Introduction
Research has linked prenatal maternal or postnatal environmental tobacco smoke (ETS) exposure to neurodevelopmental and behavioural problems in children, including deficits in intellectual ability and academic achievement, decreased attention span, and hyperactivity (Eskenazi & Castorina, Reference Eskenazi and Castorina1999; Linnet et al. Reference Linnet, Dalsgaard, Obel, Wisborg, Henriksen, Rodriguez, Kotimaa, Moilanen, Thomsen, Olsen and Jarvelin2003). Langley et al. (Reference Langley, Rice, van den Bree and Thapar2005) reported a significant correlation between maternal smoking during pregnancy and attention deficit hyperactivity disorder (ADHD), with a pooled odds ratio of 2.39. However, recent studies using sibling designs, which compared siblings who differed in their exposure to prenatal nicotine, indicated that there is no or only a minimal effect of maternal smoking during pregnancy on ADHD offspring after controlling for confounders (D'Onofrio et al. Reference D'Onofrio, Van Hulle, Waldman, Rodgers, Harden, Rathouz and Lahey2008; Lindblad & Hjern, Reference Lindblad and Hjern2010; Obel et al. Reference Obel, Olsen, Henriksen, Rodriguez, Järvelin, Moilanen, Parner, Linnet, Taanila, Ebeling, Heiervang and Gissler2011). Other emerging findings have suggested that the association between maternal smoking during pregnancy and ADHD might be affected by important confounders such as postnatal child ETS (Knopik, Reference Knopik2009; Thapar et al. Reference Thapar, Rice, Hay, Boivin, Langley, van den Bree, Rutter and Harold2009). Recently, several studies have suggested that postnatal ETS exposure is associated with ADHD, independent of prenatal ETS exposure (Kollins et al. Reference Kollins, Garrett, McClernon, Lachiewicz, Morrissey-Kane, FitzGerald, Collins, Anastopoulos and Ashley-Koch2009; Twardella et al. Reference Twardella, Bolte, Fromme, Wildner and von Kries2010; Xu et al. Reference Xu, Cook, Ilacqua, Kan and Talbott2010).
With regard to the relationship between ETS exposure and academic problems, previous studies have identified an association between maternal smoking during pregnancy and deficits in reading, writing, mathematics, and visuospatial skills (Batstra et al. Reference Batstra, Hadders-Algra and Neeleman2003; Yolton et al. Reference Yolton, Dietrich, Auinger, Lanphear and Hornung2005). However, little is known about the effect of postnatal ETS exposure on learning disabilities, although a recent longitudinal study reported that arithmetic and spelling problems were more pronounced when the mother continued to smoke after the child's birth (Batstra et al. Reference Batstra, Hadders-Algra and Neeleman2003).
Recent evidence has indicated that prenatal nicotine exposure might influence ADHD symptoms and cognitive/academic deficits via the disruption of the dopamine neurocircuits (Biederman, Reference Biederman2005). A recent study reported that heavy maternal smoking during pregnancy was associated with slower response times and response time variability on the continuous performance test (CPT; Motlagh et al. Reference Motlagh, Sukhodolsky, Landeros-Weisenberger, Katsovich, Thompson, Scahill, King, Peterson, Schultz and Leckman2011). The CPT has often been employed to measure neurocognitive functioning and deficits in ADHD, and its variables have been reported to possess the largest effect size for the diagnosis of ADHD (Frazier et al. Reference Frazier, Demaree and Youngstrom2004). Rodent studies have suggested that postnatal nicotine exposure may affect synaptic function and brain development in a manner that is similar to prenatal exposure (Gospe et al. Reference Gospe, Zhou and Pinkerton1996; Britton et al. Reference Britton, Vann and Robinson2007; Slotkin et al. Reference Slotkin, MacKillop, Rudder, Ryde, Tate and Seidler2007). Therefore, postnatal nicotine exposure may be also associated with ADHD and/or learning disabilities via its impact on attention and inhibitory control, as indexed by CPT measures.
