Introduction
Heptacodium miconioides (syn. H. jasminoides) is the seven-son's flower in the Caprifoliaceae. Both the Latin and common names are in reference to the seven flowers in the terminal dichasial cyme (Koller, Reference Koller1986). Heptacodium is a monotypic genus that is endemic to China where it is considered endangered (Jin and Li, Reference Jin and Li2007). It was reintroduced into cultivation as an ornamental large shrub or small tree in 1980 after being initially described in the west by E.H. Wilson in 1907. Heptacodium can be commercially propagated vegetatively by softwood cuttings (Lee and Bilderback, Reference Lee and Bilderback1990) and the recommended seed pretreatment has been 5 months warm stratification followed by 3 months cold stratification, but the authors indicate that results can be variable (Dirr and Heuser, Reference Dirr and Heuser2006).
Seeds with a small under-developed embryo usually display morphological or morphophysiological dormancy (MPD). There are at least eight types of MPD differentiated by the sequences of warm or cold stratification exposure required for dormancy release and the temperature for embryo growth prior to germination (Baskin and Baskin, Reference Baskin and Baskin2004). In the Caprifoliaceae, the major genera have been shown to have either non-deep simple, deep simple, or non-deep complex MPD (Hidayati et al., Reference Hidayati, Baskin and Baskin2000a, Reference Hidayati, Baskin and Baskinb, Reference Hidayati, Baskin and Baskin2001; Rosner et al., Reference Rosner, Harrington, Dreesen and Murray2002). Heptacodium has been projected to have morphophysiological dormancy based on seed morphology and its phylogenetic position between the Diervilla and Lonicera clades (Baskin et al., Reference Baskin, Hidayati, Baskin, Walck, Huang and Chien2006). However, the specific type of MPD in Heptacodium has not been elucidated. Therefore, the objectives of this study were to establish the type of MPD found in Heptacodium as well as the morphological changes in the seed associated with dormancy release. Of particular interest is the interaction between the growing embryo within the seed and specialized endosperm channel cells (Finneseth et al., Reference Finneseth, Layne and Geneve1998) that accommodate the expanding cotyledons.
Materials and methods
Plant material
The dispersal unit for H. miconioides is a single-seeded fruit hereafter referred to as a seed. Seeds were collected from trees on the University of Kentucky, Lexington, KY in November. Seeds were cleaned by rubbing the adhering sepal tissue from the fruit and stored dry in sealed plastic containers at 10°C until used (within 2 months).
Stratification and germination conditions
Seeds were placed in Petri dishes (100 × 15 mm) containing 30 ml of moist washed sand and sealed with Parafilm (Pechiney Plastic Packaging, Chicago, IL, USA). Each Petri dish contained 24 seeds and there were four dishes per treatment. Treatment temperatures for warm stratification and cold stratification were 20 and 5°C, respectively. Seed germination was in incubators at a constant 20°C in 8 hours light and 16 hours dark. Germination was evaluated weekly.
Initial germination
Seeds were placed in Petri dishes with wetted sand and incubated at 15/20, 20 and 25°C and germination evaluated after 30 days. There were 24 seeds per Petri dish and four replications per treatment.
Embryo growth
Stratification treatments included warm stratified for 16 weeks; 8 weeks warm followed by 8 weeks cold; and 8 weeks cold followed by 8 weeks warm. Thirty seeds were evaluated every 2 weeks for embryo growth. Seeds were halved along the long axis and placed on a flatbed scanner (HP Scanjet 5370 C with transparency adapter) for imaging as 600 d.p.i. grey-scale JPEG files. Seed to embryo length ratio (embryo length/seed length × 100) was measured using the trace function in SigmaScan Pro 5.0 for Windows (SPPC Science, Chicago, IL, USA).
Impact of gibberellic acid
Seeds were left intact or the top one-fifth (opposite the embryo) of the seed was removed and placed in Petri dishes containing 6 ml of water or treatment solution, two pieces of Grade 8001 germination paper (Stults Scientific, Mt Holly Springs, PA, USA) and sealed with Parafilm. Seeds were treated with gibberellic acid at 0, 100, 300 and 500 µM for 7 days prior to being moved to Petri dishes with wetted sand and incubated at 20°C as previously described for germination. There were 30 seeds per Petri dish; replicated four times.
