved in these effects of chronic administration.
Vortioxetine
In contrast, vortioxetine should have markedly different effects on the output of frontal cortex networks that will be driven by its direct pharmacological actions on several serotonergic receptors. Vortioxetine’s potent antagonism of 5-HT3 receptors should remove a source of 5-HT-mediated fast excitatory drive on a selective population of non-PV-IR cortical interneurons localized in shallow cortical layers. Based on the model presented here, this action should disinhibit cortical pyramidal neurons, although it is unclear whether this effect would be segregated purely in layer II/III pyramidal neurons or if it would generalize to layer V pyramidal output cells. 5-HT3 receptor antagonism can also result in increased extracellular 5-HT concentrations when dosed along with an SSRI,Reference Bétry, Overstreet and Haddjeri75 which may lead to more stimulation of serotonergic receptors that are not directly targeted by vortioxetine. Additionally, 5-HT1A receptor agonism should induce a fast inhibitory effect mediated by inwardly rectifying potassium channels in both PV-IR and non-PV-IR interneurons, which may also disinhibit pyramidal neuron activity that would be balanced by inhibitory actions on the pyramidal neurons themselves. Additionally, vortioxetine’s partial agonist action at 5-HT1B receptors, which acts to attenuate 5-HT1B receptor actions in vivo,Reference El Mansari, Lecours and Blier76 may increase serotonergic tone in the PFC by reducing inhibitory feedback at the site of the serotonergic terminal, which may again lead to greater stimulation of serotonergic targets not directly targeted by vortioxetine. Furthermore, this partial agonist effect at 5-HT1B receptors may also attenuate 5-HT1B receptor mediated inhibition of cortical glutamatergic terminals, leading to increased glutamate release from layer II/III pyramidal neurons at their local cellular targets. Finally, it is unclear at this time what effect vortioxetine’s 5-HT7 receptor antagonist properties will have on PFC activity. But we expect that the net effect of vortioxetine in the PFC will be to reduce aspects of GABAergic neurotransmission and indirectly increase the activity of glutamatergic pyramidal cells.
In support of these ideas, recent in vivo electrophysiology experiments by Riga et al Reference Riga, Celada, Sanchez and Artigas18 in anesthetized rats demonstrated that acute administration of the SSRI escitalopram had no effect on the overall firing rate of putative frontal cortex pyramidal neurons, although a relatively equal minority of cells exhibited excitatory or inhibitory responses. However, in stark contrast, acute i.v. administration of vortioxetine led to an overwhelmingly excitatory response in cortical pyramidal neurons. This research group also found that an acute combination of escitalopram and the selective 5-HT3 receptor antagonist ondansetron led to significant increases in pyramidal neuron firing, at least implicating a combination of 5-HT reuptake inhibition and 5-HT3 receptor antagonism in vortioxetine’s excitatory effects.
In summary, SSRIs appear to have mixed effects on the function of the PFC circuit that do not clearly modulate activity either toward excitation or inhibition under acute conditions, while acute vortioxetine leads to an increase in PFC pyramidal neuron activity that is driven by a reduction in GABAergic inhibitory processes. After long-term administration, SSRI antidepressants lead to an overall reduction of PFC pyramidal neuron firing that is likely driven by the desensitization of some serotonergic receptors. At this time, empirical data on the long-term effects of vortioxetine on PFC pyramidal neuron activity are not available; however, we hypothesize that vortioxetine will still have an overall excitatory effect on these cells after chronic administration.
The Striatum
Functional role and organization
The striatum, which is a term we will use to refer specifically to the caudate and putamen, is a subcortical structure that receives afferent inputs from a number of different brain regions including association and sensorimotor cortices as well as the thalamus, and is considered the primary input structure for the basal ganglia. Thus, the striatum can be viewed as an assimilation zone for information related to central nervous system functions as broad as sensorimotor integration and motor function, motivation and reinforcement, and some aspects of cognitive function such as working memory and executive function.Reference Lindgren, Wickens, Tait, Brown and Dunnett77, Reference Rolls78
From a structural perspective, the striatum has a number of important differences compared to the PFC. First, the laminar structure observed in the PFC is absent in the striatum. Additionally, while the PFC has a mix of inhibitory interneurons and excitatory cells that are native to the region, the vast majority of striatal neurons are GABAergic and thus inhibitory in nature. In fact, there are at least four separate types of GABAergic cells in the striatum. These include the medium spiny neuron (MSN), which is this region’s principle projection cell, as well as at least three chemically differentiated types of striatal GABAergic interneuron.Reference Kawaguchi, Wilson, Augood and Emson79 In addition, a fifth type of striatal interneuron is cholinergic, rather than GABAergic.Reference Kawaguchi, Wilson, Augood and Emson79 The section below will focus on describing medium spiny neurons, PV-IR fast-spiking interneurons, and cholinergic interneurons and their relationships to one another. These relationships, as well as the influence of 5-HT on their behavior, are depicted in Figure 4.