Given the dearth of human data investigating the association between postnatal ETS and ADHD or learning disabilities, we examined the relationship between ETS exposure (measured by urine cotinine), CPT measures, and ADHD or learning disabilities in school-aged children. Cotinine is a major metabolite of nicotine and a biomarker of ETS exposure (Puig et al. Reference Puig, Garcia-Algar, Monleon, Pacifici, Zuccaro, Sunyer, Figueroa, Pichini and Vall2008). We hypothesized that children with ADHD would have higher urinary cotinine levels than children without ADHD and that the levels of urinary cotinine would be associated with higher scores on parent and teacher ratings of ADHD, more errors on the CPT, and lower performance on parental ratings of learning disabilities. We also hypothesized that attention and inhibitory control operations, as measured by the CPT, would mediate the relationship between urine cotinine levels and symptoms of ADHD or learning disabilities.
Method and materials
Participants
We conducted this study as the second- and third-year processes of a 3-year research project named, ‘Effects of pollution on neurobehavioral development, and future policies to protect our children’, funded by the Korean Ministry of Environment's Eco-Technopia 21 Project. Based on our experience with the preliminary survey conducted in the first year (Kim et al. Reference Kim, Cho, Kim, Shin, Yoo, Kim, Yang, Kim, Bhang and Hong2009), we modified the research design and applied it to the study of cotinine, as follows. In brief, we recruited participants from five different administrative regions in Korea: Seoul and Seongnam are urban districts, Incheon and Ulsan are industrial cities, and Yeoncheon is a rural district. We selected 2–3 schools from each region that best represented the local demographics, for a total of 13, and sent parents of third- and fourth-grade children (age range 8–11 years, n = 1712) letters inviting them to participate in our study. Schools in the centre of each region were chosen, to reflect a microcosm of each region. We gave the parents and children detailed information about the study and then obtained written informed consent before any child was enrolled the study. From the initial 1712 subjects, a total of 1089 (response rate 63.6%) participated in this study. The study participants' geographical distribution was as follows: 463 (42.5%) from urban districts, 422 (38.8%) from industrial cities, and 202 (18.7%) from the rural district. The response rates between urban districts, industrial cities, and the rural district were not significantly different (62.2%, 65.3%, and 63.4%, respectively). The study protocol was approved by the institutional review board of the Seoul National University Hospital.
The parents completed an extensive questionnaire about demographics and other relevant information concerning the children, including questions about family structure, socioeconomic status, paternal education, maternal age at conception, tobacco and alcohol use by the mothers during pregnancy (yes or no), indirect smoking status of the children (yes or no), and medical, obstetrical, and neurodevelopmental, and educational histories of the children.
Assessment of the children's ADHD and learning disabilities
We used the Diagnostic Interview Schedule for Children – version IV (DISC-IV) ADHD module for diagnosing ADHD (Shaffer et al. Reference Shaffer, Fisher, Lucas, Dulcan and Schwab-Stone2000). Trained laypersons conducted face-to-face interviews with the parents at each participant's school and administered the DISC-IV – Parent Version. A previous study has ascertained the reliability and validity of the Korean version DISC-IV (Cho et al. Reference Cho, Kim, Kim, Kim, Choi, Jung, Yang, Chungh, Go, Kim, Shin, Yoo, Yoo, Lee, Lee, Lee, Lee, Jeon, Jung, Hong, Hwang and Han2006). For the ADHD diagnostic assessment, we assessed both full-syndrome ADHD (all DSM-IV criteria met) and subthreshold ADHD, operationally defining subthreshold ADHD as the presence of at least three and no more than five inattentive and/or hyperactive–impulsive symptoms, provided some impairment from the symptoms was present in two or more settings. A child also had to meet the DSM-IV ADHD age-of-onset and impairment criteria to be diagnosed with subthreshold ADHD.
We also used the ADHD Rating Scale-IV (ADHD-RS; DuPaul et al. Reference DuPaul, Power, Anastopoulos and Reid1998) to evaluate ADHD symptom severity. This scale is composed of 18 items reflecting the DSM-IV diagnostic criteria and uses a 4-point rating scale ranging from 0 to 3. Of the 18 items, nine reflect symptoms related to inattention, and nine reflect symptoms related to hyperactivity and impulsivity. The reliability and validity of the Korean version ADHD-RS (K-ADHD-RS) are well-established (So et al. Reference So, Noh, Kim, Ko and Koh2002). In this study, both parents and teachers completed the scales.
