Introduction
The muscid Synthesiomyia nudiseta (van der Wulp, 1883) is widespread in Old and New World tropics and subtropics, with 20 °C annual isotherms (Skidmore, Reference Skidmore1985). This species has been involved in forensic investigations in different countries, such as Costa Rica, India, Malaysia, Thailand and the United States (i.e. southern USA and Hawaii) (Jirón et al., Reference Jirón, Vargas and Vargas-Alvarado1983; Lord et al., Reference Lord, Adkins and Catts1992; Omar et al., Reference Omar, Marwi, Mansar, Rahman and Oothuman1994; Kulshrestha et al., Reference Kulshrestha, Satpathy and Dubey1997; Goff, Reference Goff2000; Lee et al., Reference Lee, Krishnasamy, Abdullah and Jeffery2004; Sukontason et al., Reference Sukontason, Narongchai, Kanchai, Vichairat, Sribanditmongkol, Bhoopat, Kurahashi, Chockjamsai, Piangjai, Bunchu, Vongvivach, Samai, Chaiwong, Methanitikorn, Ngern-Klun, Sripakdee, Boonsriwong, Siriwattanarungsee, Srimuangwong, Hanterdsith, Chaiwan, Srisuwan, Upakut, Moopayak, Vogtsberger, Olson and Sukontason2007; Kumara et al., Reference Kumara, Abu Hassan, Che Salmah and Bhupinder2009). It has also been associated with human cadavers in archaeological contexts in Mexico and Peru (Huchet & Greenberg, Reference Huchet and Greenberg2010).
In Europe, S. nudiseta has been recorded mainly from insular territories, such as Malta, the Azores, Madeira, the Canary Islands and two small islands close to the coast of Alicante (SE Spain) (Pont, Reference Pont, Soós and Papp1986; Ebejer & Gatt, Reference Ebejer and Gatt1999; Martínez-Sánchez et al., Reference Martínez-Sánchez, Marcos-García and Rojo2005). This species was recorded for the first time on the European mainland in 1996 from Marbella (Bowden, Reference Bowden1997), a locality situated on the coast of southern Spain, about 70 km from North Africa (36°N 4°W). Until now, the distribution of S. nudiseta seemed to be restricted to insular territories and Mediterranean coastal areas because it has not been cited previously in studies about sarcosaprophagous dipteran communities in the interior of the peninsula.
This is the only known species under the genus Synthesiomyia Brauer & Bergenstamm, 1893 (Muscidae: Azeliinae: Reinwardtiini). The larvae are commonly found in animal and human faeces, as well as in decaying vegetable matter and garbage. However, it has been reported that this species prefers carrion as the food source of its choice and will successfully pupate in confined environments (Byrd & Castner, Reference Byrd, Castner, Byrd and Castner2010; Byrd & Tomberlin, Reference Byrd, Tomberlin, Byrd and Castner2010). Facultative carnivorous larvae feed just below the surface of the flesh and, often, post-feeding larvae secrete a silky white substance in which the puparium forms, frequently with adhered debris (Siddons & Roy, Reference Siddons and Roy1942). This species is considered eusynanthropic because adults present a marked preference for human environments (Nazni et al., Reference Nazni, Nooraidah, Jeffery, Azahari, Mohd Noor, Sadiyah and Lee2007; Uribe-M. et al., Reference Velásquez, Magaña, Martínez-Sánchez and Rojo2010) and are frequently associated with corpses discovered indoors at urban locations (Goff, Reference Goff2000; Lee et al., Reference Lee, Krishnasamy, Abdullah and Jeffery2004; Sukontason et al., Reference Sukontason, Narongchai, Kanchai, Vichairat, Sribanditmongkol, Bhoopat, Kurahashi, Chockjamsai, Piangjai, Bunchu, Vongvivach, Samai, Chaiwong, Methanitikorn, Ngern-Klun, Sripakdee, Boonsriwong, Siriwattanarungsee, Srimuangwong, Hanterdsith, Chaiwan, Srisuwan, Upakut, Moopayak, Vogtsberger, Olson and Sukontason2007). Developmental studies of S. nudiseta are limited, and they have been performed in South America and Asia at 20, 26 and 28 °C (Siddons & Roy, Reference Siddons and Roy1942; Rabinovich, Reference Rabinovich1970; Krüger et al., Reference Krüger, Ribeiro, Carvalho and Costa2002; Kumara et al., Reference Kumara, Abu Hassan, Che Salmah and Bhupinder2009).
