Vancomycin-resistant enterococcus (VRE) is endemic in many healthcare facilities, accounting for ~60% of bacteremiasReference Wisplinghoff, Bischoff, Tallent, Seifert, Wenzel and Edmond 1 and >80% of healthcare-associated infections due to Enterococcus faecium in some settings.Reference Weiner, Webb and Limbago 2 Furthermore, VRE infections are associated with significant mortality and morbidity,Reference Prematunge, MacDougall and Johnstone 3 in part due to limited antimicrobial treatment options.Reference Arias, Contreras and Murray 4 Given the clinical impact of this pathogen, efforts to reduce cross-transmission have been implemented in many hospitals. However, the optimal approach to VRE control remains controversial.Reference Morgan, Murthy and Munoz-Price 5 , Reference Humphreys 6
In late 2013, a shift from vanB to vanA VRE occurred across Australia.Reference Coombs, Pearson and Daley 7 , Reference van Hal, Espedido and Coombs 8 Unlike the vanB gene, which usually integrates into the E. faecium chromosome, the vanA gene is often located on a plasmid.Reference Cetinkaya, Falk and Mayhall 9 , Reference Gold 10 The ease with which horizontal transfer of plasmids occurs suggests that the emergence of vanA VRE will likely lead to an overall larger burden of VRE. Indeed, there was a dramatic increase in vanA VRE incidence in our institution between 2013 and 2014, despite improvement in methicillin-resistant Staphylococcus aureus acquisition rates during this period (from 5.7 per 10,000 patient days to 3.4 per 10,000 patient days).
We therefore undertook this molecular epidemiological study to better characterize the emergence of vanA VRE in our intensive care unit (ICU) using whole-genome sequencing of patient and environmental isolates to delineate transmission chains. We hypothesized that the development of a substantial environmental reservoir played a key role in the emergence and sustained transmission of vanA VRE in the unit.
METHODS
Study Design, Setting, and Participants
This retrospective cohort study was conducted at a 911-bed tertiary-care referral hospital in Sydney, Australia. The hospital provides solid-organ transplantation, hematopoietic stem-cell transplantation and pelvic exenteration services. The 2 general ICU wards (ICU-1 and ICU-2) care for both medical and surgical patients. The ICUs are close to each other, with potential movement of patients, staff, and equipment between units. After the emergence of vanA VRE was noted in 2013, VRE isolates from patients admitted to the ICUs from January to November 2014 were systematically stored and included in this study.
VRE Screening and Infection Control Precautions
Patients in the ICU undergo routine screening for VRE with rectal swabs collected on admission, weekly, and on discharge from the unit. Individuals colonized or infected with VRE are placed on contact precautions (using gowns and gloves) and are isolated in single rooms when available. ICU-1 has 13 beds with 3 (23%) single rooms, while ICU-2 has 17 beds including 7 (41%) single rooms. Bed spaces are terminally cleaned with a hypochlorite disinfectant when VRE-colonized or -infected patients are discharged from the ICU.
Environmental Sampling
Environmental sampling was performed in the ICUs in September 2014 to determine whether there was a reservoir to explain the increasing vanA VRE incidence. Samples were collected by premoistening swabs with normal saline then swabbing an area ≥5 cm in diameter. High-touch areas from bed spaces (ie, bed rails, bedside tables, infusion pumps, drawer handles, counters, patient stethoscopes, monitors, and computers), waste rooms (ie, door handle, pan sanitizer and taps), bathrooms (ie, light switch, shower taps, rails, call button, and sink taps) and shared equipment (ie, blood gas analyzer, point-of-care coagulation timer, patient slide, patient lifter, air-assisted patient transfer system (Hovermatt, HoverTech International, Allentown, PA), chlorhexidine wipe warmer, ultrasound, intravenous poles, electrocardiogram (ECG) machine, and ECG leads) were sampled. The bed spaces were randomly selected within each of the following categories in each ICU: current occupant VRE positive, previous occupant VRE positive, current occupant colonized with a multiresistant organism other than VRE (eg, methicillin-resistant S. aureus), and current occupant not colonized with a multiresistant organism.