The Learning Disability Evaluation Scale (LDES; McCarney, Reference McCarney1996) consists of 88 items describing the observed characteristics of students with a learning disability. Each item is rated on a 3-point scale ranging from 1 (rarely or never) to 3 (all or most of the time). The LDES has been factor analysed and it consists of seven subscales: listening, thinking, speaking, reading, writing, spelling, and mathematical calculations. The sum of each subscale's item scores are converted to age-adjusted standard scores, in which better performance is indicated by higher scores. In addition, as a global measure of learning disability, the learning quotient (LQ) is derived from the sum of the seven subscales' standard scores. The Korean version of the LDES has been age-standardized and found to be a valid and reliable instrument for screening specific learning disorders (Shin et al. Reference Shin, Hong, Kim and Cho1998). In this study, the parents completed the LDES.
Assessment of the children's cognitive and neuropsychological functioning
A trained examiner, blinded to the children's cotinine levels, administered the following tests to each of the children in a quiet room. A licensed specialist in clinical psychology (S.M.S) coordinated the tests and supervised the examiners. Our previous paper extensively described the training process for this study's examiners (Cho et al. Reference Cho, Bhang, Hong, Shin, Kim, Kim, Yoo, Cho and Kim2010).
We administered the abbreviated form of the Korean Educational Development Institute's Wechsler Intelligence Scales for Children (KEDI-WISC; Park et al. Reference Park, Yoon, Park, Park and Kwon1996), which tests vocabulary, arithmetic, picture arrangement, and block design, for each child. The sums of the first two subtests' age-adjusted t scores were used to estimate verbal intelligence quotient (VIQ), and the sums of the last two were used to estimate performance IQ (PIQ; Park et al. Reference Park, Yoon, Park, Park and Kwon1996). Scores from the abbreviated battery correlate well with the WISC full-scale IQ (FSIQ) in the widely translated original instrument, the revised version of the WISC, and the standardized Korean version, the KEDI-WISC; Kim & Kim, Reference Kim and Kim1986).
We assessed the children's attention and response inhibition using a standardized, visual version of a computerized CPT (Greenberg & Waldman, Reference Greenberg and Waldman1993) called Attention deficit hyperactivity disorder Diagnostic System (ADS; Shin et al. Reference Shin, Cho, Chun and Hong2000). In this test, the examinee is shown visual stimuli on a screen, one every 2 s, for 100 ms. The examinee is required to respond to a square containing a triangle (target) but not to a square containing another square or a circle (non-target). The target stimulus was presented 22.5% of the time during the first half and 77.5% of the time during the second half of the test. In this study, we used the school version of ADS, which measures four variables: omission errors (failure to respond to a target, i.e. a measure of inattention); commission errors (erroneous response to a non-target, i.e. a measure of impulsivity); response time for correct responses to targets (a measure of information processing and motor speed); and the standard deviation of these response times (response time variability, i.e. a measure of variability or consistency of attention).
Assessment of the mothers' cognitive functioning
Each mother completed the short form of the Korean Wechsler Adult Intelligence Scale (K-WAIS), which tests vocabulary, arithmetic, picture arrangement, and block design, under the guidance of a trained examiner who was blinded to the children's IQs. We used vocabulary and arithmetic scores to estimate VIQ and picture arrangement, and block design to estimate PIQ. Short-form scores correlate well with FSIQ (Silverstein, Reference Silverstein1990).