Figure 4 Serotonergic influence on a theoretical striatal microcircuit. The effects of serotonin on the behavior of striatal medium spiny neurons (MSNs) are mediated by a number of different factors. Serotonergic afferents from the raphe have a mostly excitatory direct influence on MSNs that are mediated through 5-HT2A/2C, 5-HT4, and 5-HT6 receptors, although 5-HT5 receptors have an inhibitory influence on these cells. 5-HT1B receptors are prominently expressed in the striatum, but are thought to exist primarily as presynaptic autoreceptors in this region (A). Similarly, serotonin has an excitatory direct effect on parvalbumin-immuoreactive (PV-IR) interneurons, which are at least mediated by 5-HT2C receptors (B). The excitatory effect of 5-HT observed in choline-acetyltransferase immunoreactive interneurons ChAT-IR cells is mediated by a combination of 5-HT2C, 5-HT6, and 5-HT7 receptors (C). MSN behavior is also modulated by the behavior of PV-IR and ChAT-IR cells. PV-IR cells have a powerful inhibitory effect on MSN firing that is mediated by GABAergic neurotransmission localized at the MSN soma or dendrites. ChAT-IR cells have a relatively complex mix of excitatory and inhibitory effects on the behavior of PV-IR cells and MSNs. Cholinergic transmission from ChAT-IR cells has a short-term activating effect on PV-IR cells that are mediated by nicotinic acetylcholinergic receptors (D), but can reduce GABA release from PV-IR cells via presynaptic M2 receptors (E). ChAT-IR cells can also excite MSNs via M1 receptors (E), and can reduce release from glutamatergic afferent terminals via M2 receptors (F).
Important striatal cell types
PV-IR fast-spiking interneurons
One of the most important classes of striatal GABAergic interneuron is identified immunohistochemically by the expression of the calcium binding protein parvalbumin (PV). As in the frontal cortex, PV-IR interneurons are characterized electrophysiologically by their fast firing rate and short-duration action potential. Therefore we will refer to these cell types interchangeably as striatal PV-IR interneurons or as striatal fast-spiking interneurons.
PV-IR interneurons are believed to make up between 3% and 5% of striatal neurons (for an excellent review, see Kawaguchi et al Reference Kawaguchi, Wilson, Augood and Emson79). These interneurons are thought to receive a strong excitatory input from the neocortex, as well as an inhibitory input from other striatal PV-IR cells.Reference Kawaguchi, Wilson, Augood and Emson79, Reference Wilson80 In addition to these inputs, PV-IR cells are reported to form electrical networks with one another via gap junctions,Reference Kawaguchi, Wilson, Augood and Emson79 which may allow for the formation of neural assemblies with other striatal fast-spiking interneurons.
The axons of striatal PV-IR cells have a basket-like arborization pattern,Reference Kawaguchi, Wilson, Augood and Emson79 with synapses directed at both the dendritic tree and the soma of MSNs.Reference Wilson80 Studies on the synaptic connections between this class of interneurons and MSNs have suggested that each MSN receives connections from multiple PV-IR cells.Reference Wilson80 Thus, given the ideas that multiple PV-IR interneurons form inhibitory synapses on the soma of each MSN cell, and further that these cells may easily form inhibitory neural ensembles, it can be expected that PV-IR interneurons will have a powerful inhibitory effect on the output of striatal MSNs. Moreover, this idea is consistent with electrophysiological studies that have suggested that the majority of inhibitory processes in the striatum are mediated by PV-IR cells.Reference Jaeger, Kita and Wilson81
Although there are at least 2 other types of GABAergic interneurons that have been chemically or morphologically identified in the striatum,Reference Kawaguchi, Wilson, Augood and Emson79 there is relatively little accumulated knowledge on the calretinin-IR and nitric oxide/neuropeptide Y/somatostatin-IR interneurons in the striatum, particularly in terms of their relationship to serotonergic neurotransmission. Therefore, this review will not consider these cell types further.
Cholinergic interneurons
Striatal non-GABAergic interneurons are differentiated from other striatal interneurons by a number of morphological, histochemical, and electrical properties, including a large soma with broadly branching dendritic and axonal arbors; the expression of the acetylcholine-producing enzyme choline acetyltransferase (ChAT); and a tonic, irregular firing pattern with low activation thresholds.Reference Kawaguchi, Wilson, Augood and Emson79 These cells receive excitatory inputs from the cortex and thalamus, although the thalamic inputs are thought to predominate in this cellular population.Reference Kawaguchi, Wilson, Augood and Emson79, Reference Goldberg and Reynolds82 Throughout this article, we will refer to this cellular population as ChAT-IR interneurons.