Microbiology Methods
Screening and environmental samples were inoculated directly onto selective chromogenic agar (chromID VRE Agar, bioMérieux, Marcy-l'Étoile, France), incubated at 37°C, and read at 24 and 48 hours. Characteristically colored colonies were identified as E. faecium by the MALDI-TOF biotyper (Bruker Daltonics, Bremen, Germany). Presence of vanA or vanB genes was confirmed by polymerase chain reaction (PCR).Reference Adams 11 The first available patient and all environmental vanA VRE isolates were included in the study.
Data Collection
Data regarding admissions, patient days, hand hygiene compliance, and newly identified VRE patients were prospectively collected. Hand hygiene compliance was calculated as the number of compliant moments divided by total moments directly observed by trained auditors according to the National Hand Hygiene Initiative, 12 based on the World Health Organization’s “Five Moments for Hand Hygiene.”Reference Sax, Allegranzi, Uckay, Larson, Boyce and Pittet 13 For VRE patients, admission date, admitting specialty, ward and bed movements, and single-room isolation were also recorded. VRE acquisition was defined as isolation of VRE with no prior history of VRE colonization or infection, and VRE infection was defined as isolation of VRE from a sterile site or other specimen accompanied by signs of infection. ICU-acquired VRE was defined as new detection of VRE >48 hours after admission to the unit.
Statistical Analysis
Descriptive statistics included calculation of means for normally distributed variables and medians for nonparametric variables. For differences in proportions, the χ2 test was used. Poisson regression was used to calculate differences in rates using 1,000 patient days as the exposure, VRE acquisition count as the dependent variable, and time period as the independent variable. All P values were 2-tailed, and P<.05 was considered statistically significant. Data were analyzed using Stata version 11.0 software (StataCorp, College Station, TX).
Genomic Analysis
Isolate sequencing was performed using a bench-top Illumina MiSeq sequencer and MiSeq V3 chemistry following library preparation (NextEra XT kit, Illumina, San Diego, CA) according to the manufacturer’s instructions, generating 75 nucleotide paired-end reads. Single-nucleotide variants (SNVs) were determined from the pan-genome using kSNP3Reference Gardner, Slezak and Hall 14 with vancomycin resistance and multilocus sequence typing (MLST) obtained from de novo assemblies. A maximum-likelihood phylogeny was generated on the SNV matrix using RaxML version 8.2.9 (Exelixis, San Francisco, CA)Reference Stamatakis 15 with hierarchical clustering.Reference Cheng, Connor, Siren, Aanensen and Corander 16 Links between isolates were analyzed using the R package “outbreaker” software (R Foundation for Statistical Computing, Vienna, Austria). 12 , Reference Jombart, Cori, Didelot, Cauchemez, Fraser and Ferguson 17 This model determines the directionality of isolates based on genetic distance and sample isolation date assuming a single introduction event with no molecular clock rate. To minimize the impact of these assumptions, we limited this analysis to isolates from (1) the single dominant cluster (cluster 1) and (2) those obtained within a ±2 month window from the time of the environmental sampling, based on previous observations of VRE survival on surfaces for up to 2 months.Reference Bonilla, Zervos and Kauffman 18
RESULTS
In total, 1,729 patients were admitted to the 2 ICUs during the study period; 92 (5.3%) were VRE-positive on admission. Most patients colonized or infected on admission had vanB VRE (55 of 92; 60%), while 36 patients (39%) had vanA VRE and 1 patient was colonized with both vanA and vanB VRE. VRE acquisition rates in the ICUs rose from 3.1 per 1,000 patient days in 2013 to 7.0 per 1,000 patient days in 2014 (incidence rate ratio [IRR], 2.2; 95% CI, 1.4–3.5; P<.001), predominantly due to an increase in vanA VRE from 0.3 per 1,000 patient days to 3.9 per 1,000 patient days during this period (IRR, 11.2; 95% CI, 3.4–36.3; P<.001). Acquisition of vanB VRE remained relatively stable at 2.8 per 1,000 patient days in 2013 and 3.1 per 1,000 patient days in 2014 (IRR, 1.1; 95% CI, 0.6–1.9; P=.69).
Overall, 62 patients (3.6%) acquired VRE in the ICUs during the study period; 34 (55%) had vanA VRE and 28 (45%) had vanB VRE. Among the ICU-acquired vanA VRE, most (74%) were detected in ICU-1. For 31 patients with ICU-acquired vanA VRE, isolates had been stored and were therefore available for sequencing. Among these patients, 18 (58%) were male and the median age was 62 years (range, 26–87 years). Patients with vanA VRE were most frequently admitted under gastroenterology/hepatology (n=10), gastrointestinal surgery (n=6), or hematology (n=4) specialties (Table 1). Overall, 19 screening isolates (61%) and 12 clinical isolates (39%) were identified (Table 1).
