INTRODUCTION
The lesser weever, Echiichthys vipera (Cuvier, 1829) is a small benthic fish usually restricted to clean sand substrates and much disliked by commercial fishermen because of the painful injuries inflicted by the venomous dorsal spines (Evans, Reference Evans1907; Carlisle, Reference Carlisle1962; Cain, Reference Cain1983; Lopacinski et al., Reference Lopacinski, Bak, Fiszer, Czerniak and Krakowiak2009). The geographic range extends from the north of the British Isles to the Atlantic coast of Morocco (Lewis, Reference Lewis1980); it is notably abundant in the southern North Sea where densities >0.1/m2 have been recorded (Creutzberg & Witte, Reference Creutzberg and Witte1989). Ellis et al. (Reference Ellis, Maxwell, Schratzberger and Rogers2011) reported lesser weever to be the most abundant fish along the crests of the North Norfolk sand banks at depths of <20 m and concluded that these shallow waters were an important nursery habitat for the species. Historically, the North Sea distribution was heavily biased towards the south within ICES fishing area 4C, probably because it favours warmer summer temperatures. This view is supported by the increase in abundance in the more northerly waters of ICES fishing area 4B following recent seawater warming (Beare et al., Reference Beare, Burns, Greig, Jones, Peach, Kienzle, McKenzie and Reid2004). An offshore winter migration controlled by temperature occurs (Lewis, Reference Lewis1980; Creutzberg & Witte, Reference Creutzberg and Witte1989) and growth ceases in winter (Creutzberg & Witte, Reference Creutzberg and Witte1989) when weever remain buried in the sand (Lewis, Reference Lewis1980; Creutzberg & Witte, Reference Creutzberg and Witte1989). During the summer regular movements with the tide into the lower shore intertidal habitat are undertaken (Lewis, Reference Lewis1976).
Lesser weever hunt both as ambush predators hidden on the seafloor and up in the water column (Vasconcelos et al., Reference Vasconcelos, Prista, Cabral and Costa2004). The hunting strategy is flexible, some studies report higher night-time (Lewis, Reference Lewis1980; Nash & Santos, Reference Nash and Santos1993) and others higher daylight feeding activity (Creutzberg & Witte, Reference Creutzberg and Witte1989; Vasconcelos et al., Reference Vasconcelos, Prista, Cabral and Costa2004). Motile epibenthic crustaceans, particularly mysids, and small fish are important prey (Vasconcelos et al., Reference Vasconcelos, Prista, Cabral and Costa2004). In the North Sea, gobies dominate the diet, but crustaceans, Nereis and cephalopods are also eaten (Creutzberg & Witte, Reference Creutzberg and Witte1989). The main known predators are brill, Scophthalmus rhombus, and turbot, Scophthalmus maximus.
In the North Sea, lesser weever eggs are in the water column from May to September and post-larvae are found until November (Creutzberg & Witte, Reference Creutzberg and Witte1989). Peak egg abundance is in July. Lesser weever is reported to attain a total length (TL) of about 160 mm (Creutzberg & Witte, Reference Creutzberg and Witte1989; Nash & Santos, Reference Nash and Santos1993). Creutzberg & Witte (Reference Creutzberg and Witte1989) report a TL of about 140 mm at age 6 and a von Bertalanffy asymptotic TL of 150.3 mm. The maximum reported age off the Dutch coast is 13 years in a study on ecology in the vicinity of a proposed windfarm (Tien et al., Reference Tien, Tulp and Grift2004).