Preimaginal stages have been described in several papers (Siddons & Roy, Reference Siddons and Roy1942; Skidmore, Reference Skidmore1985; Pires de Alencar & Rios, Reference Pires de Alencar and Rios1992; Calderón-Arguedas et al., Reference Calderón-Arguedas, Troyo and Solano2005; Sukontason et al., Reference Sukontason, Narongchai, Kanchai, Vichairat, Sribanditmongkol, Bhoopat, Kurahashi, Chockjamsai, Piangjai, Bunchu, Vongvivach, Samai, Chaiwong, Methanitikorn, Ngern-Klun, Sripakdee, Boonsriwong, Siriwattanarungsee, Srimuangwong, Hanterdsith, Chaiwan, Srisuwan, Upakut, Moopayak, Vogtsberger, Olson and Sukontason2007; Ubero-Pascal et al., Reference Ubero-Pascal, Arnaldos, López-Esclapez, García, Méndez-Vilas and Díaz2010b), and identification keys to mature larvae of this species can be found in Ishijima (Reference Ishijima1967), Skidmore (Reference Skidmore1985) and Velásquez et al. (Reference Velásquez, Magaña, Martínez-Sánchez and Rojo2010). Although methods of scanning electron microscopy (SEM) have been used in some studies (Pires de Alencar & Rios, Reference Pires de Alencar and Rios1992; El-Alfy, Reference El-Alfy1994; Ubero-Pascal et al., Reference Ubero-Pascal, Arnaldos and García2010a,Reference Ubero-Pascal, Arnaldos, López-Esclapez, García, Méndez-Vilas and Díazb), no exhaustive documentation of larval morphology has been published until now.
Due to the potential usefulness of S. nudiseta in forensic investigations in southwestern Europe, the aims of this paper are (i) to describe details of larval morphology for all instars using combined methods of light microscopy and SEM, (ii) to provide development data of this species under four constant temperatures, and (iii) to review the cases where S. nudiseta has appeared breeding in human corpses in Spain.
Materials and methods
Colony of Synthesiomyia nudiseta
Larvae were collected from a human corpse during autopsy procedures at the Institute of Legal Medicine of Alicante (IMLA, Spain) and reared on pork liver ad libitum until development was completed. A colony was established at the Department of Environmental Sciences and Natural Resources, University of Alicante from emerged adults. The colony was maintained at 23 °C, 60–70% RH with a photoperiod of 12:12 (L:D) in mesh cages (40 × 40 × 40 cm) with sugar and water. Fresh fish was used for oviposition and pork liver as a food source for larvae.
Larval morphology study
Larvae for morphological study were obtained from the colony. They were killed by soaking in hot water (≈97 °C), cleaned with a fine brush and preserved in 70% ethanol. For light microscopy study, larvae were slide-mounted in Hoyer's medium using concave slides. Photographs were taken with a Nikon 8400 digital camera mounted on a Nikon Eclipse E200 microscope (Nikon Corp., Tokyo, Japan). For additional observations, larvae were prepared with methyl salicylate according to Niederegger et al. (Reference Niederegger, Wartenberg, Spieß and Mall2011). Preparation for SEM study involved dehydration through 80%, 90% and 99.5% ethanol, critical point drying in CO2, mounting on aluminium stubs with double-sided tape and coating with platinum. Images were taken with variable-pressure SEM (LEO 1455). Larval terminology follows Skidmore (Reference Skidmore1985) and Courtney et al. (Reference Courtney, Sinclair, Meier, Papp and Darvas2000) with a few modifications proposed by Szpila & Pape (Reference Szpila and Pape2005).
Development under constant temperatures
Growth and developmental time were evaluated under four constant temperatures (15 °C, 20 °C, 25 °C, 30 °C), with 60–70% RH and a photoperiod of 12:12 (L:D). After oviposition, egg masses (±12 h old) were introduced into an environmental chamber at one of the four temperatures proposed. These eggs were checked every 12 h until larvae hatched. Then, 150 larvae were placed in a pot with 50 g of pork liver covered with mesh. For each temperature, ten replicates were carried out. Five of the largest larvae were collected daily, killed by soaking in hot water (≈97 °C) for five minutes and preserved in 70% ethanol. As a preliminary trial, five replicates at 12 °C were performed. In this case, eggs were incubated at 25 °C and then larvae were introduced into the environmental chamber at 12 °C. Larvae were collected every 24 h until the sixth day and after that, every 72 h until pupation.
To build growth curves, total length was measured using a digital vernier (0.01 mm) and the mean of the maximum length of larvae measured daily was plotted against time for each temperature regime. The minimal developmental time for larval and pupal stages was determined when the first five individuals in each replicate had changed to the next stage and it was recorded as the average of all pots. At 12 °C, only the larval stage was analyzed. For practical use in forensic investigation, developmental times from oviposition to adult emergence were represented against temperature in the isomorphen-diagram, where lines represent morphological changes and areas between lines represent identical morphological stages. Normality and homogeneity of variance of data were tested. A Kruskal-Wallis test (H) was carried out to compare development between temperatures. If significant differences were found, post hoc pairwise comparison procedures were carried out using Dunn's method (SigmaStat for Windows 3.5).
Accumulated degree-days (ADD) for larval and total development at all studied temperatures were calculated from the formula: ADD=y (t−t 0), where y is the developmental time in days, t is the rearing temperature and t 0 is the minimum development threshold temperature. The t 0 was estimated from the linear regression of the development rate (developmental time–1) on constant temperature.