TABLE 1 Characteristics of ICU Patients With vanA VRE

NOTE. ICU, intensive care unit; VRE, vancomycin-resistant enterococcus.
Of the 92 environmental samples, 14 (15%) were positive for vanA VRE, compared with only 1 (1%) positive for vanB VRE. ICU-1 had widespread environmental contamination, particularly surrounding the VRE-colonized patient (Figure 1). VRE was also detected, although at fewer sites, around other patients. Notably, however, VRE was not isolated from the bed space where the prior room occupant had been VRE colonized. In contrast, in ICU-2, vanA VRE was only recovered from 1 site. Importantly, more than half of the sampled equipment shared between the ICUs was also contaminated (Figure 1). The patient transfer system and ultrasound machine, items which come into direct patient contact, were particularly heavily colonized.
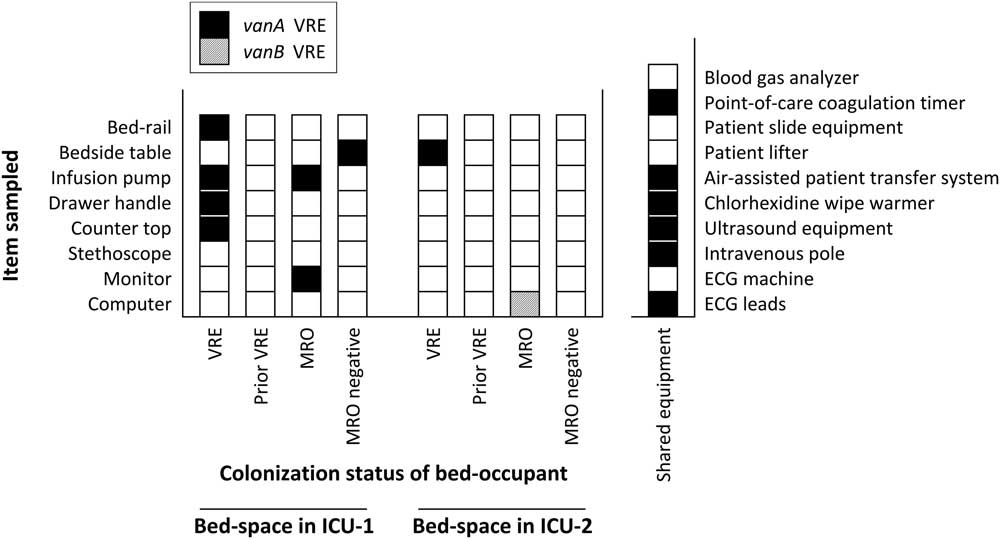
FIGURE 1 Isolation of vancomycin-resistant enterococcus (VRE) from environmental samples. Detection of VRE from environmental samples collected from ICU-1, ICU-2 and shared equipment. Results from sampling of bed spaces are labelled with the colonization status of the bed occupant at the time of sampling. NOTE. VRE, vancomycin-resistant enterococcus; MRO, multiresistant organism; ECG, electrocardiogram.
Genomic Analysis Results
The phylogeny (based on the pan-genome SNV matrix) revealed 4 distinct clusters. In silico MLST supported the clustering with identical sequence types within each cluster. A single cluster (Figure 2) predominated (84% of isolates), within which all isolates were not typeable as a result of deletion of the pstS allele.Reference Carter, Buultjens and Ballard 19 Cross-transmission events were observed with identical isolates between patient and environmental genomes (median SNV between isolate pairs 13 SNVs; range, 5–55).

FIGURE 2 Maximum-likelihood phylogenetic tree. The phylogeny of all sequenced isolates (n=45) with the isolate identifier and source of isolation depicted by the legend to the left of the tree. Four clusters were observed (see text for details). Members of the largest cluster (cluster 1 outlined by the top grey box) all classified as a single multilocus sequence type. Further analysis was directed at sequences within the predominant cluster that met inclusion criteria (ie, isolates with an identifier). Identifiers are shown to allow for cross-referencing between Figures 2 and 3. NOTE. SNV, single-nucleotide variant.