The paucity of reported ecological studies on lesser weever is notable given the high abundance in the southern North Sea (Lewis, Reference Lewis1980; Creutzberg & Witte, Reference Creutzberg and Witte1989; Beare et al., Reference Beare, Burns, Greig, Jones, Peach, Kienzle, McKenzie and Reid2004; Pearce, Reference Pearce2008), especially as it is suspected of having greatly increased in abundance in recent years possibly linked to climate change or other anthropogenic disturbance of the North Sea ecosystem (Beare et al., Reference Beare, Burns, Greig, Jones, Peach, Kienzle, McKenzie and Reid2004). This research was stimulated by the notably high relative abundance of lesser weever impinging on the cooling water screens of Sizewell B nuclear power station, Suffolk and the regular occurrence of individuals >160 mm TL, which is well above the maximum size previously reported. Intensive sampling over the period February 2009–2013 generated a large data set with which to generate a growth curve and estimate adult mortality. The availability of samples collected throughout the year allowed age-related and seasonal movements to be identified. Data collected at Sizewell in the 1980s between May 1981 to April 1982 (Turnpenny et al., Reference Turnpenny, Utting, Millner and Riley1983) were also available to check for any long-term changes possibly linked to recent seawater warming.
MATERIALS AND METHODS
Water temperature records between 2008 and 2013 were automatically recorded at noon each day from a thermistor placed in the condenser cooling water inlet to turbo-generator 1 at Sizewell B nuclear power station. The water was pumped from 600 m offshore at between 10 to 5 m depths.
Sampling
Fish samples were taken from the power station cooling water intakes located on the southern North Sea coast of Suffolk, England at 52°12′28.6″N 1°37′5.27″E. The intakes are situated 600 m offshore in a shallow coastal bay and were sampled between February 2009 to July 2012. Sampling intensity throughout this period ranged from 34 to 40 24 h periods per annum which were randomly selected from quarterly blocks. These 24 h periods comprised an 18 h bulk sample and 6 subsequent 1 h samples, all fish in each sample were identified, weighed to the nearest 1 g and their standard length (SL) measured from the tip of the nose to the end of the vertebral column recorded to the nearest mm. The lesser weever caught were retained and frozen for later analysis. A sample of 250 individuals was collected over the size range of 27 to 147 mm SL to determine the relationship between total and standard length. Total Length (TL) was not generally measured because of tail damage. SL and TL in mm were found to be related by the equation:
Age determination
Age was determined for 94 lesser weever using sagittal otoliths. The fish were selected to cover the observed size range, the smallest was 44 mm SL and the largest 135 mm SL and their otoliths showed between 1 and 15 growth checks (see Figure 3). After removal, otoliths were cleaned in 70% ethanol to remove residual tissue; they were then weighed and measured. Otoliths were burned under a gas flame for 10 to 30 s, depending upon size, they were then broken using a needle to expose a transverse section. Sections were visualized under a microscope and digital images adjusted to make the growth checks easier to count. When one otolith was insufficiently clear to confidently determine age, the second from the same fish was analysed. Two out of 94 individuals could not be aged because their multiple growth checks were very close together, suggesting they were not all annual checks, but caused, in part, by some other stress. Lesser weever reproduce during the summer and fish were assumed to be born on 1 July for ageing purposes. To check that this rapid burning technique fully visualized the otolith structure a small number of otoliths were mounted in resin, sectioned, stained with neutral red and polished. These preparations showed clear growth checks which were consistent with the results from the burn and break method.
Of the age validation methods listed by Campana (Reference Campana2001) the three methods potentially applicable to the samples collected were (1) length frequency analysis, (2) marginal increment analysis and (3) analysis of daily increments. Creutzberg & Witte (Reference Creutzberg and Witte1989) using 10 mm stretched mesh beam trawl samples were able to easily distinguish the 0, I, II and in some cases III group lesser weevers as distinct modes in the length frequency distribution. At the end of July, individuals approximately 1 year old which had experienced one winter had an average length of 50 mm TL, individuals 2 years old which had experienced two winter checks had an average length of about 78 mm TL and 3-year-old fish with three winter checks about 94 mm TL. The smallest individual analysed in the present study, captured on 25 July 2012, was 53 mm TL and the otolith showed a single growth check close to the margin of the otolith. The second smallest individual captured on 25 July 2012 was 75 mm TL and had two growth checks. Between May and July 2012 eight individuals with three growth checks were recorded with lengths of 95, 91, 95, 91, 87, 81, 101 and 86 mm TL with an average length of 90.87 mm TL. Over the first 3 years of life, when the individual age classes are distinguishable by length frequency analysis, it was concluded that weever only laid down one main check over the winter period.
