Introduction
Cystic echinococcosis (CE) is ranked second in the list of food-borne parasites worldwide, listed among the most severe parasitic diseases in humans (World Health Organization and Food and Agriculture Organization of the United Nations, 2014) and prioritized by the World Health Organization as one of the 17 neglected tropical diseases (WHO, 2015). It is also known to be the most important parasitic zoonosis in the Mediterranean region (Dakkak, Reference Dakkak2010; Ismail et al., Reference Ismail, Eassa, Mahgoub and El-Dib2018; Borhani et al., Reference Borhani, Fathi, Lahmar, Ahmed, Abdulhameed and Harandi2020), with stable endemicity in the Middle East due to the high diversity of intermediate hosts, mainly sheep and goats, followed by camels and cattle (Al Kitani et al., Reference Al Kitani, Al Riyami, Al Yahyai and Hussain2015). CE is creating a serious public health problem and is a burden to national economy for most countries in this region (Grosso et al., Reference Grosso, Gruttadauria, Biondi, Marventano and Mistretta2012; Thys et al., Reference Thys, Sahibi, Gabriël, Rahali, Lefèvre, Rhalem, Marcotty, Boelaert and Dorny2019).
CE occurs as a result of infection at the larval stage of the tapeworms belonging to the species complex Echinococcus granulosus sensu lato (s.l.). To complete the lifecycle, the cestode needs as intermediate hosts a wide range of herbivorous and omnivorous mammals and as definitive hosts dogs and wild canids (Thompson et al., Reference Deplazes, Rinaldi, Alvarez Rojas, Torgerson, Harandi, Romig, Antolova, Schurer, Lahmar, Cringoli, Magambo, Thompson and Jenkins2017; Kinkar et al., Reference Kinkar, Laurimäe, Balkaya, Casulli, Zait, Irshadullah and Saarma2018a). The adult worm develops in the small intestine of the definitive host while the larval stage grows into a hydatid cyst in intermediate hosts, causing CE (Kinkar et al., Reference Kinkar, Laurimäe, Balkaya, Casulli, Zait, Irshadullah and Saarma2018a; Thompson, Reference Thompson2020). Transmission of the infection occurs through egg ingestion either by direct contact with definitive hosts or indirectly by drinking water or consuming contaminated fruits and vegetables (Possenti et al., Reference Possenti, Manzano-Román, Sánchez-Ovejero, Boufana, La Torre, Siles-Lucas and Casulli2016). However, the infection in definitive hosts does not cause morbidity, while it is considered severe in intermediate hosts (including humans) (WHO, 2020).
The taxonomy of E. granulosus s.l. has been a challenging issue for decades. Studies have identified a number of genotypes/species within E. granulosus s.l. (Knapp et al., Reference Knapp, Nakao, Yanagida, Okamoto, Saarma, Lavikainen and Ito2011; Kinkar et al., Reference Kinkar, Laurimäe, Acosta-Jamett, Andresiuk, Balkaya, Casulli, Gasser, González, Haag, Zait, Irshadullah, Jabbar, Jenkins, Manfredi, Mirhendi, M'rad, Rostami-Nejad, Oudni-M'rad, Pierangeli, Ponce-Gordo, Rehbein, Sharbatkhori, Kia, Simsek, Soriano, Sprong, Šnábel, Umhang, Varcasia and Saarma2018b) on the basis of mitochondrial DNA. At least 10 strains (G1–10) of E. granulosus s.l. have been described, forming four major clades (G1–G3, G4, G5 and G6–G10) (Nakao et al., Reference Nakao, Lavikainen, Yanagida and Ito2013). Some of these genotypes are now considered as distinct species: E. granulosus sensu stricto (s.s.; comprising genotypes G1 and G3) (Kinkar et al., Reference Kinkar, Laurimäe, Acosta-Jamett, Andresiuk, Balkaya, Casulli, Gasser, González, Haag, Zait, Irshadullah, Jabbar, Jenkins, Manfredi, Mirhendi, M'rad, Rostami-Nejad, Oudni-M'rad, Pierangeli, Ponce-Gordo, Rehbein, Sharbatkhori, Kia, Simsek, Soriano, Sprong, Šnábel, Umhang, Varcasia and Saarma2018b), Echinococcus equinus (G4) and Echinococcus ortleppi (G5) (Thompson and McManus, Reference Thompson and McManus2002), whereas the status of genotypes G6–G10 is under dispute (Lymbery et al., Reference Lymbery, Jenkins, Schurer and Thompson2015; Nakao et al., Reference Nakao, Lavikainen and Hoberg2015; Laurimäe et al., Reference Laurimäe, Kinkar, Romig, Omer, Casulli, Umhang, Gasser, Jabbar, Sharbatkhori, Mirhendi, Ponce-Gordo, Lazzarini, Soriano, Varcasia, Rostami Nejad, Andresiuk, Maravilla, González, Dybicz, Gawor, Šarkūnas, Šnábel, Kuzmina and Saarma2018). Some authors have proposed the consideration of G6–G10 provisionally as single species, Echinococcus canadensis awaiting further data from the nuclear genome (Nakao et al., Reference Nakao, Lavikainen, Yanagida and Ito2013; Addy et al., Reference Addy, Wassermann, Kagendo, Ebi, Zeyhle, Elmahdi, Umhang, Casulli, Harandi, Aschenborn, Kern, Mackenstedt and Romig2017), while others consider them as two distinct species: G6/G7 as Echinococcus intermedius and G8/G10 as E. canadensis (Thompson, Reference Thompson2008; Saarma et al., Reference Saarma, Jõgisalu, Moks, Varcasia, Lavikainen, Oksanen, Simsek, Andresiuk, Denegri, Gonzalez, Ferrer, Garate, Rinaldi and Maravilla2009; Laurimäe et al., Reference Laurimäe, Kinkar, Romig, Omer, Casulli, Umhang, Gasser, Jabbar, Sharbatkhori, Mirhendi, Ponce-Gordo, Lazzarini, Soriano, Varcasia, Rostami Nejad, Andresiuk, Maravilla, González, Dybicz, Gawor, Šarkūnas, Šnábel, Kuzmina and Saarma2018) or even as three species: G6/G7 as E. intermedius, G8 as Echinococcus borealis and G10 as E. canadensis (Lymbery et al., Reference Lymbery, Jenkins, Schurer and Thompson2015).