Analysis of forensic cases
A review was conducted of the cases where S. nudiseta was involved, in autopsies carried out at the Anatomical Forensic Institute of Madrid (IAFM, Spain) and the IMLA. Fourteen cases were processed by the IAFM, one by the Forensic Anthropology Laboratory of the General Commissariat of the Scientific Police of Madrid and eight at the University of Alicante. One part of the larval sample was preserved, and the remainder of the larval and pupal samples were reared to the adult stage in an environmental chamber under controlled conditions (23–25 °C, 60–70% RH). The larvae were reared with pork liver and pupae were kept individually. Specimens of S. nudiseta were identified using Skidmore (Reference Skidmore1985), Pont (Reference Pont1991) and Velásquez et al. (Reference Velásquez, Magaña, Martínez-Sánchez and Rojo2010). Collected specimens were deposited in the Entomological Collection of the University of Alicante (CEUA).
Results
Larval morphology
Pseudocephalon
Each lobe of the bilobate pseudocephalon is equipped with an antenna, maxillary palpus, ventral organ and labial lobe (figs 1B–E; 2C–D and 3B–D). The antennal complex consists of an antennal dome situated on a basal ring (fig. 1C). The length of the antennal dome is similar to the height of the basal ring in the first instar and slightly shorter in the successive instars. The maxillary palpus in all instars consists of three sensilla coeloconica, three sensilla basiconica and one or more small additional sensilla, all in a tight cluster. Two typical sensilla coeloconica of possible non-maxillary origin are present laterodorsally on the surface of the maxillary palpus (figs 1D, 2B and 3C). In the second and third instars, maxillary palpus are surrounded by several rings of semicircular folds (figs 2B and 3C). A facial mask surrounding the functional mouth opening in the first instar is composed of cirri and oral ridges (fig. 1B). Cirri are arranged in one row and have a shape of stripes tapering distally, without distinct sclerotization (fig 4A, B). In the successive instars, oral ridges are more numerous, and sclerotized suprabuccal teeth are present in the anterior part of the buccal cavity (figs 2C, 3E, 4C–E and 5B, C). In the third instar, cutaneous teeth are present on the sides of the buccal cavity (figs 3E and 5C).

Fig. 1. First instar of S.nudiseta. (A) Anterior end of body, lateral view; (B) anterior end of body, ventral view; (C) antennal complex; (D) maxillary palpus; (E) ventral organ; (F) Keilin's organ; (G) lateral sensillum; and (H) anterior spiracle. an, antennal complex; as, anterior spiracle; cir, cirri; ll, labial lobe; mp, maxillary palpus; ns1 [ns2], first [second] additional sensillum coeloconicum; or, oral ridges; sb1 [sb2/sb3], first [second/third] sensillum basiconicum; sc1 [sc2/sc3], first [second/third] sensillum coeloconicum; vo, ventral organ.

Fig. 2. Second instar of S. nudiseta. (A) Anterior end of body, lateral view; (B) maxillary palpus; (C) anterior end of body, ventral view; (D) ventral organ; (E) posterior spiracles; (F) abdominal segments, ventral view; (G) posterior end of body, latero-dorsal view; and (H) posterior end of body, ventral view. A1 [A2/A3], first [second/third] abdominal segment; an, antennal complex; ap, anal plate; as, anterior spiracle; cr, transverse crevice; ex, extra-anal papilla; ko, Keilin's organ; ll, labial lobe; lo, labial organ; mp, maxillary palpus; ns1 [ns2], first [second] additional sensillum coeloconicum; or, oral ridges; pa, postanal papilla; paa, para-anal papilla; pre, pre-anal papilla; sa, subanal pailla; sb1 [sb2/sb3], first [second/third] sensillum basiconicum; sc1 [sc2/sc3], first [second/third] sensillum coeloconicum; sub, suprabuccal teeth; vo, ventral organ.

Fig. 3. Third instar of S. nudiseta. (A) Anterior end of body, lateral view; (B) anterior end of body, ventral view; (C) maxillary palpus; (D) ventral organ; (E) facial mask; (F) anterior spiracle; (G) Keilin's organ; and (H) lateral spinulation of second thoracic segment. an, antennal complex; as, anterior spiracle; cut, cutaneous teeth; ko, Keilin's organ; ll, labial lobe; lo, labial organ, mp, maxillary palpus; ns1 [ns2], first [second] additional sensillum coeloconicum; or, oral ridges; sb1 [sb2/sb3], first [second/third] sensillum basiconicum; sc1 [sc2/sc3], first [second/third] sensillum coeloconicum; sub, suprabuccal teeth; vo, ventral organ.

Fig. 4. Larvae of S. nudiseta. (A) First instar, cephaloskeleton, lateral view; (B) first instar, cephaloskeleton, ventral view; (C) second instar, cephaloskeleton, lateral view; (D) second instar, cephaloskeleton, ventral view; (E) third instar, cephaloskeleton, lateral view; and (F) third instar, posterior spiracles.