ChAT-IR interneurons modulate the behavior of other striatal neuronal populations in a complex manner, having a mix of excitatory and inhibitory effects on both MSN and PV-IR cell populations. These influences have been reviewed in detail in Goldberg and Reynolds,Reference Goldberg and Reynolds82 but for the purpose of understanding how serotonergic neurotransmission affects neuronal activity in the striatum, we will briefly discuss these effects here (summarized in Figure 4). ChAT-IR neurons have a fast excitatory influence on PV-IR cells that is mediated by nicotinic acetycholinergic receptors expressed on the soma.Reference Luo, Janssen, Partridge and Vicini83 However, this excitatory effect is balanced by a slower, inhibitory influence on GABA release that is mediated by muscarinic M2 receptors expressed on striatal GABA terminals.Reference Goldberg and Reynolds82 Additionally, Goldberg and ReynoldsReference Goldberg and Reynolds82 suggest that M2 receptors can have a similar effect on the release of glutamate from cortical or thalamic afferent terminals and that striatal ChAT-IR neurons can have a direct stimulatory effect on MSN firing that is mediated via muscarinic M1 receptors.
Medium spiny neurons
Whereas principle output cells in the cortex are excitatory glutamatergic pyramidal neurons, principle cells in the striatum are GABAergic in nature, and thus efferents originating from the striatum have an inhibitory effect on their projection regions. These striatal principle cells are MSNs, which are thought to represent between 80% and 98% of the neurons present in the neostriatum (for a review, see Tepper et al Reference Tepper, Koos and Wilson84). From a morphological perspective, MSNs are characterized by their medium size and spiny dendritic arbors.Reference Kawaguchi, Wilson and Emson85 MSNs can be further subdivided into at least 2 separate but equally represented populations based on neuropeptide and neurotransmitter receptor expression: those expressing enkephalin and dopamine (DA) D2 receptors, and those expressing dynorphin, substance P, and DA D1 receptors.Reference Augood and Emson86–Reference Pollack, Harrison, Wooten and Fink89 MSNs receive excitatory glutamatergic afferents from the neocortex and thalamus,Reference Wilson80 as well as serotonergic afferents from the midbrain raphe nuclei. However, the MSN subpopulations noted above preferentially send efferent projections to separate portions of the basal ganglia. MSNs expressing enkephalin and DA D2 receptors project preferentially to the globus pallidus, while those expressing dynorphin, substance P, and DA D1 receptors send efferents to the substantia nigra.Reference Gerfen, Engber and Mahan87, Reference Gerfen and Young88
In addition to MSN axon efferents sent to basal ganglia targets, MSN neurons also send axon collaterals to other MSN cells in the striatum that synapse in the dendritic arbor.Reference Kawaguchi, Wilson, Augood and Emson79 This phenomenon, in combination with the inhibitory nature of these cells, originally led to a hypothesis that striatal MSNs form a competitive lateral inhibitory network, where activation of one MSN leads to inhibition of surrounding MSNs. However, more recently this theory has been modified in light of electrophysiological evidence demonstrating that the inhibitory effect of a given MSN on the firing of surrounding MSNs is very weak, owing probably to the dendritic insertion of MSN-MSN axon collaterals.Reference Wilson80, Reference Jaeger, Kita and Wilson81 A modified theory suggests that lateral inhibition may still be possible in MSN networks, based on the fact that each MSN sends axon collaterals to about 1 in 6 of its surrounding MSNs and receives inputs from approximately 450 other local MSNs. In this theory, MSNs that are not connected to one another via axon collaterals but receive similar excitatory inputs may form neural assemblies that can inhibit surrounding MSNs in the aggregate (reviewed in WilsonReference Wilson80). Thus, it is possible that MSNs have a relatively weak lateral inhibitory influence on other MSNs in the striatum, but this is currently considered to represent a small minority of the total phasic inhibitory drive on striatal MSN activity.Reference Wilson80
In the following section we will review evidence on the regional and cellular pattern of 5-HT receptor expression for each of the above-mentioned striatal cell types.
Serotonergic receptor expression within the striatum
5-HT1 receptors
5-HT1A receptor binding in the caudate and putamen it is extremely lowReference Aznar, Qian, Shah, Rahbek and Knudsen29, Reference Pompeiano, Palacios and Mengod90 (Figure 2, panels A and B). Thus, although there may be some very weak expression of 5-HT1A receptors within the striatum of the rodent brain,Reference Ghavami, Stark, Jareb, Ramboz, Segu and Hen91 we will consider it to be essentially absent in this region.