Morphology
For anatomical observations, seeds were fixed in formalin-acetic-alcohol (FAA) and vacuum-infiltrated for 24 h. Tissue was rinsed twice with 50% ethyl alcohol and dehydrated using a tertiary butyl alcohol dehydration series. Tissue was then embedded in paraffin blocks and sectioned (12–15 µm thick). Tissue sections were affixed to glass slides (8 × 3 cm) and stained with Safranin O-Fast Green. Sections were observed and photographed under a light microscope (Olympus BX40) equipped with a digital camera (Olympus DP25).
Results and discussion
Seed (fruit) morphology
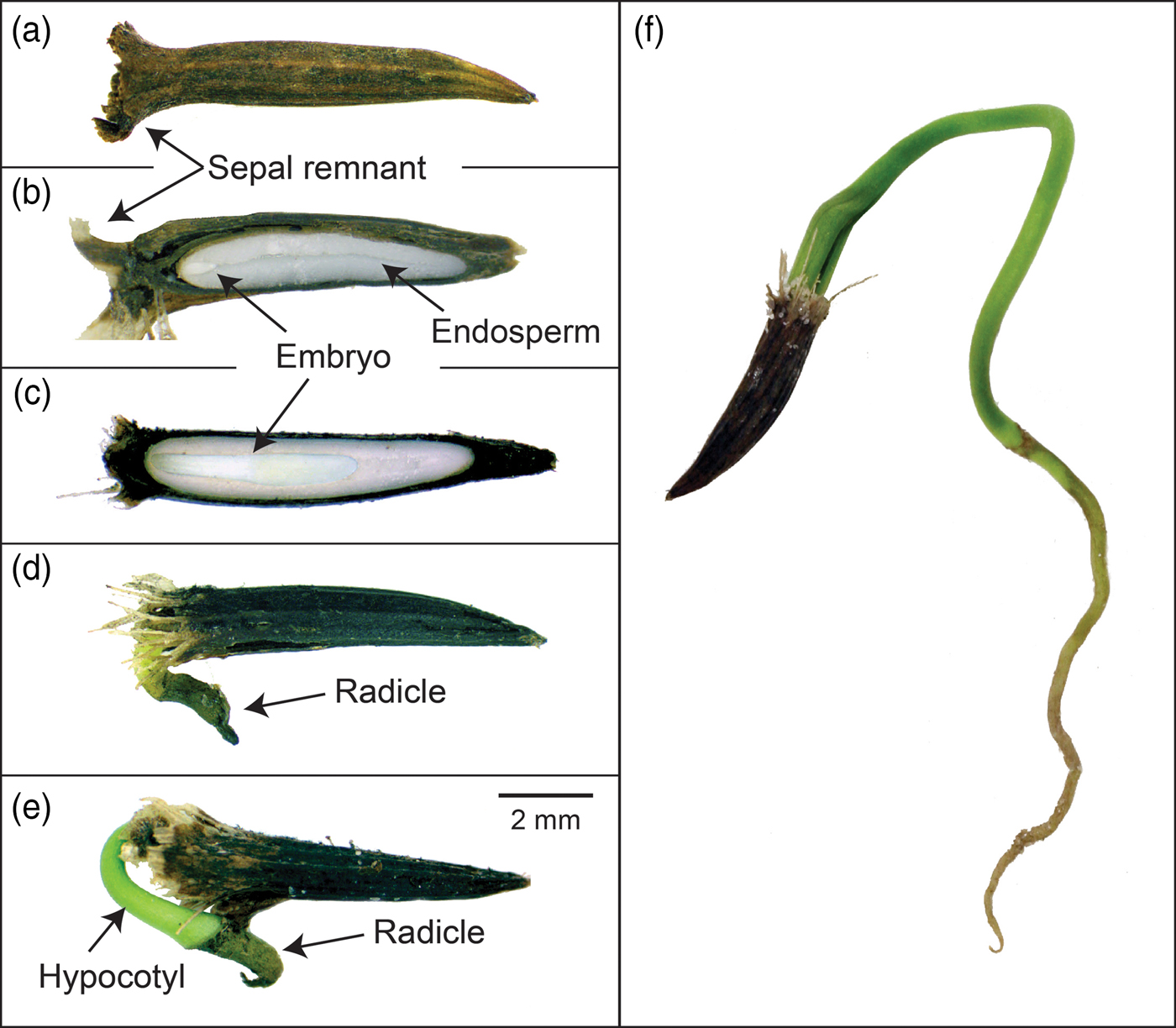
The dispersal unit in Heptacodium is a linear, indehiscent achene (Jacobs et al., Reference Jacobs, Lens and Smets2009) with a sepal remnant attached to the calyx end of the fruit (Figs 1 and 2). The pericarp is multilayered and the lower endocarp contains sclerified fibres (Fig. 1c). The seed is separated from the pericarp by a compressed parenchyma seed coat (Jacobs et al., Reference Jacobs, Lens and Smets2009). The seed coat is generally a few layers thick except over the radicle where it is multicellular (Fig. 1b and d). There is an apparent suberized layer between the seed coat and endosperm (Fig. 1d). The linear embryo is under-developed and at dispersal occupies less than 15% of the seed length (Figs 1 and 2). Although small, the embryo has differentiated cotyledons, apical meristem and a radicle (Fig. 1b). The embryo is embedded in endosperm that fills most of the seed. Endosperm cells are generally thick walled, isodiametric in shape and packed with storage vesicles (Fig. 1d).