In the Middle East, the most dominant species responsible for CE infections in both wild and domestic animals is E. granulosus s.s. (G1–G3) (Kim et al., Reference Kim, Yong, Shin, Lee, Park, Suvonkulov, Kovalenko and Yu2020) followed by genotype G6 (Rostami et al., Reference Rostami, Torbaghan, Dabiri, Babaei, Mohammadi, Sharbatkhori and Harandi2015; Khademvatan et al., Reference Khademvatan, Majidiani, Foroutan, Hazrati Tappeh, Aryamand and Khalkhali2019). Information on strains and species from the Middle East, especially Iraq, Lebanon, Palestine, Syria and the Persian Gulf countries, is currently scant (Thompson, Reference Thompson2017). Regarding Lebanon, in 1930 the prevalence of 41.5% CE in cattle was reported in Beirut (Goodale and Krischner, Reference Goodale and Krischner1930; Matossian et al., Reference Matossian, Rickard and Smyth1977). In 1936, the epidemiological surveys conducted in Beirut revealed a prevalence of 22.1% in sheep and 45.1% in cattle (Turner et al., Reference Turner, Dennis and Kassis1936a), and 20–25% in dogs (Turner et al., Reference Turner, Berberian and Dennis1936b). Another study demonstrated prevalence rates of 11.6% in sheep, 67.4% in camels, 47% in cattle and 11.75% in dogs (Pipkin et al., Reference Pipkin, Rizk and Balikian1951). Another survey undertaken in 1963 reported that almost one-third of mature swine, one-third of cattle and one-fourth of sheep and goats were infected with CE in Beirut (Luttermoser and Koussa, Reference Luttermoser and Koussa1963). In 1964, the prevalence rates of echinococcosis were 100% in camels and 42% in donkeys in Lebanon and Syria, whereas an infection rate of 28% was documented in dogs in Lebanon (Dailey et al., Reference Dailey, Sweatman and Schacher1966). These historical reports concerning CE in Lebanon suggest the endemic nature of CE in the country (Frayha, Reference Frayha1970).
CE has been an important public health problem in Lebanon; however, no recent information is available regarding the prevalence and impact of the different genotypes of E. granulosus s.l. in the country. Moreover, Lebanon has a high number of free-ranging dogs, illegal slaughtering and other cultural and socioeconomic conditions that can contribute to the transmission and perpetuation of CE, particularly in sheep and goats. Therefore, the aim of this study was to investigate the prevalence of CE in sheep and goats of Lebanon and determine genotypes of isolated metacestodes to get a better understanding of E. granulosus s.l. species and genotypes that are represented in this part of the Mediterranean region.
Materials and methods
It was decided to proceed through a multidimensional approach combining: (i) exhaustive sampling and post-mortem inspection of slaughtered animals, (ii) morphological characterization of hydatid cyst, (iii) statistical analysis of the gathered data and (iv) molecular identification of the circulating genotypes.
Sample collection
According to the Raosoft sample size calculator (http://www.raosoft.com/samplesize.htm), the sample size number was determined in a population of 400 000 sheep and 400 000 goats, which supposed prevalence for CE of 60% and confidence level of 95%.
During three consecutive years (2018, 2019 and 2020), 369 sheep and 335 goats have been examined in various abattoirs from five different Lebanese regions (Mount Lebanon, Beirut, Bekaa, North and South of Lebanon). The animals’ ages were estimated by examining teeth, and only animals above 2 years old were enrolled in this study (Varcasia et al., Reference Varcasia, Canu, Kogkos, Pipia, Scala, Garippa and Seimenis2007).
Liver and lungs were examined for cysts by post-mortem visual inspection and palpation. When hydatid cysts were found, these were counted and examined to verify exact location, fertility and morphological characteristics.