Genomic analyses of directionality (of the dominant cluster 1) confirmed the importance of the environment, including shared equipment, as the potential source of ongoing transmission (Figure 3). For example, an infusion pump (labeled “A” in Figure 3) was the source for several patient colonization and infection episodes, as well as further environmental contamination. Most transmission events from environmental sources were to patients close to the contaminated area (ie, within 1 bed space). In contrast, most transmission events that occurred at a distance (>1 bed space away) within the same ICU or between the 2 ICUs were related to patient sources, suggesting healthcare workers as potential conduits of transmission. Interestingly, isolates from VRE-infected patients were not linked with any additional isolates.

FIGURE 3 Inter- and intra-intensive care unit (ICU) transmission dynamics. Transmission chains and directionality of cluster 1 sequenced isolates within ±2 months of the date of environmental sampling. Arrows between samples indicate the likely ancestor or transmission chain of each isolate with darker arrow colors representing higher likelihoods of the parent isolate being the true ancestor. The time scale is provided on the x-axis with isolate source depicted using colors according to the legend at the top left of the figure. Environmental isolates are further categorized into shared equipment and high-touch areas in the legend. Circular shapes indicate a nonisolation area, and square shapes indicate that the patient was in a single room at the time the first positive VRE sample was collected. All shapes are highlighted with either dark blue or turquoise to reflect adjacent (within 1 bed space either side of the index isolate) and distant (>1 one bed space away) intra-ICU transmission respectively. Grey borders represent inter-ICU transmission events. For example, isolate 9 (a screening isolate on day 56) obtained from a nonisolated patient led to contamination of a high-touch area (G4, in the other ICU on day 60). This high-touch region was subsequently the source for a distant (>1 bed space apart) colonization (patient 24) and an infection event (patient 31) ~26 and 41 days later in the same ICU (intra-ICU events). Both patients were isolated at the time of first VRE detection. NOTE. IV, intravenous; ECG, electrocardiogram; POC, point-of-care.
Enhanced Infection Control Interventions and Monitoring of VRE Rates
Review of the environmental data led to implementation of a number of interventions. These included enhanced monitoring and feedback of VRE acquisition, hand hygiene, and environmental contamination data. These measures were facilitated by meetings with key stakeholders including ICU (medical and nursing), executive, environmental services, infection control and infectious diseases staff (Figure 4 and Table S1 in Supplementary Appendix). Where widespread VRE contamination was documented, cleaning in the unit was intensified, with particular attention to ICU-1 and shared equipment.

FIGURE 4 Vancomycin-resistant enterococcus (VRE) acquisition and hand hygiene compliance rates. The long arrow indicates the time point at which environmental sampling occurred in the intensive care units (ICUs). The short arrows labelled with “M” indicate the timing of multidisciplinary meetings between ICU, executive, environmental services, infection control and infectious diseases staff. NOTE. VRE, vancomycin-resistant enterococcus; M, multidisciplinary meeting.
Hand hygiene compliance rates were lower in ICU-1 than in ICU-2 during the period of environmental sampling (46% and 75% respectively; P<.001), but it improved to 76% (P<.001) over the following 12 months (Figure 4). VanA VRE acquisition rates continued to increase in the ICUs between 2014 and 2015 then remained stable in 2016 (Figure 4 and Table S2 in Supplementary Appendix). The shift from predominantly vanB to vanA VRE observed in 2014 persisted in subsequent years (Figure 4).
DISCUSSION
Increasing VRE incidence in the ICU was explained by multiple concurrent outbreaks of vanA VRE, with a single clone of a recently characterized lineageReference Carter, Buultjens and Ballard 19 emerging as the dominant circulating strain. The spread of VRE continued from patient to patient, with colonized patients acting as sources of transmission. In addition, patients transmitted VRE to the environment, including to fixed and shared equipment, which was then implicated as the source of further transmission events both within the same unit and across units.