It was not possible to undertake a study of the growth of the marginal increment of the otoliths because growth only occurs between June and October (Creutzberg & Witte, Reference Creutzberg and Witte1989) and weevers are rarely caught at Sizewell during July (Figure 1). However, otoliths examined in May and June always had a hyaline band at or close to the edge whereas otoliths from fish caught in November had a clear margin between the last hyaline band and the edge of the otolith. It was concluded that no growth checks were laid down within the growing period.
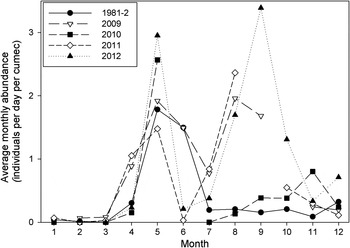
Fig. 1. Average monthly abundance (m3 s−1) of lesser weever, E. vipera off Sizewell beach, Leiston, Suffolk. Samples were collected May 1981 to April 1982 and then monthly between January 2009 and December 2012. The daily catch is standardized to a pumping rate of 1 m3 s−1. At full capacity the standard pumping rate is 50 m3 s−1, so an abundance of 3 corresponds to a capture rate of 150 individuals per day.
Otoliths were sectioned and polished to examine the fine structure and the presence of daily increments. While numerous fine increments could be discerned between the major checks, the presence of daily increments could not be confirmed.
Analytical methods
Abundance is expressed per cubic metre by dividing the number found by the volume of water sampled. A growth curve was fitted using non-linear regression, this enabled age to be estimated for all fish using their standard length. A logarithmic curve gave the most useful description of growth. A von Bertalanffy curve was found to be inappropriate because some individuals had a length greater than the fitted asymptote. Annual mortality was calculated using the relative abundance of each age class using data combined from all sample dates between 2009 and 2012. The abundance of each age class was estimated from the length distribution using the length-age relationship given by the fitted growth curve.
RESULTS
Annual variation in abundance and temperature
The seasonal changes in abundance of lesser weever for all years sampled shows a consistent pattern of low abundance between November and March followed by a spring maximum in May (Figure 1). Abundance then declines during June to reach a minimum in July. Late summer abundance varies greatly between years; in 2009, 2011 and 2012 a second peak in abundance occurred in August or September, whereas in 1981 and 2010 no late summer maximum was observed. The levels of abundance and timing of the spring peak showed no appreciable change between 1981/2 and 2009/12.
The annual seawater temperature cycles for the years 2008 to 2013 are shown in Figure 2. Temperatures typically ranged from about 3 to 21°C with the annual maximum in August and the minimum in February.
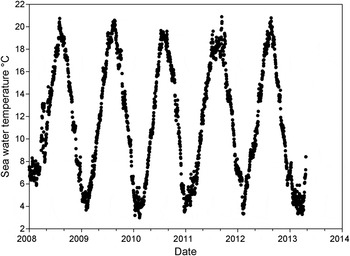
Fig. 2. Daily seawater temperature at Sizewell Beach, Leiston, Suffolk. Water temperature was recorded daily at noon at the condenser cooling water intake of Sizewell B nuclear power station. The water was extracted from 600 m offshore at a depth of 5–10 m.