In contrast, studies of 5-HT1B receptor expression in the striatum showed moderate to strong expression levels of mRNAReference Boschert, Amara, Segu and Hen92 as well as proteinReference Bruinvels, Palacios and Hoyer33, Reference Sari, Miquel and Brisorgueil35 (Figure 2, panels C and D), which may indicate that 5-HT1B receptors are postsynaptically expressed in the striatum or are expressed on interneurons. A mouse study by Ghavami et al,Reference Ghavami, Stark, Jareb, Ramboz, Segu and Hen91 using a conditional 5-HT1B receptor knockout approach, demonstrated that 5-HT1B receptors made by striatal MSNs are primarily sent to the basal ganglia projection regions. These data might suggest that striatal 5-HT1B receptors are localized primarily on terminals originating from the midbrain raphe.Reference Boschert, Amara, Segu and Hen92, Reference Sari93 However, dual-labeling immunohistochemistry has provided evidence demonstrating that 5-HT1B receptors are also present on striatal PV-IR and ChAT-IR interneurons.Reference Egeland, Warner-Schmidt, Greengard and Svenningsson38 It is not known whether there is a somatodendritic expression of 5-HT1B receptors in these cell types, and it is possible that 5-HT1B receptors act as terminal heteroreceptors in striatal PV-IR and ChAT-IR interneurons.
5-HT1D receptors are closely related to 5-HT1B receptors in the brain and often have a similar, although much more rarified, expression pattern. However, Bruinvels et al Reference Bruinvels, Palacios and Hoyer33 demonstrated that, in the striatum, 5-HT1D receptor specific binding constituted about 11% of the total binding of a radioligand that is nonselective for 5-HT1B/1D receptors. However, given evidence that 5-HT1D receptors form a heterodimer with 5-HT1B receptors when they are co-expressed,Reference Xie, Lee, O’Dowd and George94 it can be questioned whether these receptors should be considered separately.
Based on these data, striatal 5-HT1B receptors may act primarily to modulate extracellular concentrations of 5-HT by acting as an inhibitory autoreceptor at serotonergic terminals. Moreover, this idea is supported by data from microdialysis experiments.Reference de Groote, Klompmakers, Olivier and Westenberg36
If 5-HT1B receptors are present in the somatodendritic region of PV-IR and ChAT-IR interneurons, then stimulation of these receptors should drive down the activation state of these interneurons. Alternatively, 5-HT1B receptors expressed as terminal heteroreceptors in these cells may act to reduce release of GABA or acetylcholine, respectively, without markedly modulating cellular excitability at the soma. Which of these subcellular expression patterns is present in striatal interneurons is essentially unknown at this time.
5-HT2A/2C receptors
5-HT2A receptors are only moderately expressed in the striatum,Reference Cornea-Hebert, Riad, Wu, Singh and Descarries41 although it should be noted that at least one research group has noted a gradient in 5-HT2A receptor mRNA expression, with more dense expression in the caudal portions of the striatum.Reference Ward and Dorsa95 Cornea-Hebert et al Reference Cornea-Hebert, Riad, Wu, Singh and Descarries41 have confirmed 5-HT2A receptor immunoreactivity in the majority of striatal MSNs. Furthermore, Ward and DorsaReference Ward and Dorsa95 found that 5-HT2A receptor mRNA is present in MSNs that project to both the globus pallidus and substantia nigra. Cornea-Hebert et al Reference Cornea-Hebert, Riad, Wu, Singh and Descarries41 also observed some 5-HT2A receptor immunoreactivity in a population of neurons characterized by a large soma. These cells are presumably cholinergic interneurons based on the large soma.
5-HT2C receptors are present in the striatum at moderate levelsReference Abramowski, Rigo, Duc, Hoyer and Staufenbiel44 and are expressed in striatal MSN cells,Reference Ward and Dorsa95, Reference Eberle-Wang, Mikeladze, Uryu and Chesselet96 and once again do not appear to discriminate between MSN cells projecting to the globus pallidus vs the substantia nigra.Reference Ward and Dorsa95 Although we have not been able to identify any systematic immunohistochemical studies that have examined whether 5-HT2C receptors are present in striatal interneuron populations, electrophysiological experiments would suggest 5-HT2C receptor expression on striatal fast spiking interneuronsReference Blomeley and Bracci97 and ChAT-IR cells.Reference Bonsi, Cuomo and Ding98
Within the frontal cortex, 5-HT2A receptors have mixed effects on the behavior of the microcircuitry by activating pyramidal neurons and GABAergic interneurons. Within the striatum, a similar picture of the effects of 5-HT2A/2C receptor stimulation emerges, with activation of these receptors likely to stimulate the striatal MSN projection cells as well as PV-IR and ChAT-IR interneurons.
5-HT3 receptors
5-HT3 receptor expression in the striatum is extremely lowReference Gehlert, Gackenheimer, Wong and Robertson46 (Figure 2, panels E and F). There are reports of a few strongly 5-HT3 receptor-IR cells present within the striatumReference Morales, Battenberg and Bloom48; however, the identity of these cells is not known.