Measurement of urine cotinine levels
We used cotinine direct ELISA kits (BioQuant, USA) to measure each child's urine cotinine, diluting the urine 1:100 and applying 10 μl samples, in duplicate, to the 96-well microtitre plate provided. Then, the urine was incubated with 100 μl enzyme conjugate, at room temperature, for 60 min. We washed the wells with 300 μl distilled water and applied 100 μl substrate to each well. The substrate was incubated at room temperature for 30 min, and we measured the sample absorbencies at a dual wavelength of 450 nm, using a Versamax Microplate Reader (Molecular Device, USA). This method of urine cotinine detection provided a limit of detection (LOD) of 1.0 ng/dl. For the detection limit data below, half of the detection limit values (LOD/2) were used for our calculations (Polissar et al. Reference Polissar, Hopke and Poirot2001). The coefficients of variation (CVs) were 5.8–14.7% for inter-assay and 4.2–8.4% for intra-assay at environmental exposure levels. For creatinine measurement, CREA (Roche, USA) reagent was used, in a Hitachi 7600 machine (Hitachi, Japan) with a kinetic colorimetric assay (rate-blanked and compensated). Reportedly, creatine-corrected urine cotinine concentrations show less correlation with parental smoking history than the uncorrected values do, and correcting cotinine concentrations for creatinine may not enhance the data's information value (Jatlow et al. Reference Jatlow, McKee and O'Malley2003; Puig et al. Reference Puig, Garcia-Algar, Monleon, Pacifici, Zuccaro, Sunyer, Figueroa, Pichini and Vall2008). Thus, we used creatinine-unadjusted urine cotinine values for the analyses in this study.
Statistical analysis
We conducted a generalized linear mixed model (GLMM) for assessing associations between urine cotinine concentrations, neuropsychological variables, ADHD symptoms, and learning disabilities. To achieve normal distributions of the variables, we log-transformed (ln) the cotinine concentrations.
To identify possible confounders mediating the association between urine cotinine level and ADHD, we compared potentially relevant variables between ADHD (full syndrome and subthreshold) and non-ADHD groups. Group differences were computed using the t test for continuous variables and χ2 test for categorical variables. Statistical significance was defined as an alpha level <0.1. There were significant group differences in gender (p = 0.004), child's IQ (p < 0.001), paternal educational years (p = 0.040), yearly income (p = 0.001), and maternal IQ (p = 0.028).
Based on these results and clinical consideration, we selected age, gender, residential area, paternal educational level (in years), yearly income, alcohol use during pregnancy, child's IQ, and maternal IQ as covariates. Although age, residential area, and alcohol use during pregnancy did not differ between the ADHD and non-ADHD groups, we included these variables as covariates because they are generally considered as clinically important confounders in the study of children's neurocognitive function and academic achievement (Kim et al. Reference Kim, Cho, Kim, Shin, Yoo, Kim, Yang, Kim, Bhang and Hong2009; Burden et al. Reference Burden, Westerlund, Muckle, Dodge, Dewailly, Nelson, Jacobson and Jacobson2011). The above-mentioned sociodemographic variables of age, gender, residential area, paternal educational level (in years), yearly income, and alcohol use during pregnancy were considered as fixed effects (Littell et al. Reference Littell, Stroup and Freund2002) because we selected five different regions with known sociodemographic characteristics for inclusion in the study. The child's IQ and/or maternal IQ were considered as random effects because IQ was not a selection variable for inclusion in the study. We controlled for child IQ and maternal IQ and were interested in the extent to which these random effects accounted for variance in the neuropsychological variables, ADHD symptoms, and learning disabilities. A recent review of research on attention problems and academic achievement suggested that such research should control for IQ performance and thereby control for the potential influence of cognitive competence on ADHD symptoms and/or academic achievement (Polderman et al. Reference Polderman, Boomsma, Bartels, Verhulst and Huizink2010). To identify the influence of children's IQ, which we expected to have a sizable impact on the association between cotinine and both ADHD and learning disabilities (Polderman et al. Reference Polderman, Boomsma, Bartels, Verhulst and Huizink2010), we did not include children's IQ as a random effect in the first model (mixed model 1) but added it as random effect in the second model (mixed model 2).
To conduct the path analyses, explore the effects' pathways, and determine the best-fitting model, we used the AMOS version 19.0 statistical program (SPSS Inc., USA). The model fit was based on generally accepted thresholds for root mean square error of approximation (RMSEA), normed fit index (NFI), non-normed fit index (NNFI), and comparative fit index (CFI). The RMSEA assesses closeness of fit, with values approximating 0.08, 0.05, and 0 indicating reasonable, close, and exact fits, respectively (Browne & Cudeck, Reference Browne and Cudeck1992). The NFI, NNFI, and CFI values range from 0 to 1, with values >0.9 indicating an acceptable fit.