Length-frequency distribution
The length-frequency distributions for each month for the years 2009–2012 combined show a seasonally varying pattern generated more by movement than in situ growth (Figure 3). In April–May, when lesser weever are first present in large numbers, the length ranges between 27 and 150 mm SL which covers the majority of the size (age) range known for this population. In June the length range is at the maximum observed due to the capture in 2009 of four exceptionally large individuals >170 mm SL. Few individuals <50 mm SL were present between June and August inclusive although this absence could simply reflect sampling error as abundance greatly declined between May and June so that if they maintained the same relative abundance within the population few would have been sampled. However, by November, when abundance was close to the winter minimum, the length range extended from 30 to 150 mm SL, the same as in May. In autumn the population in the study area had declined considerably, but it again comprised the full size (age) range found within the population.
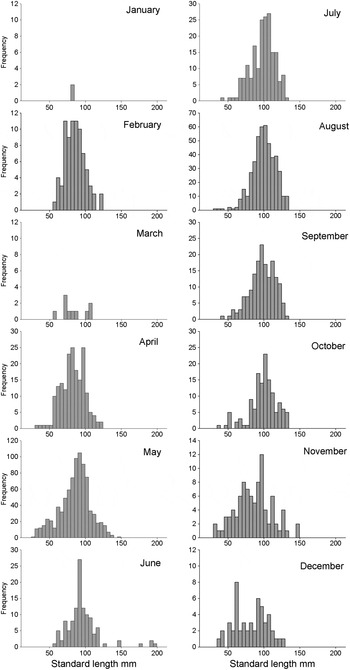
Fig. 3. Length frequency distribution of lesser weever E. vipera for each month of the year. Samples collected from 600 m off Sizewell Beach, Leiston, Suffolk. Data are summed for the years 2009 to 2012 inclusive.
Growth checks and length at age
Lesser weever sagittal otoliths were found to carry from 1 to 15 growth checks. The largest fish examined for growth checks was 135 mm SL (157.3 mm TL) which had 11 growth checks. Fish without checks and presumably <1 year old were unavailable, the smallest fish caught for ageing was 54 mm TL (44 mm SL) which is above the average size reached during their first winter of 37 mm TL. Figure 4 shows a plot of the growth of lesser weever under the assumption that the growth checks are all winter checks. This plot also presents the growth curve calculated by Creutzberg & Witte (Reference Creutzberg and Witte1989) for individuals up to 118 mm TL which was used for comparison and to check that the ages obtained were consistent with other workers.
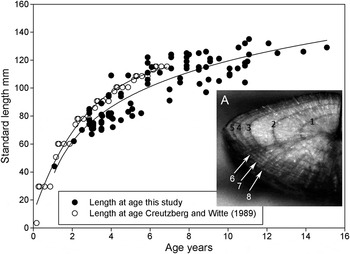
Fig. 4. The growth curve for lesser weever E. vipera in the southern North Sea. ● Data collected in this study between 2009 and 2012 inclusive off Sizewell beach, Leiston, Suffolk. A logarithmic curve is fitted by regression to the data. ○ The von Bertalanffy growth curve calculated by Creutzberg & Witte (Reference Creutzberg and Witte1989) using data collected in the southern North Sea 1972–1984. A. Image of an 8-year-old otolith from a fish caught off Sizewell beach with charred growth checks marked.
A search for a suitable growth model using non-linear regression found a logarithmic curve
gave the best fit to the data (n = 92, R 2 = 0.799).
The commonly used von Bertalanffy growth curve was unsuitable because there was no indication that growth was asymptotic; the fitted asymptotic value was below the length that fish from Sizewell are known to regularly attain.
Recruitment to the studied Sizewell population and adult survival
Using all the fish length data collected at Sizewell between 2009 and 2012 the fish in each 5 mm size class was given an estimated age using the logarithmic growth curve fitted above. The total abundance observed at ages from 1 to 21 years for the population shows that recruitment to the local population continues until at least age 5 after which abundance starts to decline indicating that death and emigration now exceeds immigration (Figure 5). From 6 years of age there is a decline in abundance which was fitted by regression analysis to an exponential curve
where N t is the number observed at age t, N o is the initial number at age 5 and t time in years.