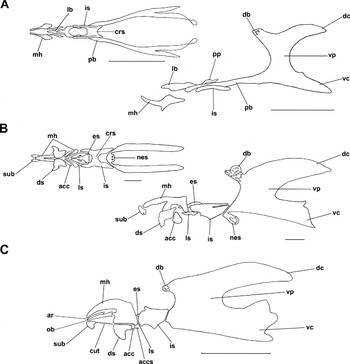
Fig. 5. Cephaloskeletons of S. nudiseta larvae. (A) First instar, ventral and lateral view; (B) second instar, ventral and lateral view; and (C) third instar, lateral view. acc, accessory stomal sclerite; accs, supplementary accessory stomal sclerite; ar, anterior ribbon, crs, crossbeam, cut, cutaneous teeth; db, dorsal bridge; dc, dorsal cornua; ds, dental sclerite; es, epistomal sclerite; is, intermediate sclerite; lb, labrum; ls, labial sclerite; mh, mouthhooks; nes, necklace structure; ob, oral bar; pb, parastomal bar; pp, process diverging from the parastomal bar; sub, suprabuccal teeth; vc, ventral cornua; vp, vertical plate. Scale bare 0.1 mm (A, B) and 1 mm (C).
Cephaloskeleton
The cephaloskeleton of the first instar (figs 4A, B and 5A) consists of paired mouthhooks, an unpaired labrum, an unpaired intermediate sclerite and a basal sclerite with paired parastomal bars and paired vertical plates connected by a dorsal bridge, with ventral and dorsal cornua. The mouthhooks are shaped like a slightly up-curved rod, with a widened basal part. The mouthhooks are not equipped with teeth apically. The labrum is shaped like a knife blade with a broader basal part and narrower apical part and is fused with the tips of the parastomal bars. Each parastomal bar is long and, in the anterior part, equipped with a short process directed posteriorly. The H-shaped intermediate sclerite is elongated, and the crossbeam is broad. The vertical plate is slightly broader than the width of the dorsal and ventral cornua. The dorsal cornua are shorter than the ventral ones. The dorsal bridge has few perforations and is less sclerotized than the vertical plates and the ventral and dorsal cornua.
The cephaloskeleton of the second instar (figs 4C, D and 5B) consists of three main parts, paired mouthhooks, an unpaired intermediate sclerite and a basal sclerite. The mouthhooks are F-shaped with a robust basal part and slender distal part slightly curved ventrally. Paired suprabuccal teeth are present anteriorly to the mouthhooks; each consists of about three teeth. The suprabuccal teeth are not connected to the mouthhooks. Below the anterior ventrolateral extension of the mouthhooks, paired angular dental and paired tooth-like accessory stomal sclerites are present. The intermediate sclerite is elongated, H-shaped with a broad crossbeam in lateral view directed anteroventrally. A longitudinal incision is present on the dorsal surface of the intermediate sclerite, forming a bar-like structure that does not reach the basal sclerite. Anterodorsally, an angular epistomal sclerite lies freely between the anterior arms of the intermediate sclerite, and a pair of labial sclerites lie anteroventrally. The epistomal sclerite is equipped with two rounded perforations. The dorsal bridge is well perforated. A necklace structure with two rounded perforations is present in the antero-ventral part of the vertical plate. Vertical plates are wider than the width of the dorsal and ventral cornua.
The cephaloskeleton of the third instar (figs 4E and 5C), as in the second instar, consists of three main parts and additional sclerites. Among the additional sclerites, paired oral bars with serrated tips and anterior ribbons adjoining dorsally, a pair of dental sclerites connected ventrally, paired accessory stomal sclerite, paired labial sclerite and an epistomal sclerite equipped with four rounded perforations are found. Posteriorly to the accessory stomal sclerites, a pair of supplementary accessory stomal sclerites is present in mature larva. Paired suprabuccal teeth, each consisting of about three teeth are not connected with mouthhooks. Between suprabuccal teeth and the dental sclerite, a row of cutaneous teeth is present on both sides of the buccal cavity. The dorsal bridge is finely perforated.
Thoracic and abdominal segments
Pair of trichoid sensilla of Keilin's organ are present on the ventral side of the thoracic segments (figs 1F, 2A and 3A, G). Anterior spiracles consist of four to six lobes (figs 2A, and 3A, F), except for the first instar, where a simple aperture is present (fig. 1A, H). Openings of posterior spiracles are surrounded by four branched spiracular tufts (figs 2E, 6D and 7C). Slits of the posterior spiracles are straight in the first, slightly curved in the second and S-shaped in the third instar. The spiracular disc is surrounded by four pairs of papillae, located on the surface of the anal division in the first instar (fig. 6C, E) and on the small protuberances in the second (fig. 2G) and third (fig 7E, F) instars. The anal plate is triangular, relatively small and surrounded with well-developed papillae (figs 2G, H; 6C, F and 7D–F). It is hardly visible in the lateral view (figs 2G and 6E) and in the ventral view does not reach the base of para-anal papilla (figs 2H, 6F and 7D). Subanal papillae of all instars are equipped with a small sensory papilla in the middle. Small sensilla coeloconica are present on thoracic and abdominal segments (fig. 1G). In the middle of the ventral surface of abdominal segments 1–7, transverse crevice is present (figs 2F, 6A, B and 7A, B, D). Spinulation pattern (figs 2F, 3H, 6A, B and 7A, B, D) found in our study are in accordance with the description by Siddons & Roy (Reference Siddons and Roy1942).