All statistical analyses except the path analyses were performed using SPSS version 19.0 (SPSS Inc., USA) with the statistical significance defined as an alpha level <0.05.
Results
Participants' characteristics
This study recruited 1089 children, with a mean age of 9.1 ± 0.7 years (range 8–11 years), of whom 571 (52.4%) were male. The study participants' geographical distribution was as follows: 463 (42.5%) from urban districts, 422 (38.8%) from industrial cities, and 202 (18.7%) from the rural district. Of the 1089 children, 1007 (92.4%) produced urine amounts sufficient to measure cotinine. We excluded the remaining 82 from the study and also excluded an additional four participants because two had histories of seizure disorders, one had a history of neonatal hypoxia, and one had a history of head trauma accompanied by cerebral haemorrhage. We also excluded five participants who had been exposed to maternal smoking during pregnancy in order to exclude the influence of prenatal smoking exposure. Finally, a total of 998 subjects were included in the statistical analysis. There were significant differences in mean IQ (110.2 ± 14.3 for included participants v. 104.4 ± 13.7 for excluded participants, p < 0.001), paternal educational years (13.8 ± 2.2 for included participants v. 13.1 ± 2.3 for excluded participants, p = 0.013), and percentage of alcohol use during pregnancy (3.2% for included participants v. 8.8% for excluded participants, p = 0.007). The children's demographic characteristics are summarized in Supplementary Table S1. The mean urine cotinine level was 4.7 ng/dl (s.d. = 11.6). The geographical mean concentrations of cotinine were 4.3 ng/dl (s.d. = 8.0) in urban districts, 5.1 ng/dl (s.d. = 15.3) in industrial cities, and 4.5 ng/dl (s.d. = 9.6) in the rural district, showing no significant differences between the residential areas (p = 0.641). The geometric mean (ln) concentration of urine cotinine was 2.0 ng/dl [geometric s.d. (g.s.d.) = 0.2].
Association between urine cotinine and ADHD
Tables 1 and 2 present the results of the GLMM of urine cotinine effects on CPT and ADHD-RS scores, respectively. Mixed model 1 showed a significant relationship between urine cotinine levels and CPT scores on omission errors [β = 1.75, 95% confidence interval (CI) 0.40–3.10, p = 0.011], commission errors (β = 2.08, 95% CI 0.59–3.56, p = 0.006), response times (β = 0.88, 95% CI 0.14–1.61, p = 0.020), and response time variability (β = 2.80, 95% CI 0.96–4.64, p = 0.003). Further analysis with mixed model 2 also showed these associations, although to lesser extent than mixed model 1 (Table 1). After Bonferroni correction [0.05/4 (number of variables of CPT), p = 0.013], the association between urine cotinine levels and response time did not reach statistical significance; however, other associations remained significant. With regard to ADHD-RS scores, mixed model 1 showed urinary cotinine levels were significantly associated with hyperactive-impulsive (β = 0.31, 95% CI 0.08–0.54, p = 0.008) and total (β = 0.59, 95% CI 0.11–1.07, p = 0.015) scores on the parent-rated ADHD-RS and with inattention (β = 0.44, 95% CI 0.09–0.79, p = 0.015) and total (β = 0.68, 95% CI 0.06–1.29, p = 0.031) scores on the teacher-rated ADHD-RS. Further analysis with mixed model 2 also showed these associations, although to lesser extent than mixed model 1 (Table 2). Following Bonferroni correction (0.05/3 (number of subscales ADHD-RS, p = 0.017), the association between urine cotinine levels and parent-rated ADHD-RS scores remained significant.
Table 1. Association between urine cotinine levels and continuous performance test variables
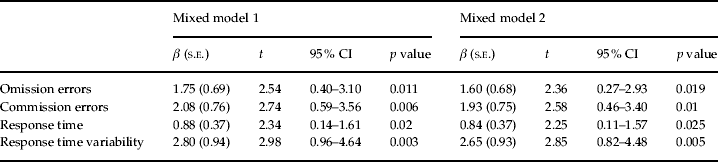
CI, Confidence interval.