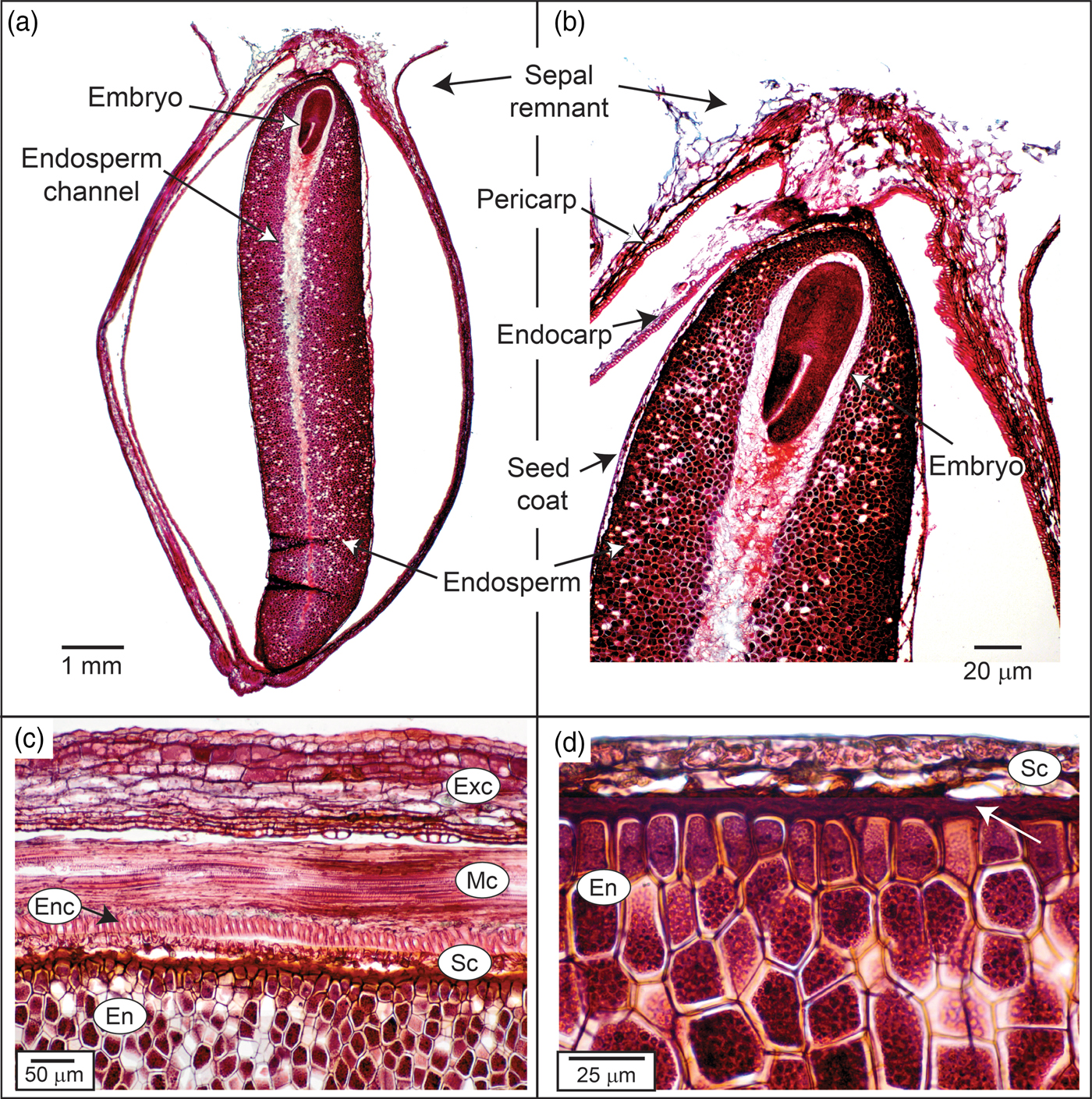
Figure 1. Photomicrograph of Heptacodium fruit (seed). (a) Whole seed. (b) Embryo end of seed. (c) Pericarp layers, seed coat and endosperm. Arrow indicates sclerified endocarp layer. (d) Seed coat and endosperm. Arrow indicates apparent suberized layer. Exc, exocarp; Enc, endocarp; En, endosperm; Mc, mesocarp; Sc, seed coat.