Hydatid cysts were classified as fertile, sterile, calcified or caseous on the basis of morphological examination. Fertility was determined by microscopic observation, without staining protoscolices, at 10× to assess morphologic characteristics along with protoscoleces’ flame cell movements (Varcasia et al., Reference Varcasia, Canu, Lightowlers, Scala and Garippa2006). Protoscoleces and germinal layers were then removed and preserved in ethanol (95%) before being further analysed.
Statistical analysis
Data were recorded on a spreadsheet (Excel® Microsoft Corp., Redmond, WA, USA) and prevalence values were calculated according to the type of hydatid cyst (fertile, sterile, caseous and calcified cysts). Differences in prevalence were statistically tested using the chi-square test for independence (Epi-Info® 7.0, CDC/WHO, Atlanta, GA, USA).
Molecular studies
DNA was extracted from 77 samples of hydatid material obtained from sheep (65) and goats (12) using NucleoSpin Tissue (Macherey-Nagel GmbH & Co. KG, Düren, North Rhine-Westphalia, Germany). Germinal layers of the hydatid cysts were lysed using proteinase K and the samples were incubated at 56°C until digestion of the membrane. Following digestion, absolute ethanol was added to each sample and the sample was transferred to the NucleoSpin Tissue column and centrifuged at 12 000 g for 1 min. Samples were subsequently washed with buffers provided in the kit and finally the DNA was eluted in a pre-labelled Eppendorf tube with BE buffer at 12 000 g centrifugation. Polymerase chain reaction (PCR) was conducted using primer pairs F/COI (TTGAATTTGCCACGTTTGAATGC) and R/COI (GAACCTAACGACATAACATAATGA) for the amplification of partial mitochondrial cox1 gene (Nakao et al., Reference Nakao, Sako, Yokoyama, Fukunaga and Ito2000). Twenty-nine isolates were further screened through nad5 mitochondrial gene employing the primers EGnd5F1 (GTTGTTGAAGTTGATTGTTTTGTTTG) and EGnd5R1 (GGAACACCGGACAAACCAAGAA) for the correct identification of genotypes G1 and G3 (Kinkar et al., Reference Kinkar, Laurimäe, Acosta-Jamett, Andresiuk, Balkaya, Casulli, Gasser, González, Haag, Zait, Irshadullah, Jabbar, Jenkins, Manfredi, Mirhendi, M'rad, Rostami-Nejad, Oudni-M'rad, Pierangeli, Ponce-Gordo, Rehbein, Sharbatkhori, Kia, Simsek, Soriano, Sprong, Šnábel, Umhang, Varcasia and Saarma2018b). A G7 isolate was also amplified using G7for (GTGTTGTGTTGTTGATAGATTG) and G7rev (GTAAAAATAATCACCACCCAAC) primers for nad2 gene (Laurimäe et al., Reference Laurimäe, Kinkar, Varcasia, Dessì, Sgroi, D'alessio, Veneziano and Saarma2019).
PCR products were purified using a NucleoSpin Gel and PCR cleanup (Macherey-Nagel GmbH & Co. KG, Düren, North Rhine-Westphalia, Germany) and sent to an external sequencing service (Eurofins Genomics, Ebersberg, Germany) for bidirectional sequencing.
The base-calling errors on sequenced chromatograms were checked on Finch TV viewer (Geospiza Inc., Seattle, WA, USA). Reference sequence (GenBank number: MG672237) from Kinkar et al. (Reference Kinkar, Laurimäe, Acosta-Jamett, Andresiuk, Balkaya, Casulli, Gasser, van der Giessen, González, Haag, Zait, Irshadullah, Jabbar, Jenkins, Kia, Manfredi, Mirhendi, M'rad, Rostami-Nejad, Oudni-M'rad, Pierangeli, Ponce-Gordo, Rehbein, Sharbatkhori, Simsek, Soriano, Sprong, Šnábel, Umhang, Varcasia and Saarma2018c) was used for multiple alignment of all E. granulosus s.s. sequences, whereas E. canadensis sequence was aligned with a reference sequence (GenBank number: MH301020) from Laurimäe et al. (Reference Laurimäe, Kinkar, Romig, Omer, Casulli, Umhang, Gasser, Jabbar, Sharbatkhori, Mirhendi, Ponce-Gordo, Lazzarini, Soriano, Varcasia, Rostami Nejad, Andresiuk, Maravilla, González, Dybicz, Gawor, Šarkūnas, Šnábel, Kuzmina and Saarma2018). Cox1 dataset (795 bp) for E. granulosus s.s. was exported to DnaSP (Rozas et al., Reference Rozas, Ferrer-Mata, Sánchez-Del Barrio, Guirao-Rico, Librado, Ramos-Onsins and Sánchez-Gracia2017) for the analysis of basic genetic variability indices (polymorphism, number of mutations, singleton variable and parsimony informative sites). For computation of haplotype and nucleotide diversities, neutrality indices (Tajima's D and Fu's F s) and genetic differentiation (F st), the data were analysed using the package Arlequin 3.5 (Excoffier and Lischer, Reference Excoffier and Lischer2010). Haplotype network formation was executed on PopArt software using a median joining network (Leigh and Bryant, Reference Leigh and Bryant2015). The estimation of pairwise divergence was performed on MEGA X software (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018).