Mixed model 1 (fixed effect: age, gender, residential area, paternal education level, yearly income, alcohol during pregnancy; random effect: maternal IQ).
Mixed model 2 (fixed effect: age, gender, residential area, paternal education level, yearly income, alcohol during pregnancy; random effect: children's IQ, maternal IQ).
Table 2. Association between urine cotinine level and parent- or teacher-rated ADHD Rating Scale – IV (ADHD-RS) scores

CI, Confidence interval.
Mixed model 1 (fixed effect: age, gender, residential area, paternal education level, yearly income, alcohol during pregnancy; random effect: maternal IQ).
Mixed model 2 (fixed effect: age, gender, residential area, paternal education level, yearly income, alcohol during pregnancy; random effect: children's IQ, maternal IQ).
Of the 998 participants, 885 (88.7%) participated in the diagnostic interview with the DISC-IV ADHD module. Full syndrome and subthreshold ADHD children (n = 143, 16.2%) had higher mean (ln) cotinine levels than did non-ADHD control children (n = 742), after adjustment for age and sex (mean 0.76, s.d. = 1.25 v. mean 0.46, s.d. = 1.23; odds ratio (OR) 1.19, 95% CI 1.03–1.37, p = 0.016). We found the two groups trended towards difference after we adjusted for residential area and paternal education level (OR 1.16, 95% CI 0.99–1.35, p = 0.057), but we could not observe significance in this trend after we adjusted for the children's IQs (OR 1.14, 95% CI 0.98–1.33, p = 0.098). Full-syndrome ADHD children (n = 42, 4.7%) and non-ADHD control children did not differ statistically in mean (ln) cotinine levels (mean = 0.80, s.d. = 1.18 for the ADHD group; OR 1.18, 95% CI 0.93–1.50, p = 0.184), after adjustment for age and sex.
Association between urine cotinine and learning disabilities
Table 3 shows the results of the GLMM of urine cotinine effects on LDES scores. Mixed model 1 showed a significant relationship between urine cotinine levels and spelling (β = −0.19, 95% CI −0.33 to −0.06, p = 0.006) and mathematical calculation (β = −0.12, 95% CI −0.22 to −0.02, p = 0.016). Further analysis with mixed model 2 also showed these associations, although to lesser extent than mixed model 1 (Table 1). After Bonferroni correction [0.05/8 (number of subscales of LDES), p = 0.006], these associations did not reach statistical significance.
Table 3. Association between urine cotinine levels and Learning Disability Evaluation Scale (LDES) variables

CI, Confidence interval.
In LDES, better performance is indicated by higher scores.
Mixed model 1 (fixed effect: age, gender, residential area, paternal education level, yearly income, alcohol during pregnancy; random effect: maternal IQ).
Mixed model 2 (fixed effect: age, gender, residential area, paternal education level, yearly income, alcohol during pregnancy; random effect: children's IQ, maternal IQ).
Associations between urine cotinine, neuropsychological functioning, and symptoms of ADHD and learning disabilities
We hypothesized that attention and inhibitory control operations, as measured by the CPT, would mediate the relationship between urine cotinine levels and ADHD symptoms based on a meta-analytical review by Frazier et al. (Reference Frazier, Demaree and Youngstrom2004). This review reported that the CPT measures possess the largest effect size for the diagnosis of ADHD. Furthermore, the measures of CPT were recently proposed as a promising endophenotype for ADHD (Kollins et al. Reference Kollins, Anastopoulos, Lachiewicz, FitzGerald, Morrissey-Kane, Garrett, Keatts and Ashley-Koch2008). To evaluate whether neuropsychological functioning mediated the relationship between urine cotinine and symptoms of ADHD or learning disabilities, we performed structural equation modelling (SEM) and estimated mediation effects using the method of Baron & Kenny (Reference Baron and Kenny1986).