5-HT4 receptors
5-HT4 receptor expression is moderate to high in the striatum with an increasing rostro-caudal gradient.Reference Vilaro, Cortes and Mengod51, Reference Vilaro, Cortes, Gerald, Branchek, Palacios and Mengod99 Given that the regional profile of 5-HT4 receptor mRNA is very similar to that seen using autoradiography within the striatum,Reference Vilaro, Cortes and Mengod51, Reference Vilaro, Cortes, Gerald, Branchek, Palacios and Mengod99 it can be hypothesized that 5-HT4 receptors primarily have a somatodendritic expression pattern. Egeland et al Reference Egeland, Warner-Schmidt, Greengard and Svenningsson38 found that striatal PV-IR and ChAT-IR interneurons do not express 5-HT4 receptors. Given the prominence of 5-HT4 receptors in the striatum, it is therefore likely that MSN cells express 5-HT4 receptors; however, this awaits a definitive confirmation.
5-HT5 receptors
Striatal 5-HT5 receptor immunoreactivity is weak, and there is a paucity of evidence on the cell types expressing 5-HT5 receptors. The little that is available suggests that these receptors are expressed by MSNs but not cholinergic cells.Reference Oliver, Kinsey, Wainwright and Sirinathsinghji53 Thus, 5-HT5 receptor activation might have an inhibitory effect on striatal MSN activity, and in fact this is one of the few serotonergic inhibitory receptors that have been found in the striatum. It remains to be seen whether 5-HT5 receptors are present in any of the GABAergic interneurons of the striatum.
5-HT6 receptors
5-HT6 receptors are densely expressed in the striatum, where they are primarily associated with a postsynaptic, dendritic expression pattern.Reference Gerard, Martres and Lefevre55 5-HT6 receptors have been observed in striatal MSN cellsReference Ward and Dorsa95, Reference Helboe and de Jong100 in a manner that does not differentiate between MSNs projecting to the globus pallidus or the substantia nigra,Reference Ward and Dorsa95 continuing with the apparent theme that serotonergic receptors do not discriminate between these alternate projections to the basal ganglia. In human striatal tissue, Marazziti et al Reference Marazziti, Baroni and Pirone101 found that 5-HT6 receptors were present in putatively PV-IR cells. However, in rodent tissue, another group found no evidence of 5-HT6 receptor mRNA in PV-IR cells.Reference Helboe and de Jong100 Electrophysiological data have suggested the presence of 5-HT6 receptors in striatal cholinergic (ChAT-IR) interneurons.Reference Bonsi, Cuomo and Ding98 Thus, stimulation of striatal 5-HT6 receptors can be expected to activate striatal MSN and ChAT-IR interneurons, whereas it is unclear that these receptors are present in striatal PV-IR interneurons.
5-HT7 receptors
Within the striatum, Neumaier et al Reference Neumaier, Sexton, Yracheta, Diaz and Brownfield57 reported moderate levels of 5-HT7 receptor-like immunoreactivity, and 5-HT7 receptor-selective autoradiographic binding is present at relatively low levels in the rodent (see Figure 2, panels G and H) and human striatum.Reference Varnas, Thomas, Tupala, Tiihonen and Hall102 We have not been able to identify any anatomical studies that characterize the striatal cellular subtypes expressing 5-HT7 receptors. However, Bonsi et al Reference Bonsi, Cuomo and Ding98 found that cholinergic interneurons expressed 5-HT7 receptor mRNA, and further that the activating effect of 5-HT on striatal cholinergic cells could be attenuated by application of the 5-HT7 receptor selective antagonist SB269970. Thus, 5-HT7 receptors are likely to be expressed at the least in striatal ChAT-IR interneurons, where they have a stimulatory effect.
Serotonergic modulation of striatal neuronal subtypes and overall striatal output
PV-IR fast-spiking interneurons
In striatal PV-IR fast-spiking interneurons, there is evidence supporting the presence of excitatory 5-HT2C receptors as well as inhibitory 5-HT1B receptors. It is not known whether the 5-HT1B receptor immunoreactivity is associated with a somatodendritic expression, as has been observed in the hippocampus,Reference Peddie, Davies, Colyer, Stewart and Rodriguez39, Reference Peddie, Davies, Colyer, Stewart and Rodriguez40 or if it is expressed primarily as a terminal heteroreceptor. Additionally, it is unknown whether 5-HT1B and 5-HT2C receptors constitute the entire complement of serotonergic receptors expressed by striatal PV-IR interneurons. Furthermore, it is somewhat difficult to gauge the relative level of affinity at these targets, as the range of observed affinities for 5-HT at these 2 receptors overlaps (Table 1). Based on these uncertainties, it is unclear how physiological levels of 5-HT will alter the behavior of striatal PV-IR interneurons, although one might expect to find mixed effects of 5-HT stimulation. However, it should be noted that electrophysiological investigations into the effects of 5-HT stimulation on PV-IR interneuron activity have not found any 5-HT1B receptor mediated inhibitory currents in striatal PV-IR interneurons (see discussion below), which may either suggest this receptor is expressed as a terminal heteroreceptor, or may call its presence in these cells into question altogether. Taken together, these data may suggest that striatal PV-IR interneurons will be primarily excited by 5-HT, via a 5-HT2C receptor mediated mechanism.