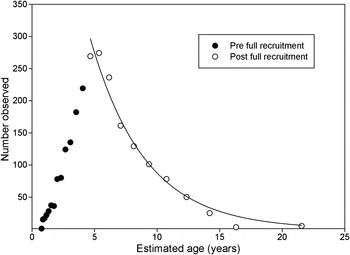
Fig. 5. Lesser weever E. vipera log10 abundance at age for the Sizewell population sampled between 2009 and 2012 inclusive. An exponential curve was fitted to the data using non-linear regression from 5 years of age when recruitment to the local population was assumed complete.
Survival from 6 to approximately 20 years of age is constant at about 80% per year.
Four extraordinarily large individuals of 177 to 195 mm SL captured in June 2009 were excluded from this analysis.
DISCUSSION
The Sizewell shore population of lesser weever holds individuals larger in size than any previously reported population. Thirty-seven individuals >151.6 mm TL, (>130 mm SL) were captured during regular sampling between 2009 and 2012 and five individuals were observed in the 170–175 mm TL size class alone. Wheeler (Reference Wheeler1978) states that the species attains a maximum of 140 mm TL while Creutzberg & Witte (Reference Creutzberg and Witte1989) in a major study of the southern North Sea population calculated the asymptotic length for their fitted von Bertalanffy growth curve as 150.3 mm TL. Four extraordinarily large individuals ranging between 205 to 225 mm TL (177 to 195 mm SL) were captured in June 2009. Unfortunately, this occurred before samples were collected for ageing. Their size is inconsistent with the observed size at age and fitted growth curve, they would be predicted to have an age >40 years. They have therefore been omitted from the calculated survival rate. There are three possible explanations for these extraordinary animals: (1) they were migrants from another population with different growth characteristics; (2) measurement error or misidentification; or (3) they were affected by some growth abnormality, which could occur with parasite infection. We have no reason to suspect error. The British record for a rod-caught lesser weever is 95.67 g wet weight off Weymouth in the English Channel. Using the length–weight relationships given in Creutzberg & Witte (Reference Creutzberg and Witte1989) this individual would have been ~198 mm TL, close in length to the largest animals reported here.
The maximum age of Sizewell lesser weever estimated from otolith winter growth checks is also greater than that previously reported for the southern North Sea. Lesser weever of 151 mm TL (129 mm SL) were estimated to be up to 15 years old and the growth curves indicate that individuals >20 years old may occur. These considerable ages are given support by the observed age of 13 years reported by Tien et al. (Reference Tien, Tulp and Grift2004). This notable longevity is consistent with the large maximum size of the fish. Creutzberg & Witte (Reference Creutzberg and Witte1989) reported fish of up to 6 years old at approximately 135 mm TL or less. As shown in Figure 4 their size at age observations are compatible with those observed at Sizewell for the first 6 years of life; the increased ages reported here are for fish notably larger than they observed.
Creutzberg & Witte (Reference Creutzberg and Witte1989) found ‘a notable dominance of adults (II–IV –group fishes). Most of the mortality, apparently, occurs towards the end of the lesser weever's life span.’ They viewed the lifespan as about 6 years. Their samples were collected between 1972 and 1984 from a large number of sampling stations extending from the Dutch coast to as far as 54°30′N. Their most westerly samples were about 2°30′E, well offshore of the Sizewell sampling location at about 1°37′E. As shown in Figure 5 recruitment to the Sizewell population is completed between 5 and 6 years of age which corresponds to the age at which Creutzberg & Witte (Reference Creutzberg and Witte1989) noted an ‘apparent’ sudden increase in mortality. The present results therefore indicate that lesser weever in the southern North Sea are not dying at around 6 years of age, but migrating to favoured regions – one of which, for part of the year, is close to the shore at Sizewell about 600 m from the beach at a depth of −10 m CD. Of equal note to the large size and extended longevity reported here is the seasonal mobility shown in Figure 1. From when they first appear off Sizewell at up to 6 years of age, until their final loss at 15 to 20 years of age, lesser weever make regular seasonal migrations into the area in both spring and autumn. During June and July the majority have moved elsewhere. The peak abundance of lesser weever post larvae at the Texel light vessel in the southern North Sea is in August and early September (Fonds & Boerman, Reference Fonds and Boerman1981). It is therefore likely that this summer movement away from Sizewell is linked to their spawning behaviour.