Figure 2. Embryo growth and germination in Heptacodium. (a) Intact fruit (seed). (b) Longitudinal section showing initial embryo size relative to endosperm size. (c) Longitudinal section showing embryo growth after 8 weeks warm stratification. (d–f) Epigeal germination and seedling emergence.
The central portion of the endosperm contains an endosperm channel with larger, less densely packed cells with few storage vesicles (Figs 1 and 3). This channel is the space the enlarging embryo will occupy during subsequent growth following dispersal and prior to germination (Figs 2c and 3e). There are few references to endosperm channel cells in seeds with underdeveloped embryos and this is the first description in the Caprifoliaceae. Seeds with underdeveloped embryos mainly occur in the gymnosperms and primitive angiosperms (Baskin and Baskin, Reference Baskin and Baskin2004). However, several advanced orders of plants also display under-developed embryos. This includes the Dipsacales that contains genera in the Adoxaceae and the Caprifoliaceae. By comparison, the endosperm channel in Heptacodium is similar to endosperm cells observed in the relatively primitive pawpaw (Asimina triloba) except that pawpaw seeds have a pair of channels corresponding to the cotyledons that grow through these cells prior to germination (Finneseth et al., Reference Finneseth, Layne and Geneve1998). These cotyledons in pawpaw are haustorial and never emerge from the seeds in a cryptocotylar germination pattern. Cotyledons emerge from the seed in Heptacodium following a typical epigeal germination pattern (Fig. 2f).
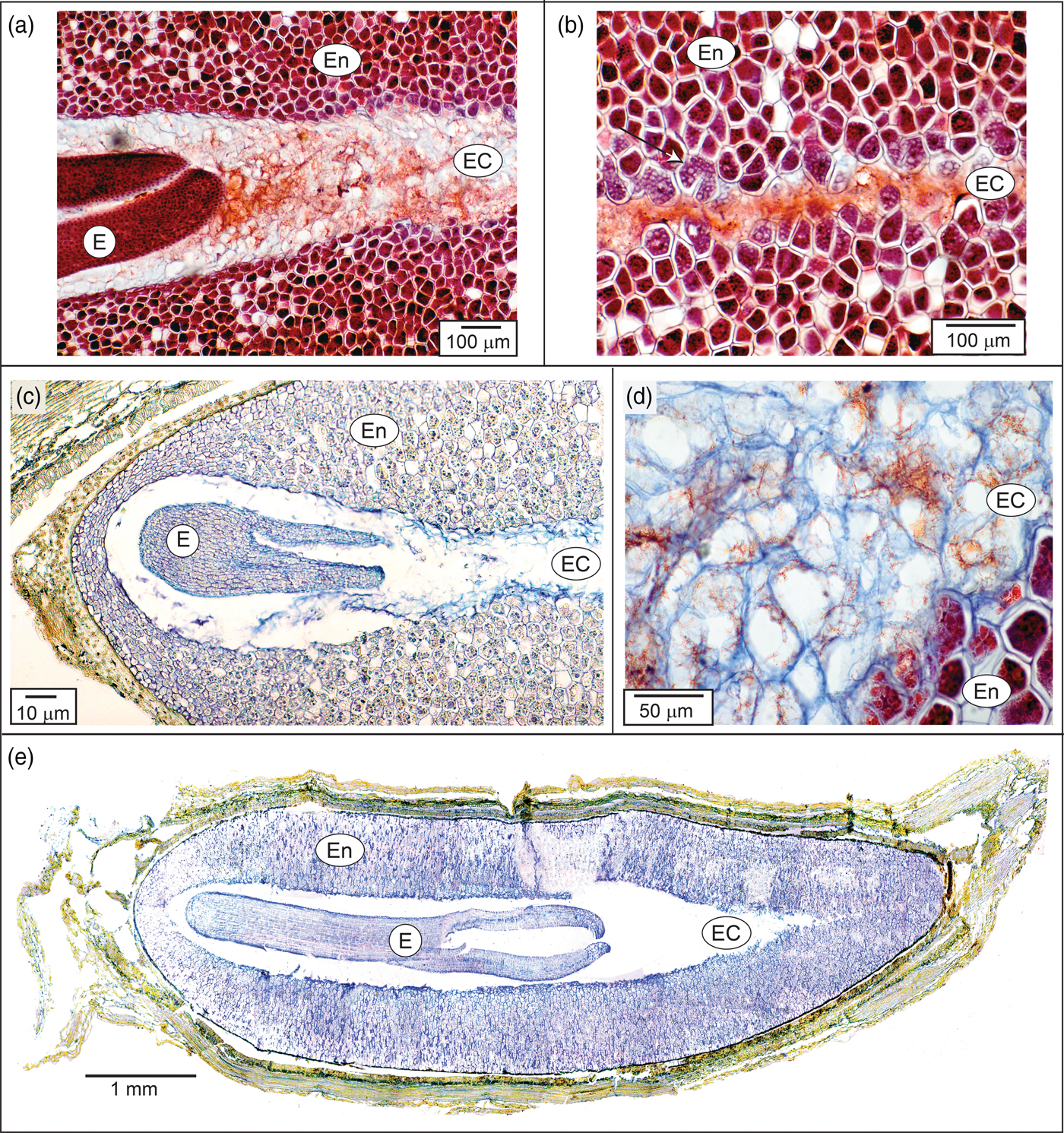