Results
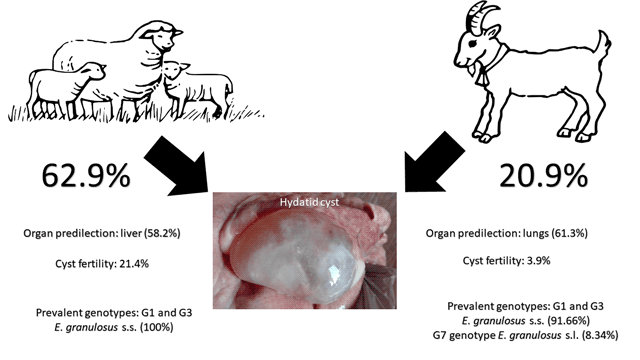
Among 369 examined sheep, 232 sheep were found positive (62.9%) with a total number of 2707 hydatid cyst. Parasitological examination showed a fertility rate of 21.4%: 79 out of the 369 examined animals harboured at least one fertile hydatid cyst. The prevalence of sheep infected with caseous cysts, calcified cysts and sterile cysts was 11.6, 54.5 and 24.7%, respectively. The overall prevalence of hydatid cyst infection and the prevalence of the different cyst categories are summarized in Table 1.
Table 1. Percentage of types of hydatid cysts in infected Lebanese sheep and goats

Note: A significant number of sheep and goats show more than one type of hydatid cyst. Only a restrained number of observed targets are limited to one type of hydatid cyst.
The presence of hydatids in sheep varied between the main target organs, showing a prevalence of 44.4% (164/369) in lungs and higher (48.5%; 179/369) in the liver (χ 2 = 1.2256; P = 0.2682). Hydatid cysts were found in both liver and lungs in 30.1% of the examined animals (111/369). Cyst abundance (number of cysts/examined animal) was 7.3 (range: 0–129), while cyst intensity (number of hydatids/positive animal) was 11.6. Of the infected animals, 32% had 1–5 cysts, while 21.9% of animals had massive infection harbouring 10–129 cysts. The fertility rate of hydatid cyst was higher in lungs (17.1%) compared with the liver (14.6%) but no statistical differences were observed (χ 2 = 0.8227; P = 0.3643).
In relation to the morphology of the recovered hydatid cyst, 49.5% of the hydatids were calcified, while 23.9, 19.2 and 7.4% were found to be fertile, sterile and caseous, respectively. Looking at all the 2707 recovered hydatid cysts in the 369 concerned sheep, it was underlined that 41.8% (1132/2707) were prevalent in lungs and 58.2% (1575/2707) in the liver. Conversely, fertile cysts were found more often in lungs (13.4%) compared with the liver (10.5%) (Table 2).
Table 2. Number and percentage of the different categories of hydatid cysts and site of infections in Lebanese sheep and goats

Among 335 investigated goats, 70 goats were CE positive, with an overall prevalence of 20.9% and a total number of 204 hydatid cyst. Parasitological examination showed a fertility rate of 3.9% (13/335) in the examined animals. The prevalence of sheep infected with caseous cysts, calcified cysts and sterile cysts was 3.3, 12.5 and 1.2%, respectively (Table 1). Contrary to what was found in sheep, the prevalence of hydatids in goats was found to be higher in lungs (11.9%; 40/335) than in the liver (9.9%; 30/335), but no statistical differences were observed (χ 2 = 1.5952; P = 0.2065). Hydatid cysts were found in both liver and lungs only in 0.9% of examined animals (3/335). None of the examined animals showed massive infection and hydatid cyst abundance.
The abundance was 0.6 (range: 0–9), while the mean intensity was 2.9. Fertility rate was higher in lungs (3.0%; 10/335) compared to the liver (1.8%; 6/335) having no statistical difference (χ 2 = 1.0245; P = 0.3114). In relation to the morphology of the recovered hydatid cyst, 64.7% of the hydatids were calcified, while 14.2, 11.8 and 9.3% were found to be caseous, fertile and sterile, respectively. The presence of hydatids in goats varied when considering the examined organs, showing that 61.3% (125/204) of hydatid cyst were found in lungs and 38.7% (79/204) in the liver. Fertile cysts were found more often in lungs (7.4%) compared with the liver (4.4%) (Table 2).