The observed low winter abundance may be caused both by lesser weever burying under the sand and also migrating to deeper, warmer, waters offshore. Creutzberg & Witte (Reference Creutzberg and Witte1989) also caught low numbers of lesser weever at offshore sites in the southern North Sea during winter. Lewis (Reference Lewis1976) reported burrowing in sand to depths of over 10 cm and gave a critical temperature of 5°C below which offshore migration occurred. At Sizewell seawater temperature typically stayed below 6°C from late December to early March and a movement offshore would be expected to be complete by January when the temperature is typically below 5°C (see Figure 2). Lesser weever have been found washed up on the shore after winter storm events suggesting they may burrow into the sand instead of migrating offshore (Lewis, Reference Lewis1980). Echiichthys vipera does not feed between November and May (Bagge, Reference Bagge2004) and low levels of activity would reduce the rate of capture at a cooling water intake. The greater weever Trachinus draco L., 1758 in the Kattegat also shows seasonal movements; in winter they move into warmer deeper waters and almost completely cease feeding and return inshore in May when they start to feed (Bagge, Reference Bagge2004).
Extended longevity implies a notably low annual mortality rate for a small benthic fish. The present study has estimated that from 5–6 years of age mortality is 0.23 y−1. Creutzberg & Witte (Reference Creutzberg and Witte1989) estimated a mortality rate from 0 to 5 years of age of 0.37 y−1. Judging by the fact that recruitment to the Sizewell near-shore population is not complete until 5 to 6 years of age, this offshore southern North Sea loss rate probably combines both death and migration. Both studies reach the conclusion that E. vipera has a notably low mortality rate for a small benthic fish. The unusual nature of the life-history strategy of lesser weever is illustrated by comparing the estimated maximum longevity of at least 15 years with that of other similarly sized benthic fish. The drangonet Callionymus lyra L., 1758 in the southern North Sea were found to reach a maximum of 4–5 years old with most under 3 (Van Der Veer et al., Reference Van Der Veer, Creutzberg, Dapper, Duineveld, Fonds, Kuipers, Van Noort and Witte1990), viviparous blenny Zoarces viviparous L., 1758 may live to 5 years (Jacobsson et al., Reference Jacobsson, Neuman and Thoresson1986), hooknose Agonus cataphractus L., 1758 may reach 3 or rarely 4 (Wheeler, Reference Wheeler1969) and 4-bearded rockling Enchelyopus cimbrius (L. 1776) live for 2 or 3 years (Keats & Steele, Reference Keats and Steele1990). Butterfish Pholis gunnellus (L., 1758) have been found to live up to 5 years in the Menai Straits (Qasim, Reference Qasim1957), however in colder waters off Iceland they have been found to attain 12 years (Gunnarsson & Gunnarsson, Reference Gunnarsson and Gunnarsson2002). The age reached and pattern of growth seen in lesser weever appears to be unusual amongst small benthic fish in the southern North Sea. This species has evolved a defensive strategy which allows a low mortality rate and an extended life cycle and only a short period of active growth each year. There is an extended period from November to May when lesser weever does not feed or grow. The important role this species plays in the benthic economy of the southern North Sea would support further investigation of what appears to be an unusual life history for a small benthic fish. A full picture of this life cycle will only be obtained from a spatially extensive sampling programme that sampled all age groups. This will likely show the species to be seasonally mobile, long-lived and a dominant, key, species in North Sea regions offering sandy substrates.