Fig. 6. First instar of S. nudiseta. (A) Thoracic and abdominal segments, ventral view; (B) abdominal segments, lateral view; (C) posterior end body, posterior view; (D) posterior spiracle; (E) posterior end of body, lateral view; and (F) posterior end of body, ventral view, A1 [A2], first [second] abdominal segment; ap, anal plate; ex, extra-anal papilla; pa, postanal papilla; paa, para-anal papilla; pre, pre-anal papilla; ps, posterior spiracle; sa, subanal pailla; st, spiracular tufts; T3, third thoracic segment.

Fig. 7. Third instar of S. nudiseta. (A) Thoracic and abdominal segments, lateral view; (B) abdominal segments, ventral view; (C) posterior spiracle; (D) posterior end of body, ventral view; (E) posterior end of body of young larva, posterior view; and (F) posterior end of body of mature larva, ventral view. A1 [A6/A7], first [sixth/seventh] abdominal segment; ap, anal plate; ex, extra-anal papilla; pa, postanal papilla; paa, para-anal papilla; pre, pre-anal papilla; ps, posterior spiracle; sa, subanal pailla; st, spiracular tufts; T3, third thoracic segment.
Growth and duration of developmental stages
Faster growth was observed when temperatures increased. Larval growth at all temperatures showed sigmoidal curves (fig. 8). The longest larvae always appeared the day before prepupa, at 25 °C and 30 °C on day 5, at 20 °C on day 6, at 15 °C on day 13, and at 12 °C on day 15. After this, length decreased slightly when larvae entered the post-feeding stage and abandoned the food to pupate. The maximum length reached by the larvae showed significant differences between temperatures (H = 93.40, df = 4, P = < 0.001). The largest larvae were obtained at 15 °C (17.47 ± 0.63 mm) and the smallest at 12 °C (14.60 ± 0.74 mm). The largest individual was 19.34 mm.

Fig. 8. The mean (±SD) length of S. nudiseta larvae, measured at 24-h intervals for each temperature regime (n = 50). At 12 °C, after the sixth day larvae were measured every 72 h (n = 25).
The duration of each developmental stage under all temperature regimes is presented in table 1 and illustrated in fig. 9. The increase of temperature had an effect on the duration of developmental stages, which decreased drastically. Total development varied between 46.50 ± 0.97 days at 15 °C and 15.39 ± 0.32 days at 30 °C. There were significant differences in larval duration (H = 162.41, df = 4, P < 0.001), pupal duration (H = 143.012, df = 3, P < 0.001) and total development (H = 143.019, df = 3, P < 0.001). The rate of larval development increased with temperature (y = 0.0059x–0.0179; R2 = 0.95). The t 0 was 3 °C and degree-days varied between 154.00 and 183.96 DD. For total development, linear regression (y = 0.003x–0.0219; R2 = 0.98) resulted in a t 0 of 7.3 °C and degree-days ranged from 309.75 to 358.05DD (table 1).

Fig. 9. Isomorphen-diagram for S. nudiseta, showing the duration of developmental stages from oviposition to eclosion, at 15, 20, 25 and 30 °C. ●, egg hatching; ○, pupation; ▼, adults emergence.
Table 1. Duration (mean ± SD) of developmental stages and accumulated degree-days for S. nudiseta, for each temperature regime.

abc Different letters within rows indicate significant differences (P < 0.05).
Analysis of the cases studied
Table 2 shows a summary of the 22 cases, from the IAFM and IMLA, where S. nudiseta was present. Of these, 13 were found in the centre of the country (Madrid), eight in the southeast (Alicante) and one in the northwest (Ourense). A total of 17 cases were dated from autumn, two from spring, two from summer and one from winter. With the exception of one case, S. nudiseta was always present indoors, and most corpses were mummified to some degree. Larvae of S. nudiseta were collected from different regions of the corpses, including the natural openings of the head, torso, genitals and legs. In several cases, pupae and empty pupae were located attached to the clothes and hair (fig. 10A–C). Regarding succession, analyzed cases suggest that the arrival time of S. nudiseta could be different according to environmental conditions. It is the first colonizer in autumn but arrives after Calliphora vicina Robineau-Desvoidy, 1830 during colder months, whereas in summer it arrives together with, or after Chrysomya albiceps (Wiedemann, 1819), Lucilia sericata (Meigen, 1826) and Sarcophaga sp. Meigen, 1826. Next, a case from IMLA that has been considered the most representative of the usefulness of this species in southern Europe is presented.