In this study, 77 hydatid cyst isolates originating from sheep (n = 65) and goats (n = 12) from five different regions of Lebanon were subjected to genetic analysis. Molecular characterization of these isolates was based on partial mitochondrial cox1 gene (795 bp) and yielded sufficient polymorphism for further population genetics analysis. It was identified that the majority of isolates (n = 76; 98.7%) belonged to E. granulosus s.s., of which G1 genotype was more prevalent (n = 72; 94.73%); G3 was identified from only four isolates (5.27%) belonging to sheep. Genotypic assessment was further confirmed through nad5 gene (680 bp; Kinkar et al., Reference Kinkar, Laurimäe, Acosta-Jamett, Andresiuk, Balkaya, Casulli, Gasser, González, Haag, Zait, Irshadullah, Jabbar, Jenkins, Manfredi, Mirhendi, M'rad, Rostami-Nejad, Oudni-M'rad, Pierangeli, Ponce-Gordo, Rehbein, Sharbatkhori, Kia, Simsek, Soriano, Sprong, Šnábel, Umhang, Varcasia and Saarma2018b) sequencing of 29 isolates and the presence of similar genotypes was reaffirmed. One isolate (1.3%) originating from goat was identified as G7 genotype and was found 100% similar to G7 sequence (MH301020) for the partial cox1 region, whereas nad5 and nad2 mitochondrial gene sequences reflected similarity to G7b at five fixed positions for this haplogroup [804 and 1060 bp (nad5); 6491, 6524 and 6620 bp (nad2); Laurimäe et al., Reference Laurimäe, Kinkar, Romig, Omer, Casulli, Umhang, Gasser, Jabbar, Sharbatkhori, Mirhendi, Ponce-Gordo, Lazzarini, Soriano, Varcasia, Rostami Nejad, Andresiuk, Maravilla, González, Dybicz, Gawor, Šarkūnas, Šnábel, Kuzmina and Saarma2018]. Because of only single sequence of E. canadensis (G7 genotype), it was subsequently excluded from the analysis covering E. granulosus s.s. population among the intermediate hosts.
The analysed cox1 region (795 bp) contained 24 point mutations at 24 segregating loci of which 17 (70.83%) were singleton sites and seven (29.17%) were parsimony informative. Parsimony informative sites contain at least two nucleotide variants which appear twice or more within the population for a given gene sequence. No insertions or deletions were observed in the sequences. Overall, there were more transitions (n = 23, 95.83%) than the transversions (n = 1, 4.17%) in the variable sites. The polymorphic loci had 10 non-synonymous (41.66%) and 14 synonymous substitutions (58.33%). In total, 22 haplotypes were found in 76 isolates for E. granulosus s.s. Discerning genealogical relationships between the haplotypes, it was identified that two haplotypes, EgLEB1 and EgLEB2, predominated the population appearing in 31 (40.78%) and 21 (27.63%) isolates, respectively (Fig. 1). G1 genotype was represented by 18 haplotypes (81.81%), whereas G3 genotype was represented by four haplotypes (18.19%) which were present as singleton variants. The number of mutational differences between the most common G1 haplotype and others ranged from one to five. Ten haplotypes had more than one nucleotide variation and seven of these microvariants shared similar mutation to that of the second most common haplotype, LEB2. Majority of the haplotypes (77.27%) occurred as singleton variants appearing only once in the population. Haplotypes identified on the basis of partial cox1 gene were also submitted to the NCBI database under the accession numbers: MW428227–MW428248 (Table 3).

Fig. 1. Haplotypic structure of Echinococcus granulosus s.s. among sheep and goats of Lebanon. Hatch marks correspond to the number of mutations between haplotypes and size of the circle indicates frequency of each haplotype (see also Table 4). Black dots represent hypothetical haplotypes in the population.
Table 3. Number, prevalence and geographic localization of haplotypes (n = 22) of Echinococcus granulosus s.s. in the sheep and goats of Lebanon

Table 4. Diversity and neutrality indices for E. granulosus s.s. in the sheep and goats of Lebanon

H n: haplotype number, H d: haplotype diversity, N d: nucleotide diversity.
*Significant at P < 0.05.
Population diversity and neutrality indices were also computed for E. granulosus s.s. population from the sheep and goats of Lebanon. Relatively moderate haplotype diversity (0.7614 ± 0.0391) was observed in the population along with low nucleotide diversity (0.00188 ± 0.00026). Harbouring a higher number of haplotypes (n = 6) in a lower number of isolates (n = 11), higher haplotype diversity existed for E. granulosus s.s. in goats (0.8364 ± 0.0887) compared to sheep (0.7567 ± 0.0423). In total, 20 haplotypes were identified from 65 isolates originating from sheep (Table 4). Other demographic parameters computed via neutrality indices indicated a significantly negative bias (D = −2.1627, F s = −19.891) in the overall population of E. granulosus s.s. identified on the basis of cox1 dataset. Furthermore, the estimation of gene flow among the two intermediate hosts was calculated using pairwise F st. Due to the occurrence of common haplotypes and less divergence between the E. granulosus s.s. haplotypes, F st value was very low (−0.01956, P > 0.05).