Cholinergic interneurons
There is evidence supporting the presence of excitatory 5-HT2C, 5-HT6, 5-HT7, and inhibitory 5-HT1B receptors in striatal ChAT-IR interneurons. This cellular profile may suggest that 5-HT will bias striatal ChAT-IR interneurons toward excitation.
Medium spiny neurons
In striatal MSNs, the available evidence primarily supports the presence of excitatory 5-HT receptors, including 5-HT2A, 5-HT2C, 5-HT4, and 5-HT6 receptors, although inhibitory 5-HT5 receptors are also present. Given that 5-HT5 receptors are expressed in a relatively weak manner in the striatum, and have a relatively low affinity for 5-HT by comparison to at least 5-HT2A, 5-HT4, and 5-HT6 receptors (Table 1), it is likely that 5-HT will have primarily excitatory direct effects on MSN firing behavior.
Electrophysiological effects of 5-HT in the striatum
In vivo electrophysiology
Although the available evidence suggests that primarily excitatory serotonergic receptor targets are expressed on the major cell types in the striatal circuitry, studies of the effects of 5-HT on neuronal firing have overwhelmingly shown an inhibitory effect. For example, Rebec and CurtisReference Rebec and Curtis103 found in paralyzed (not anesthetized) rodents that 5-HT application by microinjection at a 10 µM concentration suppressed firing at neurons close to the microinjection cannula. Similar results were found in other studies that investigated this issue,Reference El Mansari, Radja, Ferron, Reader, Molina-Holgado and Descarries104–Reference Olpe and Koella106 although in some neurons a brief excitation was followed by inhibition.Reference El Mansari, Radja, Ferron, Reader, Molina-Holgado and Descarries104 It should be noted that in at least one case, more units were excited than inhibited.Reference Contreras, Sanchez Estrada, Molina Hernandez and Marvan107 The identities of the recorded units in these in vivo experiments were not further characterized, but, given the ascendant proportion of cells that MSNs represent within the striatum, it is most likely that the recorded neurons are primarily MSNs. Interestingly, although excitatory 5-HT2A receptors are present on MSNs, and further that 5-HT2C receptors are present on all 3 major striatal neuronal types discussed here, 5-HT2A/2C receptor agonists mimicked the overwhelmingly inhibitory effects of 5-HT application. We were not able to identify any in vivo electrophysiology studies that examined the effects of 5-HT on identified fast-spiking interneurons or cholinergic interneurons within the striatum.
In vitro electrophysiology
In the one in vitro study we identified that attempted to examine the response of MSNs to 5-HT in acutely dissociated cells, the authors found that the majority of cells were excited by the bath application of 5-HT at 10–60 µM concentrations. This result stands in stark contrast to the behavior of putative MSNs recorded in vivo using anesthetized and paralyzed rats, but is in line with the primarily excitatory complement of 5-HT receptors that has been found on striatal MSNs. Given that the in vivo experiments reported above used similarly high 5-HT concentrations, the most probable explanation for this difference lies in the fact that the recorded MSNs were acutely dissociated from the striatal network, which divorces the subject cell from the inhibitory cells that would normally govern its behavior.
Blomeley and BracciReference Blomeley and Bracci97 used a slice electrophysiology preparation to demonstrate that high concentrations of 5-HT (30 µM) strongly increased the excitability of striatal fast-spiking interneurons. These authors further demonstrated that this excitatory response was blocked by a 5-HT2C receptor antagonist. Moreover, this research group did not report the presence of any 5-HT mediated inhibitory currents in these cells that could be attributed to 5-HT1B receptors, which would seem to be at odds with the somatic 5-HT1B receptor expression observed in striatal PV-IR interneurons by Egeland et al.Reference Egeland, Warner-Schmidt, Greengard and Svenningsson38
In vitro electrophysiological evidence from at least 2 research labs also supports the idea that high concentrations of 5-HT have an excitatory effect on striatal cholinergic interneurons. Blomeley and BracciReference Blomeley and Bracci108 found that 30 µM 5-HT excited striatal cholinergic cells and further that the 5-HT2 receptor antagonist ketanserin antagonized this effect. Similarly, Bonsi et al Reference Bonsi, Cuomo and Ding98 found that 50 µM 5-HT excited striatal cholinergic interneurons, that the 5-HT2A/2C receptor agonist DOI mimicked this effect, and finally that a 5-HT2C receptor antagonist reversed this excitation. This group also showed that the excitatory effect of 5-HT could be reduced by 5-HT6 and 5-HT7 receptor selective antagonists.