In the linear mixed model (Tables 2 and 3), child's IQ had a modest impact on ADHD and learning disability symptoms. Thus, in the SEM, we first entered CPT as a mediator between urine cotinine levels and symptoms of ADHD or learning disabilities (see Fig. 1) and then added IQ as a secondary mediator (see Fig. 2). The relationship between urine cotinine and ADHD symptoms was fully mediated by IQ [direct path from urine cotinine to ADHD symptoms: β = −0.04, p = 0.136; indirect path through IQ: β = 0.081, p = 0.010, bootstrap maximum likelihood (ML) method] (Supplementary Fig. S1A), whereas the relationship between urine cotinine and IQ was not mediated by ADHD symptoms (direct path from urine cotinine to IQ: β = −0.14, p < 0.001; indirect path through ADHD symptoms: β = −0.086, p = 0.116, bootstrap ML method) (Supplementary Fig. S1B). Therefore, we ordered the variables as follows: cotinine → child's IQ → ADHD symptoms. Figs 1 and 2 show the final models as the results of SEM.

Fig. 1. Structural equation model of the relationship between urine cotinine and symptoms of ADHD or learning disabilities, as mediated by attention and inhibitory control – Model 1. All values are standardized regression weights. For the learning quotient, higher scores indicate better performance.

Fig. 2. Structural equation model of the relationship between urine cotinine and symptoms of ADHD or learning disabilities, as mediated by IQ and attention and inhibitory control – Model 2. All values are standardized regression weights. For the learning quotient, higher scores indicate better performance.
As shown in Fig. 1, urine cotinine levels predicted impairments in attention and inhibitory control (β = 0.16, 95% CI 0.10–0.22, p < 0.001), and inefficient attention and inhibitory control predicted ADHD (β = 0.15, 95% CI 0.09–0.22, p < 0.001) and learning disability symptoms (β = −0.07, 95% CI −0.13 to −0.02, p < 0.001). This model showed a reasonable fit to the data (RMSEA = 0.008, NFI = 0.996, CFI > 0.999, χ2p value = 0.381). Urine cotinine levels also predicted ADHD symptoms directly (β = 0.07, 95% CI 0.02–0.15, p = 0.033), but the magnitude of the association was relatively weak. Urine cotinine levels did not directly predict learning disability symptoms (β = −0.05, 95% CI −0.10 to −0.01, p = 0.075).
As shown in Fig. 2, urine cotinine levels predicted low child's IQ (β = −0.15, 95% CI −0.21 to −0.08, p < 0.001), low IQs predicted impairments in attention and inhibitory control (β = −0.19, 95% CI −0.25 to −0.14, p < 0.001), and inefficient attention and inhibitory control predicted ADHD (β = 0.16, 95% CI 0.09–0.22, p < 0.001) and learning disability symptoms (β = −0.08, 95% CI −0.14 to −0.02, p < 0.001). This model showed a reasonable fit to the data (RMSEA = 0.098, NFI = 0.936, CFI = 0.941, χ2p value <0.001). Urine cotinine levels also predicted ADHD symptoms directly (β = 0.07, 95% CI 0.02–0.15, p = 0.038), but the magnitude of the association was relatively weak. Urine cotinine levels did not directly predict learning disability symptoms (β = −0.05, 95% CI −0.10 to −0.01, p = 0.079).
We conducted additional path analyses after excluding children who fulfilled all of the criteria for ADHD from the analysis (n = 843). In these additional analyses, the relationship between the variables remained constant (data not shown, but available upon request).
Discussion
In this study, we demonstrated that urinary cotinine levels are significantly associated with parental reports of ADHD or learning disability symptoms in school-aged children. We also found a positive association between urinary cotinine levels and neuropsychological control operations, such as response inhibition and response time variability, as measured by the CPT. Notably, we observed higher mean (ln) cotinine levels in children diagnosed with ADHD than in non-ADHD control children. SEM demonstrated that attention and inhibitory control operations, as measured by the CPT, might mediate the relationship between postnatal ETS exposure and symptoms of ADHD or learning disabilities.