Discussion
To our knowledge, the current paper constitutes the first molecular characterization of E. granulosus s.l. isolates from sheep and goats of Lebanon, besides being the largest epidemiological survey carried out in this country in the last 30 years. In previous CE studies carried out in Lebanon between 1951 and 1989, the prevalence of CE in sheep and goat varied between 6.6 and 22.1% (Araj and Mourad, Reference Araj and Mourad2014). According to the current survey, the prevalence of CE in sheep and goats has increased to 62.9 and 20.9%, respectively. Current results show the persistence of the disease throughout the years; and the high prevalence can be justified with the age of the examined animals, as only adult animals were included in this study, the widespread home slaughtering and feeding dogs with infected offal. An increase in prevalence with age was also described in several studies (Scala et al., Reference Scala, Garippa, Varcasia, Tranquillo and Genchi2006; El Berbri et al., Reference El Berbri, Petavy, Umhang, Bouslikhane, Fihri, Boué and Dakkak2015; Varcasia et al., Reference Varcasia, Dessì, Lattanzio, Marongiu, Cuccuru, Carta, Meloni, Tamponi and Scala2020). It is well established that in the endemic areas of echinococcosis, the prevalence of hydatid cysts increases with the age of livestock, which become more susceptible to lower immunological resistance against infections (Torgerson and Heath, Reference Torgerson and Heath2003; Fikire et al., Reference Fikire, Tolosa, Nigussie, Macias and Kebede2012).
Also, higher prevalence of hydatid disease was found among sheep compared to goats (62.9 vs 20.9%; χ 2 = 126.31; P < 0.0001), which is in agreement with other studies carried out in several Arab countries (Ibrahim, Reference Ibrahim2010; Lotfi et al., Reference Lotfi, Yusefkhani, Samavatian, Yilmaz, Cengiz and Valilou2010; El Berbri et al., Reference El Berbri, Petavy, Umhang, Bouslikhane, Fihri, Boué and Dakkak2015; Hassan et al., Reference Hassan, Mero, Casulli, Interisano and Boufana2016; Almalki et al., Reference Almalki, Al-Quarishy and Abdel-Baki2017; Abdel-Baki et al., Reference Abdel-Baki, Almalki and Al-Quarishy2018). Similarly, previous studies in other non-Arab Mediterranean regions reported higher prevalence in sheep (Seimenis et al., Reference Seimenis, Morelli and Mantovani2006; Varcasia et al., Reference Varcasia, Canu, Kogkos, Pipia, Scala, Garippa and Seimenis2007; Manfredi et al., Reference Manfredi, Di Cerbo, Zanzani, Moriggia, Fattori, Siboni, Bonazza, Filice and Brunetti2011; Chaligiannis et al., Reference Chaligiannis, Maillard, Boubaker, Spiliotis, Saratsis, Gottstein and Sotiraki2015), whereas a study conducted by Kamhawi (Reference Kamhawi1995) in Jordan, reported that infection rates were similar for sheep and goats.
Current results provided evidence that cyst fertility rates were higher in sheep (21.4%) compared to goats (3.9%; χ 2 = 47.489; P < 0.0001). Similar results have been reported in other surveys carried out in Jordan and Greece (Kamhawi et al., Reference Kamhawi, Hijjawi, Abu-Gazaleh and Abbass1995; Varcasia et al., Reference Varcasia, Canu, Kogkos, Pipia, Scala, Garippa and Seimenis2007; Chaligiannis et al., Reference Chaligiannis, Maillard, Boubaker, Spiliotis, Saratsis, Gottstein and Sotiraki2015). The lowest rate seen in goats is likely due to the feeding habits of this animal, as they eat higher part of herbs (Otero-Abad and Torgerson, Reference Otero-Abad and Torgerson2013); the latter represents difficulty for dogs to uphill to these areas for defecation and is exposed to sunlight, which decreases the viability of the E. granulosus eggs. Most importantly, high prevalence and cyst fertility rates in sheep correlate with the molecular results where majority of sheep and goat samples belonged to E. granulosus s.s., a species particularly well adapted to sheep as intermediate hosts (Deplazes et al., Reference Deplazes, Rinaldi, Alvarez Rojas, Torgerson, Harandi, Romig, Antolova, Schurer, Lahmar, Cringoli, Magambo, Thompson and Jenkins2017) whereas goats are considered to have more G6/G7 genotypes (Varcasia et al., Reference Varcasia, Canu, Kogkos, Pipia, Scala, Garippa and Seimenis2007). However, almost all other livestock species (goats, cattle, yak, camels, alpacas, pigs and donkeys) contribute to the transmission of CE and are usually known to develop fertile cysts of E. granulosus s.s., but considered less important for the life cycle due to lower prevalence, cyst fertility or availability to dogs (Deplazes et al., Reference Deplazes, Rinaldi, Alvarez Rojas, Torgerson, Harandi, Romig, Antolova, Schurer, Lahmar, Cringoli, Magambo, Thompson and Jenkins2017).
Cysts in sheep were predominantly located in the liver (58.2%), whereas in goats, lung was the most affected organ (61.3%). This has also been demonstrated in other Mediterranean Arab and non-Arab countries, where a high infection rate was seen in sheep with the liver being the most affected organ (Varcasia et al., Reference Varcasia, Canu, Lightowlers, Scala and Garippa2006, Reference Varcasia, Dessì, Lattanzio, Marongiu, Cuccuru, Carta, Meloni, Tamponi and Scala2020; Grosso et al., Reference Grosso, Gruttadauria, Biondi, Marventano and Mistretta2012; Almalki et al., Reference Almalki, Al-Quarishy and Abdel-Baki2017; Brik et al., Reference Brik, Hassouni, Youssir, Baroud, Elkharrim and Belghyti2018; Toulah and Albalawi, Reference Toulah and Albalawi2019). A different situation has been observed in epidemiological studies conducted in Iran and Pakistan, where sheep had highest infection in lungs and liver was the most infected organ in goats (Lotfi et al., Reference Lotfi, Yusefkhani, Samavatian, Yilmaz, Cengiz and Valilou2010; Mehmood et al., Reference Mehmood, Arshad, Ahmed, Simsek and Muqaddas2020a).