Hypothesized effects of 5-HT, SSRIs, and vortioxetine on the overall striatal circuit
5-HT and SSRIs
The electrophysiological data on the effects of 5-HT on striatal PV-IR and ChAT-IR firing suggest that 5-HT has an excitatory effect on these cell types. Additionally, the profile of 5-HT receptor expression on striatal MSN cells also suggests that 5-HT should have primarily excitatory direct effects. This is supported by data showing that 5-HT activates MSN cell firing when these cells are dissociated from the striatal network, but it is firmly at odds with the electrophysiological effects of 5-HT on putative MSN cells recorded in vivo.
The most plausible explanation for this apparently paradoxical effect of 5-HT on striatal MSN activity is that the concomitant activation of striatal PV-IR interneurons has a downstream inhibitory action on MSNs that overwhelms 5-HT’s direct excitatory effects on these cells. This reasoning is based on the fact that striatal PV-IR interneurons have inhibitory axo-somatic insertions on numerous MSN cells, which will tend to powerfully and negatively modulate MSN activity. Furthermore, each MSN cell receives inhibitory inputs from multiple PV-IR interneurons. This phenomenon, along with the presence of electrical gap junctions between striatal PV-IR cells, will tend to multiply the inhibitory influence of PV-IR interneurons on MSN behavior. It is possible that some of 5-HT’s effects on MSN firing are also mediated via striatal ChAT-IR interneurons. However, ChAT-IR interneuron activation is expected to have a mixture of excitatory and inhibitory effects that seem unlikely to clearly bias MSN output in either direction. Therefore, the strong inhibitory effect of 5-HT on MSN firing is most likely mediated primarily through PV-IR interneuron activation.
Based on this line of reasoning, we hypothesize that SSRIs will have primarily inhibitory local effects on striatal MSN firing under acute conditions. Given that 5-HT2C receptors are the only excitatory serotonergic targets identified on striatal PV-IR interneurons, the degree to which this local inhibitory effect of SSRIs is maintained after chronic administration might be dictated by the degree to which 5-HT2C receptors desensitize or downregulate in response to long-term stimulation. Thus, it is possible that the local inhibitory effect of SSRIs on MSN firing will be decreased after chronic administration. Additionally, given that SSRIs tend to reduce cortical pyramidal neuron firing after chronic administration, striatal PV-IR cells may also receive an attenuated excitatory drive from the cortex after chronic SSRI treatment. Thus, it seems plausible to hypothesize that long-term SSRI treatment will attenuate the reduction in MSN firing. However, we were unable to identify in vivo or in vitro electrophysiological studies of the effects of acute or long-term SSRI administration on striatal neuron firing.
Vortioxetine
Although vortioxetine has markedly different effects by comparison to SSRIs within the frontal cortex, this may not be the case within the striatum. 5-HT3 and 5-HT1A receptors are largely absent in the striatum, and thus cannot be expected to modulate the effects of this network locally. 5-HT7 receptors, where vortioxetine acts as an antagonist, have been confirmed in striatal ChAT-IR interneurons, and thus vortioxetine may remove some of the excitatory influence of 5-HT on this cell population. But it is seems unlikely that altered activation of striatal ChAT-IR interneurons will clearly bias MSN output in either direction. 5-HT1B receptors, where vortioxetine functions as a partial agonist in vivo,Reference El Mansari, Lecours and Blier76 are thought to primarily exist in the striatum as terminal autoreceptors. Thus vortioxetine’s primary 5-HT1B receptor mediated effect in this region will probably be an enhancement of extracellular 5-HT by comparison to an SSRI. However, the question of whether vortioxetine drives greater extracellular 5-HT concentrations in the striatum than an SSRI is currently untested. Additionally, if 5-HT1B receptors are expressed in a meaningful way on striatal PV-IR interneurons, then vortioxetine’s 5-HT1B receptor partial agonism may have the effect of attenuating the only inhibitory influence 5-HT would be expected to have on PV-IR interneurons, thereby increasing their excitability and further driving down the activity of MSNs.
Therefore, when considering local effects of vortioxetine within the striatum only, the emerging theme is that vortioxetine will increase striatal PV-IR interneuron activation and reduce the output of striatal MSN projection neurons downstream. Moreover, these inhibitory effects on striatal MSN behavior will be qualitatively similar to those expected from SSRIs. However, the question of how vortioxetine may modulate striatal neuron activity is complicated by the fact that striatal MSN and PV-IR interneurons receive excitatory inputs from the cortex,Reference Wilson80 where vortioxetine increases activity in projection neurons.Reference Riga, Celada, Sanchez and Artigas18 Thus, vortioxetine may have both local and non-local effects that will tend to activate striatal PV-IR interneurons, while in MSN cells, vortioxetine’s local GABA-mediated inhibitory effects and nonlocal excitatory effects may be at odds with one another. These contradictory actions may suggest that vortioxetine will produce a mix of excitatory and inhibitory effects on MSN activity, but we hypothesize that these effects will skew toward inhibition of MSN firing.