Fig. 10. Pupae of S. nudiseta embedded in the hair of the deceased. (A) Lateral and (B) frontal view, and (C) detail of the pupae.
Table 2. Summary of the cases from IAFM and IMLA where S. nudiseta appeared breeding in human corpses. Postmortem interval estimated by forensic pathologist or from witness testimonial of the last sighting of the individual alive.

1 Madrid (central Spain); Ourense (northwest Spain); Alicante (southeast Spain)
E, eggs; LI, larva of first; LII, second; LIII, third instar; P, pupa; EP, empty puparia; L?, larva of unknown instar.
IMLA Case: 794/2010
The fully clothed body of a woman was found sitting on the couch in an apartment in Torrevieja on 4 November 2010. The corpse was mummified. The body showed no signs of injury or criminal violence and death had been due to natural causes. The maximum postmortem interval (PMImax) (time of death) estimated by the pathologist was 40 days. The average temperature in the area, before the discovery of the body was 19.1 °C. The entomological samples were one pupa of C. vicina, larvae, pupae and empty puparia of Ch. albiceps (Calliphoridae), larvae and pupae of S. nudiseta and pupae of Megaselia scalaris (Loew, 1866) (Phoridae).
The species collected with the most advanced developmental stage was Ch. albiceps, with empty puparium; however, they were low in number and the majority of adults emerged two days later in the laboratory. In contrast, pupae of S. nudiseta were the most abundant sample; and, together with M. scalaris, they emerged three days after being collected. No adult emerged from the single pupa of C. vicina. According to several authors (Marchenko, Reference Marchenko2001; Al-Misned et al., Reference Al-Misned, Amoundi and Abou-Fannah2003; Grassberger et al., Reference Grassberger, Friedrich and Reiter2003), Ch. albiceps completes its cycle in approximately 19 days at 20 °C. Data from this study (table 1, fig. 9) and results obtained by Rabinovich (Reference Rabinovich1970) indicate that S. nudiseta needs about 26–27 days to complete its development at 20 °C. For M. scalaris, information on development at 20 °C is highly variable, but pupae of this species have been found in corpses indoors with a PMI of 10–20 days (Disney, Reference Disney2008). Given that S. nudiseta has the longest cycle, was more abundant and spent more time on the corpse, we can suppose that this species was the first colonizer. A minimum postmortem interval of around 24 days, based on S. nudiseta, was estimated.
Discussion
The updated distribution of S. nudiseta from European mainland includes littoral southeast Iberian Peninsula, from the provinces of Alicante (Martínez-Sánchez, Reference Martínez-Sánchez2003), Murcia (Ubero-Pascal et al., Reference Ubero-Pascal, Arnaldos, López-Esclapez, García, Méndez-Vilas and Díaz2010b), Granada (J. Marín, personal communication) and Málaga (Bowden, Reference Bowden1997). Recently, this species has also been collected from Lisbon, Portugal (Prado e Castro et al., Reference Prado e Castro, Serrano, Martins da Silva and García2012) and Naples, Italy (Lebrun & Mayer, Reference Lebrun and Mayer2011). After a review of forensic cases, we realized that the first appearance of S. nudiseta in a corpse was in 2004 in Madrid (central Spain). What is more, the record of Ourense (northwest Spain) confirms that this species has established itself in the Mediterranean Basin and is expanding its distribution northwards. In addition, continuous sampling at the Campus of the University of Alicante shows that abundance of S. nudiseta has increased during the last years, frequently being observed in large numbers during warmer months from early summer onwards (Y. Velásquez, personal observation). This species should not be rare in human remains in other areas of southern Europe. Probably, this species has been overlooked previously because it does not appear in most available ‘forensic’ identification keys and, moreover, may have been confused with other Muscidae (wrongly identified as genus Muscina Robineau-Desvoidy, 1830 or Musca Linnaeus, 1758).
Separation of the larvae of S. nudiseta from other species of Muscidae that are also found during forensic entomology examinations is restricted because of limited information on larval morphology of these flies. The identification of the third instar larvae is relatively easy, thanks to the keys of Ishijima (Reference Ishijima1967), Skidmore (Reference Skidmore1985) and recently Velásquez et al. (Reference Velásquez, Magaña, Martínez-Sánchez and Rojo2010). According to these authors, identification of the third instar is based on the presence of the suprabuccal teeth and cutaneous teeth (figs 3E and 5C), as well as on the peculiar S-shaped slits of posterior spiracles (figs 4F and 7C) and dark pigmentation of the peritreme. Providing a key or any information allowing identification of the first and second instars is seriously limited. Tentatively, it could be stated that identification of the first instar of S. nudiseta is possible because of the presence of sharply ended cirri (fig. 1B), the smooth surface of the dorsal side of thoracic segments (fig. 1A) and a small triangular anal plate surrounded by well-developed papillae (fig. 6C, E, F). For the second instar, the most valuable characters are the small triangular anal plate surrounded by well-developed papillae (fig. 2G, H) and F-shaped mouthhooks (figs 4C and 5B) (Grzywacz et al., unpublished data). On the other hand, relatively easy identification of adults is possible based on the uniquely orange flagellomere and tip of abdomen (fig. 11A) together with wing vein M1 curved forward towards vein R4 + 5 (fig. 11B), not angular (fig. 11C), and lack of the orange or reddish tip of the scutellum (Pont, Reference Pont1991; Carvalho, Reference Carvalho2002; Couri, Reference Couri2007).