Figure 3. Endosperm and endosperm channel in Heptacodium. (a) Initial seed showing the portion of the seed near the embryo. (b) Same seed showing endosperm and endosperm channel distal to the embryo. Arrow indicates dissolution of storage reserves. (c, d) Seed after 4 weeks warm stratification showing a dissolution zone in endosperm channel cells as cells begin to autolyse. (e) Seed after 8 weeks warm stratification. E, embryo; En, endosperm; EC, endosperm channel cells.
During embryo growth, cells in the endosperm channel of Heptacodium formed a dissolution zone similar to the endosperm cells surrounding the haustorial cotyledon described in palm seeds during germination (Pinheiro, Reference Pinheiro2001). Initially, channel cells began to lyse in proximity to the embryo (Fig. 3a), but activity was also observed in channel cells distal to the embryo (Fig. 3b). It has been suggested that in endospermic seeds with living endosperm cells, the embryo is the source of hormonal signalling molecules that initiate synthesis or activation of hydrolytic enzymes within endosperm cells. In celery (Apium), the embryo enlarges two to three times its size prior to germination. The endosperm cells adjacent to the embryo showed the first signs of storage reserve hydrolysis and the activity spreads radially (Jacobsen and Pressman, Reference Jacobsen and Pressman1979). In celery seeds with the embryo removed, enzymatic activity in the endosperm could be induced by treatment with gibberellin, indicating that endosperm cells could be the source of hydrolytic enzymes and suggesting that the embryo was the source of gibberellin. DeMason et al. (Reference DeMason, Stillman and Ellmore1989) demonstrated that the source of hydrolytic enzymes in several palms (Phoenix and Washingtonia) was localized to the endosperm cells rather than in the expanding haustorial cotyledon.