Conclusions
The recurrent theme underlying this article is that 5-HT modulates GABA and glutamate neurotransmission differently from brain region to brain region, based simply on the circumscribed expression pattern exhibited by each serotonergic target. Using a more complex analysis of receptor expression and electrophysiological data, we have generated several hypotheses about how 5-HT in general and vortioxetine in particular will modulate the behavior of GABAergic and glutamatergic cells in the PFC and striatum.
Within the PFC, we have hypothesized that acute SSRI administration will have mixed excitatory and inhibitory effects on GABAergic interneurons and principle pyramidal neuron firing that will not clearly bias the output of the overall network strongly in either direction. However, chronic SSRI administration may lead to a reduction in glutamatergic pyramidal neuron firing. Within the striatum, SSRIs will acutely reduce the activity of MSNs by enhancing the activity of GABAergic fast-spiking interneurons, and this effect may be attenuated by chronic administration. Recent computational modeling studies have suggested that increased activity in fast-spiking interneurons and the resulting reduction in MSN activity may contribute to reduced impulsive behavior.Reference Bahuguna, Aertsen and Kumar109 Although, to our knowledge, this idea has not yet been empirically evaluated, it is consistent with preclinical studies showing that SSRIs reduce some aspects of impulsivity.Reference Baarendse and Vanderschuren110, Reference Humpston, Wood and Robinson111
By contrast, vortioxetine is hypothesized to reduce PFC GABAergic inhibitory tone through a variety of serotonergic mechanisms, including 5-HT3 receptor antagonism, leading to an increase in the activity of excitatory glutamatergic pyramidal projection neurons. This notion is supported by electrophysiological recordings showing that vortioxetine has strong excitatory effects on putative pyramidal neurons.Reference Riga, Celada, Sanchez and Artigas18 This increase in cortical glutamate neurotransmission is thought to be responsible in part for vortioxetine’s beneficial effects on some aspects of cognitive function.Reference Pehrson and Sanchez16 Locally within the striatum, vortioxetine is hypothesized to have qualitatively similar effects as SSRIs, ie, enhancing the activity of striatal GABAergic PV-IR interneurons, leading to an overall reduction in the activation of striatal MSNs. But vortioxetine’s effects on striatal MSN function may also be due in part to effects driven by the enhancement of frontal cortex pyramidal neurons, which are thought to make excitatory connections with striatal PV-IR and MSN cells. Thus, the combination of local and nonlocal effects of vortioxetine may ultimately lead to mixed excitatory and inhibitory effects on striatal MSN output, although in our view, vortioxetine is most likely to bias MSN function toward inhibition. This hypothesis clearly needs to be confirmed by empirical data.
Future research should evaluate these hypotheses in further detail by examining the effects of vortioxetine on cortical and striatal signaling pathways. Additionally, if technically feasible, then future studies should investigate vortioxetine’s effects on GABA and glutamate neurotransmission (eg, pyramidal neuron firing) in awake animals during the conduct of a cognitive task. Ideally, it will be important to include translational demonstrations of these effects of vortioxetine in humans. However the technical feasibility of such a demonstration is uncertain, given current limitations in non-invasively measuring GABA and glutamate neurotransmission.
Limitations
There are a number of important limitations to consider when evaluating this body of work. First and foremost, there is a relative paucity of cell-type specific 5-HT receptor expression or electrophysiological data available for the striatum, which implies uncertainty around hypotheses generated for the effects of 5-HT, SSRIs, and vortioxetine. Additionally, we do not currently have electrophysiological data on vortioxetine’s effects in the striatum with which to check the validity of these hypotheses. Furthermore, the available electrophysiological data on how 5-HT modulates striatal neurotransmission are generally characterized by the use of high concentrations, which may not be physiologically relevant. It is important to note that the hypotheses of the effects of 5-HT, SSRIs, and vortioxetine are primarily developed using anatomical and electrophysiological data from rodents. The biology that drives serotonergic modulation of GABA and glutamate neurotransmission may not translate across species into humans. Additionally, vortioxetine’s affinities for some of its receptor targets, most notably 5-HT1A and 5-HT7 receptors, are approximately 10-fold weaker at the rodent versions than they are at their human counterparts.Reference Paxinos and Watson113 Therefore, the degree to which these concepts of serotonergic modulation of GABA and glutamate neurotransmission will translate into humans is unknown at this time.
Disclosures
Alan L. Pehrson, Theepica Jeyarajah, and Connie Sanchez are employees of Lundbeck Research USA, Inc.