Fig. 11. (A) Adult female of S. nudiseta with orange flagellomere (fl) and tip of abdomen (abd); (B) wing of S. nudiseta, dorsal view; and (C) wing of Musca domestica, dorsal view. M1, R4 + 5, wing veins.
Larval morphology of S. nudiseta was described by Siddons & Roy (Reference Siddons and Roy1942); we have focused here on characters insufficiently described or completely omitted. Results of our study agreed with their descriptions and most differences are due to the different terminology; however, a few contradictions were found, mainly in the cephaloskeleton structures. The parastomal bar of the first instar of S. nudiseta in Siddons & Roy's (Reference Siddons and Roy1942) fig. 5 is shown as simple and not connected with the basal sclerite. During this study, a process diverging from the parastomal bar was found, and parastomal bars are continuous with the basal sclerite (figs 4A and 5A), as has been reported in other families (Courtney et al., Reference Courtney, Sinclair, Meier, Papp and Darvas2000; Szpila & Pape, Reference Szpila and Pape2005; Szpila et al., Reference Szpila, Pape and Rusinek2008b; Szpila, Reference Szpila2010; Szpila & Villet, Reference Szpila and Villet2011). We clearly differentiate the epistomal and labial sclerites in the second and third instars, which Siddons & Roy (Reference Siddons and Roy1942) showed as an integral part of the intermediate sclerite, except for an imprecise outline on the drawing of the third instar in their fig. 15.
The cirri of the first instar were described by Siddons & Roy (Reference Siddons and Roy1942) as “the anterior margin of the first (oral) groove”. Similarly to Siddons & Roy (Reference Siddons and Roy1942) and Skidmore (Reference Skidmore1985), we did not observe any presence of the suprabuccal teeth in the first instar although these structures have been found among first instars of other Muscidae, e.g. in Muscina (Skidmore, Reference Skidmore1985), Philornis downsi Dodge & Aitken, 1968 (Fessl et al., Reference Fessl, Sinclair and Kleindorfer2006) (as cuticular teeth) and Musca domestica (Linnaeus, 1758) (Skidmore, Reference Skidmore1985; Szpila & Pape, Reference Szpila and Pape2008). For S. nudiseta, there is no doubt that suprabuccal teeth, present in the second and third instars, are separate structures because there are no signs of any connection with mouthhooks. In other families, sclerotized structures present on the facial mask have been recognized unambiguously (Szpila & Pape, Reference Szpila and Pape2005; Szpila et al., Reference Szpila, Pape and Rusinek2008b; Szpila, Reference Szpila2010; Szpila & Villet, Reference Szpila and Villet2011) or with some reserves (Grzywacz et al., Reference Grzywacz, Pape and Szpila2012) as integral parts of the mouthhooks.
Based on drawings of fig. 7 in Siddons & Roy (Reference Siddons and Roy1942), one could consider the presence of a parastomal bar in the second instar larva of S. nudiseta as Skidmore (Reference Skidmore1985) did. He has indicated the occurrence of a distinct parastomal bar (as a parastomal sclerite), but the presence of such a feature would be unusual and primitive because of the general absence of the parastomal bar in the second and third instars of Muscidae (Skidmore, Reference Skidmore1985). According to our observations, a longitudinal incision is present on the dorsal surface of the lateral arms of intermediate sclerite, forming a bar-like feature that does not reach the basal sclerite (fig. 7B). In our opinion, Siddons & Roy (Reference Siddons and Roy1942) have wrongly recognized this bar-like feature as separated from the intermediate sclerite and connected with the basal sclerite, causing mistakes in Skidmore's interpretation of literature data.