When considering cyst fertility, the probability of finding fertile cysts in lungs was higher than that in liver in both animals. Similar results have been reported in other Mediterranean regions and the Middle East (Scala et al., Reference Scala, Garippa, Varcasia, Tranquillo and Genchi2006; Daryani et al., Reference Daryani, Sharif and Amouei2009; Conchedda et al., Reference Conchedda, Seu, Capra, Caredda, Pani, Lochi, Collu, Mura and Gabriele2012; Varcasia et al., Reference Varcasia, Dessì, Lattanzio, Marongiu, Cuccuru, Carta, Meloni, Tamponi and Scala2020). However, in some surveys the fertility rates were similar for liver and lungs (Khan et al., Reference Khan, El-Buni and Ali2001) or even higher in the liver (Dalimi et al., Reference Dalimi, Motamedi, Hosseini, Mohammadian, Malaki, Ghamari and Ghaffari2002; Elham et al., Reference Elham, Hassan, Ghasem, Gholamreza and Parviz2014). The increased presence of fertile cysts in lungs can be explained by the variation in tissue resistance between organs (Fikire et al., Reference Fikire, Tolosa, Nigussie, Macias and Kebede2012).
Determination of the epidemiological role of different E. granulosus s.l. species involved in echinococcosis is of paramount importance for disease control. Current investigation involving molecular characterization of metacestodes from sheep and goats in Lebanon revealed the presence of three genotypes, G1, G3 and G7 circulating among the analysed sheep and goats. Such information on the prevalence of CE in sympatric species has epidemiological implications and helps understanding the transmission dynamics of different genotypes (Nakao et al., Reference Nakao, Li, Han, Ma, Xiao, Qiu, Wang, Yanagida, Mamuti, Wen, Moro, Giraudoux, Craig and Ito2010; Mehmood et al., Reference Mehmood, Muqaddas, Arshad, Ullah and Khan2020b). Of five different areas of Lebanon, it was revealed that a common haplotype EgLEB1 existed among the sheep and goats of these regions. Similar existence of a shared and predominant haplotype based on cox1 mitochondrial marker has been identified in different countries such as China (Nakao et al., Reference Nakao, Li, Han, Ma, Xiao, Qiu, Wang, Yanagida, Mamuti, Wen, Moro, Giraudoux, Craig and Ito2010), Iran (Yanagida et al., Reference Yanagida, Mohammadzadeh, Kamhawi, Nakao, Sadjjadi, Hijjawi, Abdel-Hafez, Sako, Okamoto and Ito2012), United Kingdom (Boufana et al., Reference Boufana, San Lett, Lahmar, Buishi, Bodell, Varcasia, Casulli, Beeching, Campbell, Terlizzo, McManus and Craig2015) and Italy (Mehmood et al., unpublished data). The presence of a common haplotype among different populations suggests expansion from an ancestral haplotype (Yanagida et al., Reference Yanagida, Mohammadzadeh, Kamhawi, Nakao, Sadjjadi, Hijjawi, Abdel-Hafez, Sako, Okamoto and Ito2012).
A double-clustered haplotype network of E. granulosus s.s. having two predominant haplotypes, both belonging to G1 genotype (EgLEB1 and EgLEB2) was identified, signifying the importance of this genotype and the two haplotypes in this region. The second most common haplotype, EgLEB2, has been identified from other geographic areas of the world such as Iran (Yanagida et al., Reference Yanagida, Mohammadzadeh, Kamhawi, Nakao, Sadjjadi, Hijjawi, Abdel-Hafez, Sako, Okamoto and Ito2012), Eastern Europe (Casulli et al., Reference Casulli, Interisano, Sreter, Chitimia, Kirkova, La Rosa and Pozio2012) and Iraq (Hassan et al., Reference Hassan, Meerkhan, Boufana, Hama, Ahmed, Mero, Orsten, Interisano, Pozio and Casulli2017), implying that no significant phylogeographic segregation has occurred globally. This haplotype results in a non-synonymous substitution which may have led to its higher frequency and selection advantage and this is the same nucleotide position at which the point mutation led to initial description of G2 strain by Bowles et al. (Reference Bowles, Blair and McManus1992). Assembled in a star-like configuration, haplotypes EgLEB3, EgLEB7, EgLEB8, EgLEB11, EgLEB16 and EgLEB18 seemed to have radiated from EgLEB2 which was separated by a single mutation from the common G1 haplotype. Genotype G3 was represented by four haplotypes having very low occurrence in the population. Furthermore, the indication of hypothetical haplotypes in the haplotype network implied that high genetic variability exists among the E. granulosus s.s. population of Lebanon which required molecular surveillance encompassing more isolates.