In line with our results, Kollins et al. (Reference Kollins, Garrett, McClernon, Lachiewicz, Morrissey-Kane, FitzGerald, Collins, Anastopoulos and Ashley-Koch2009) found that postnatal ETS exposure correlated with both parent and teacher ADHD symptom ratings, after controlling for a range of relevant covariates. However, a study by Braun et al. (Reference Braun, Kahn, Froehlich, Auinger and Lanphear2006), using serum cotinine and the data from the National Health and Nutrition Examination Survey conducted from 1999 to 2002 in the USA, did not find an association between postnatal ETS exposure and ADHD.
Finding of the association between ETS exposure and mathematical or spelling problems in the present study is consistent with previous studies. Using data from the National Health and Nutrition Examination Survey, conducted from 1988 to 1994, Yolton et al. (Reference Yolton, Dietrich, Auinger, Lanphear and Hornung2005) found a significant inverse relationship between serum cotinine and mathematic skills. A longitudinal study using a Dutch birth cohort found that children whose mothers smoked during and after pregnancy performed worse on mathematics and spelling tests than other children (Batstra et al. Reference Batstra, Hadders-Algra and Neeleman2003).
The mechanisms by which ETS exposure exerts its effects on neurocognitive functioning are unknown. Brain nicotinic acetylcholine receptors are thought to play important roles in attention, memory, and cognition by modulating synaptic transmission and plasticity in cortico-limbic circuits and, thus, participate in the pathogenesis of ADHD (Sacco et al. Reference Sacco, Bannon and George2004; Mansvelder et al. Reference Mansvelder, Mertz and Role2009). Moreover, nicotine stimulates phasic dopamine release in the striatum of both animals and human smokers (Corrigall et al. Reference Corrigall, Coen and Adamson1994; Brody et al. Reference Brody, Olmstead, London, Farahi, Meyer, Grossman, Lee, Huang, Hahn and Mandelkern2004), and such dopamine disruption may be associated with ADHD pathology. Attention and inhibitory control are among the neuropsychological functions related to dopamine neurocircuits (Castellanos & Tannock, Reference Castellanos and Tannock2002). Thus, our finding that urine cotinine levels were associated with ADHD and learning disabilities via their effect on attention and inhibitory control, measured by CPT, suggests that ETS influences ADHD symptoms and academic deficits via the disruption of dopamine neurocircuits.
This study has several limitations. First, its cross-sectional nature precluded the possibility of inferring any causal relationships between ETS exposure, neuropsychological functioning, and symptoms of ADHD and learning disabilities. Second, we were unable to examine the association between ETS exposure and ADHD subtypes due to limited sample size. Third, although we collected a range of information about the family environment including parental education levels and intelligence, which could affect the smoking exposure of children, data about the family history of ADHD and/or learning disabilities were lacking. Thus, we could not exclude the possibility that the association between urine cotinine levels and ADHD and learning disabilities may be due to shared correlation with parental ADHD symptoms. Further studies with information about family history of ADHD and learning disabilities and/or using a sibling design are required to identify direct causal effects of ETS exposure on ADHD and/or learning disabilities. Fourth, we found significant differences in background characteristics between included and excluded participants, and even non-significant differences in potential confounders between the two groups could have biased our results. However, in this study, even inclusion of the excluded participants might not weaken the associations of the major findings, due to the excluded participants' characteristics of lower IQ and paternal educational years compared to included participants. Finally, it should be noted that using a single urine cotinine measurement might not be sufficient for examining the level and severity of exposure. It is unclear whether short-term exposure (i.e. urine cotinine, which reflects a nicotine exposure of 2–3 days) represents a child's chronic exposure or indicates the short-term toxicity of ETS exposure. Thus, further studies with serial measurements of cotinine are needed, to obtain a more accurate estimate of ETS exposure.
In conclusion, the results of this study extend the previously observed association between ETS exposure and ADHD or academic impairment in children. Furthermore, our data indicate that impairments in attention and inhibitory control probably mediate these associations.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291712001109.
Acknowledgements
This work was supported by the Eco-technopia 21 project of Korea Institute of Environmental Science and Technology (091-081-059).
Declaration of Interest
None.