Regarding development, information about the growth of this species is limited. According to the description of Skidmore (Reference Skidmore1985), the range of the length of the third instar is 7.0–19.5 mm, similar to our findings. Compared with the study of Kumara et al. (Reference Kumara, Abu Hassan, Che Salmah and Bhupinder2009), the maximum length of larvae at 28 °C was 14.9 mm, considerably lower than our values between 25 and 30 °C, but they measured five larvae randomly instead of the largest as in the current study. On the other hand, we observed an increase in developmental periods with lower temperatures. Our findings were similar to those reported by Rabinovich (Reference Rabinovich1970) at 20 °C and 28 °C, but higher than those found in Asia (Siddons & Roy, Reference Siddons and Roy1942; Kumara et al., Reference Kumara, Abu Hassan, Che Salmah and Bhupinder2009) and slower than Krüger et al. (Reference Krüger, Ribeiro, Carvalho and Costa2002). Differences between development times may be due to different methods used in each study, e.g. the use of fluctuating temperatures (Siddons & Roy, Reference Siddons and Roy1942; Kumara et al., Reference Kumara, Abu Hassan, Che Salmah and Bhupinder2009) or the use of a carbohydrate diet instead of a protein diet (Krüger et al., Reference Krüger, Ribeiro, Carvalho and Costa2002). Moreover, it has been proposed that some species do not exhibit the same growth pattern in different geographical areas (Greenberg, Reference Greenberg1991; Grassberger & Reiter, Reference Grassberger and Reiter2001; Donovan et al., Reference Donovan, Hall, Turner and Moncrieff2006; Martínez-Sánchez et al., Reference Martínez-Sánchez, Smith, Rojo, Marcos-Gracía and Wall2007). In any case, generation of development data for local populations is imperative in order to permit the proper use of this species of tropical origin in forensic investigations in Europe.
Studies related to low temperatures for this species do not exist. Although in trials at 12 °C, eggs were reared at high temperatures (23–25 °C), larvae were successfully reared at 12 °C. We do not know what the development of S. nudiseta would be like at low temperatures in natural conditions in the Mediterranean region; but, the fact that adults have been captured in the field at 12 °C (Martínez-Sánchez, Reference Martínez-Sánchez2003), larvae have been collected breeding in winter and a minimum development threshold estimated of 3 °C and 7.3 °C for larval and total development, respectively, suggest that this species of tropical origin could have adapted to the lower temperatures that occur in these latitudes in colder months.
The entomological evidence from reviewed cases suggests that the presence of S. nudiseta in human remains is strongly determined by ecological conditions, and its abundance and arrival time varied throughout the year. The high frequency of this species in cases dated from autumn and the heavy infestation observed in this season agree with a phenology study with wind oriented traps (WOT traps) performed in Alicante in 1997, in which this species was present throughout the year with the highest abundance in autumn (Martínez-Sánchez, Reference Martínez-Sánchez2003). Possibly the highest values of humidity produced by autumn rainfall favour the abundance and the arrival time of S. nudiseta. Apparently, this species is able to reach bodies not only inside buildings but also outdoors. Until now, it had not been reported in outdoor remains; nevertheless, in that case, the body was wrapped by a blanket, so it is possible that the wrapping creates a particular microhabitat that allowed oviposition and development of this species outdoors.
Abundance of S. nudiseta on the corpse can give us some conclusions about the season of death, because its activity is dependent on the warm and wet months. Nevertheless, it seems that the main forensic value of this species is the estimation of minimum time of death when the death occurs during autumn, when this species seem to be one of the first colonizers and is more abundant or, in those cases, when pioneer species do not have access to the body or their maggots migrate away from the remains.
The recent records of S. nudiseta from Spain, Portugal and Italy are evidence of range expansion of this species in southern Europe as occurred in the past with other forensically important flies such as Ch. albiceps (Grassberger et al., Reference Grassberger, Friedrich and Reiter2003; Verves, Reference Verves2004; Gosselin & Braet, Reference Gosselin and Braet2008; Szpila et al., Reference Szpila, Matuszewski, Bajerlein and Konwerski2008a). Therefore, we should like to draw forensic entomologists’ attention to the possible presence of this species in entomological material collected from experimental and real cases and its potential use as a forensic indicator.
Acknowledgements
We thank the authorities of the IAFM and IMLA, especially Dr Salvador Giner, for allowing us to collect entomological samples and his close cooperation during the analysis of the cases. We thank Dr Martin J.R. Hall for help during research on larval morphology at the Natural History Museum (London, UK). We are very grateful to Dr Henry Disney at the University of Cambridge for identification of Phoridae, to José Marín for his contribution to the update of the distribution of the species (http://www.diptera.info) and to the Spanish Meteorological Agency (AEMET) for providing weather data. We also thank two anonymous reviewers and Dr Jens Amendt at the Institute of Forensic Medicine, University of Frankfurt (Germany) for valuable comments on an earlier version of the manuscript. This work is part of a PhD project supported by the Programme Alßan, (European Union Programme of High Level Scholarships for Latin America, scholarship No. E06D101359VE) and has been partially supported by the Generalitat Valenciana (GV/2011/039) and the University of Alicante (GRE09-27). Research on larval morphology received support from the SYNTHESYS Project http://www.synthesys.info/, which is financed by the European Community Research Infrastructure Action under the FP7 Integrating Activities Programme and the Polish Ministry of Science and Higher Education (grant no N N303 470838).