The importance of the environment as a VRE reservoir has previously been documented.Reference Hayden, Bonten, Blom, Lyle, van de Vijver and Weinstein 20 , Reference Grabsch, Mahony and Cameron 21 However, our study provides an in-depth understanding of the role of the environment by detailed delineation of VRE transmission chains using discriminatory genomic data showing identical isolates on a pan-genome level. Notably, reusable medical equipment was demonstrated to be an important source for healthcare-associated infections. Cleaning and disinfection of these devices are frequently overlooked, often due to a lack of designated responsible personnel.Reference Anderson, Young, Stewart, Robertson and Dancer 22 , Reference Dancer 23 This situation is particularly concerning for VRE due to its ability to survive on dry surfaces for prolonged periods and to withstand attempts at disinfection.Reference Dancer 23
It is possible that increasing vanA VRE incidence may reflect the emergence of a strain with greater ability to persistent in the environment and/or enhanced transmissibility. Although most patients colonized on admission to the ICU harbored vanB VRE, acquisition in the unit and environmental contamination was predominantly with vanA VRE. These data support the hypothesis that the emerging vanA VRE strain possessed characteristics enabling its long-term survival in the environment. Interestingly, in contrast to previous studies,Reference Huang, Datta and Platt 24 , Reference Drees, Snydman and Schmid 25 VRE was not detected in bed spaces where the prior bed occupant had been VRE positive, suggesting that terminal cleaning had been adequately performed in the ICU. Furthermore, it is possible that intensification of daily cleaning of VRE-positive patient bed spaces may have a significant impact on environmental burden and potentially reduce cross-transmission.
Patients with VRE infections were not linked to further transmission events, irrespective of single-room isolation. This finding is contrary to the expectation that infected patients (with higher VRE burden) would lead to a greater intensity of environment contamination compared to asymptomatically colonized individuals. VRE-specific antimicrobial therapy may have reduced VRE shedding and consequently lowered the risk of transmission from these patients. Other possible explanations include behavioral change (eg, greater adherence to hand hygiene and contact precautions), enhanced cleaning of bed spaces, and dedicated equipment for infected patients. Cessation of such interventions may increase VRE burden, as has occurred in settings where VRE control measures were discontinued.Reference Bodily, McMullen, Russo, Kittur, Hoppe-Bauer and Warren 26 , Reference Lam, Johnstone and Adomako 27
This study used whole-genome sequencing, a powerful epidemiological tool, to provide a deeper understanding of the transmission dynamics of VRE, including extensive environmental sampling, to characterize the contribution of this reservoir to VRE spread. Weekly, in addition to admission and discharge, screening enabled more accurate classification of acquisition events. We used culture-based rather than nucleic acid detection methods for VRE screening, using direct inoculation of a chromogenic medium. Although they are less sensitive, culture-based methods may more closely reflect a patient’s ability to transmit VRE, as positive cultures correlate with higher density of stool and in turn with skin colonization.Reference D’Agata, Gautam, Green and Tang 28 It is expected that ICU patients would have a high load of VRE carriage,Reference Gouliouris, Blane and Brodrick 29 and cultures were incubated for 48 hours, which increases the sensitivity of VRE detection.Reference Kuch, Stefaniuk, Ozorowski and Hryniewicz 30 It is therefore likely that most VRE carriers in the ICU were identified. In addition, nucleic acid detection assays have been associated with high rates of false-positive results related to fecal carriage of non-enterococcal species harboring van genes.Reference Graham, Ballard, Grabsch, Johnson and Grayson 31 We did not sample healthcare workers. Screening of this group could be incorporated into future research to enhance our understanding of transmission chains. This study is limited by its small sample size and residual confounding inherent in its retrospective nature. However, these data can be used to provide the basis for future prospective studies aimed at evaluating the utility of specific environmental interventions.
In conclusion, the transmission dynamics of VRE in the ICU were complex, emphasizing the importance of multifaceted control strategies. Notably, the environmental data indicate that hospital cleaning inadequacies, especially of equipment, can contribute to continuing VRE spread. However, infected patients were not linked with further transmission, suggesting that the interventions instituted for them were effective and providing ongoing support for such measures for VRE control. Our findings are likely generalizable to many healthcare facilities where VRE is now endemic, and they should prompt consideration of specific interventions targeting the environment, particularly shared equipment, as an underappreciated source of healthcare-associated infections.
ACKNOWLEDGMENTS
We would like to acknowledge the scientific staff in the Microbiology Laboratory at Royal Prince Alfred Hospital for storing isolates for the study.
Financial support: No financial support was provided relevant to this article.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2018.29