Echinococcus granulosus s.s. has shown to have undergone rapid radiation globally (Kinkar et al., Reference Kinkar, Laurimäe, Balkaya, Casulli, Zait, Irshadullah and Saarma2018a, Reference Kinkar, Laurimäe, Acosta-Jamett, Andresiuk, Balkaya, Casulli, Gasser, González, Haag, Zait, Irshadullah, Jabbar, Jenkins, Manfredi, Mirhendi, M'rad, Rostami-Nejad, Oudni-M'rad, Pierangeli, Ponce-Gordo, Rehbein, Sharbatkhori, Kia, Simsek, Soriano, Sprong, Šnábel, Umhang, Varcasia and Saarma2018b). Current study estimated moderate genetic variability (H d: 0.7614 ± 0.0391) among the E. granulosus s.s. specimens isolated from sheep and goats. Even though sheep harboured 20 haplotypes, the haplotype diversity was lower for sheep in comparison with goats which had six E. granulosus s.s. haplotypes. An overall negative bias from neutrality was observed among the parasitic population which suggests population expansion after bottleneck event in the past. Genetic differentiation estimate (F st) for the goat and sheep population yielded a very low value (−0.01956) suggesting the efficient transmission of E. granulosus s.s. among these hosts in this region. Transmission dynamics and spread of this parasitic species across different geographical regions have largely been influenced by humans (Kinkar et al., Reference Kinkar, Laurimäe, Acosta-Jamett, Andresiuk, Balkaya, Casulli, Gasser, van der Giessen, González, Haag, Zait, Irshadullah, Jabbar, Jenkins, Kia, Manfredi, Mirhendi, M'rad, Rostami-Nejad, Oudni-M'rad, Pierangeli, Ponce-Gordo, Rehbein, Sharbatkhori, Simsek, Soriano, Sprong, Šnábel, Umhang, Varcasia and Saarma2018c).
Echinococcus canadensis G7 genotype was isolated from a goat specimen sampled from Beirut and is the first report on molecular identification of this genotype in Lebanon. Based on mitochondrial nad5 gene (680 bp) and nad2 gene (714 bp), it was identified that this sequence belonged to the G7b haplogroup of E. canadensis which was also recognized from other Mediterranean countries such as France and Italy (Laurimäe et al., Reference Laurimäe, Kinkar, Varcasia, Dessì, Sgroi, D'alessio, Veneziano and Saarma2019). A similar occurrence of pig strain in goats was reported earlier from Greece (Varcasia et al., Reference Varcasia, Canu, Kogkos, Pipia, Scala, Garippa and Seimenis2007) and Iran (Fadakar et al., Reference Fadakar, Tabatabaei, Borji and Naghibi2015) indicating that goats could harbour this genotype despite low levels of infection. Goats are naturally more prone to E. granulosus s.s. infection, most probably due to the cosmopolitan distribution of sheep strain, however susceptibility of goats to other genotypes cannot be disregarded. Different genotypes of E. granulosus s.l. occur sympatrically across various geographical areas among different intermediate hosts (Maillard et al., Reference Maillard, Benchikh-Elfegoun, Knapp, Bart, Koskei, Gottstein and Piarroux2007), however, intrinsic barriers to gene exchange between sympatric species maintain genetic identity among the strains despite overlap in the host spectrum.
The unofficial slaughterhouses and inadequate tracking of animals are serious issues sustaining the CE in endemic countries that could have contributed to the high prevalence recorded in sheep and goats. Furthermore, the socioeconomic and cultural conditions of Lebanon could have led to reduction in the prophylactic approaches to a bare minimum contributing to the maintenance of the parasite lifecycle. In conclusion, high rates of infection in sheep and goats represent a zoonotic risk with particular relevance to urban areas. This was brought to light through a reliable assessment of the epidemiological situation that revealed the true burden of disease. Developing guidelines and an implementation plan to reduce the public health costs in endemic settings using genotype-specific approach for disease control is deemed necessary.
Data
The datasets used and/or analysed during the current study are available from the corresponding author on a reasonable request.
Acknowledgements
The authors thank Professor George Araj of the Department of Pathology and Laboratory Medicine, American University of Beirut, Medical Center, Beirut, Lebanon for the critical revision of the manuscript. The authors also thank Dr Jamal Khazaal, previous President of the Lebanese Veterinary Syndicate and owner of the Libanvet Laboratory, Zahle, Lebanon, for his generous help and support.
Author contribution
Conceptualization, A. V., G. J.; investigation, G. J., G. D., C. T., F. N.; data curation, M. N., C. T.; writing original draft preparation, G. J., A. V.; writing review and editing, C. T., U. S., A. S., M. N., C. H.; all authors have read and agreed to the published version of the manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
All the operations carried out on live animals were performed by the vet as part of the routine clinical visit and the study was carried out following the recommendations of European Council Directive (86/609/EEC) on the protection of animals.