Initial embryo growth
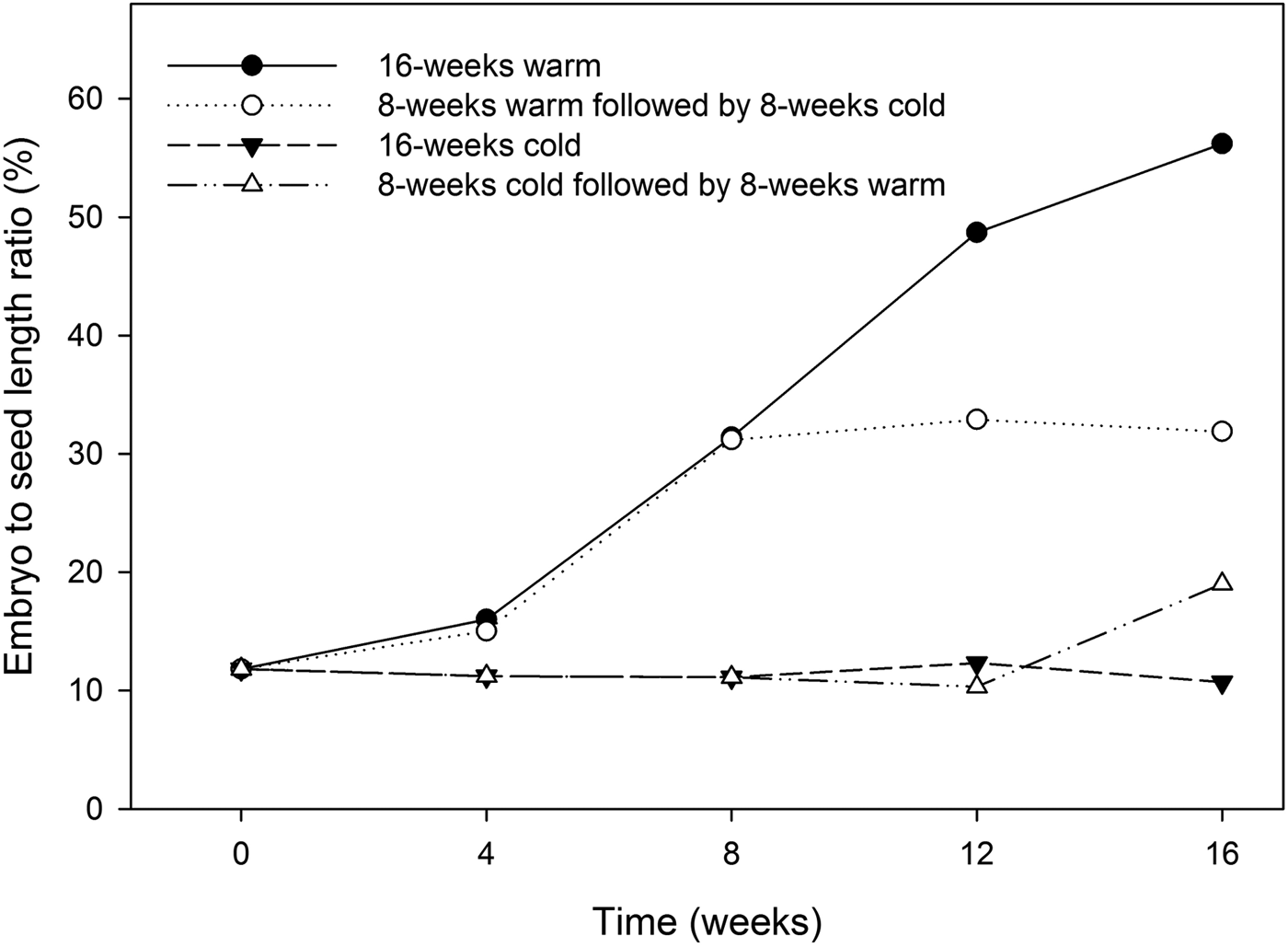
At the time of fruit dispersal, seeds displayed an under-developed embryo with an embryo to seed length ratio of approximately 12% (Fig. 4). Seeds sown at 15/20, 20 or 25°C failed to germinate within 30 days (data not shown), indicating that Heptacodium seeds have either morphological or morphophysiological dormancy. Embryo growth only took place during warm (20°C) stratification (Fig. 4). Seeds placed under warm conditions for 8 weeks had embryos that doubled in size (Fig. 2c), but this growth was arrested by moving seeds to cold (5°C) stratification (Fig. 4).
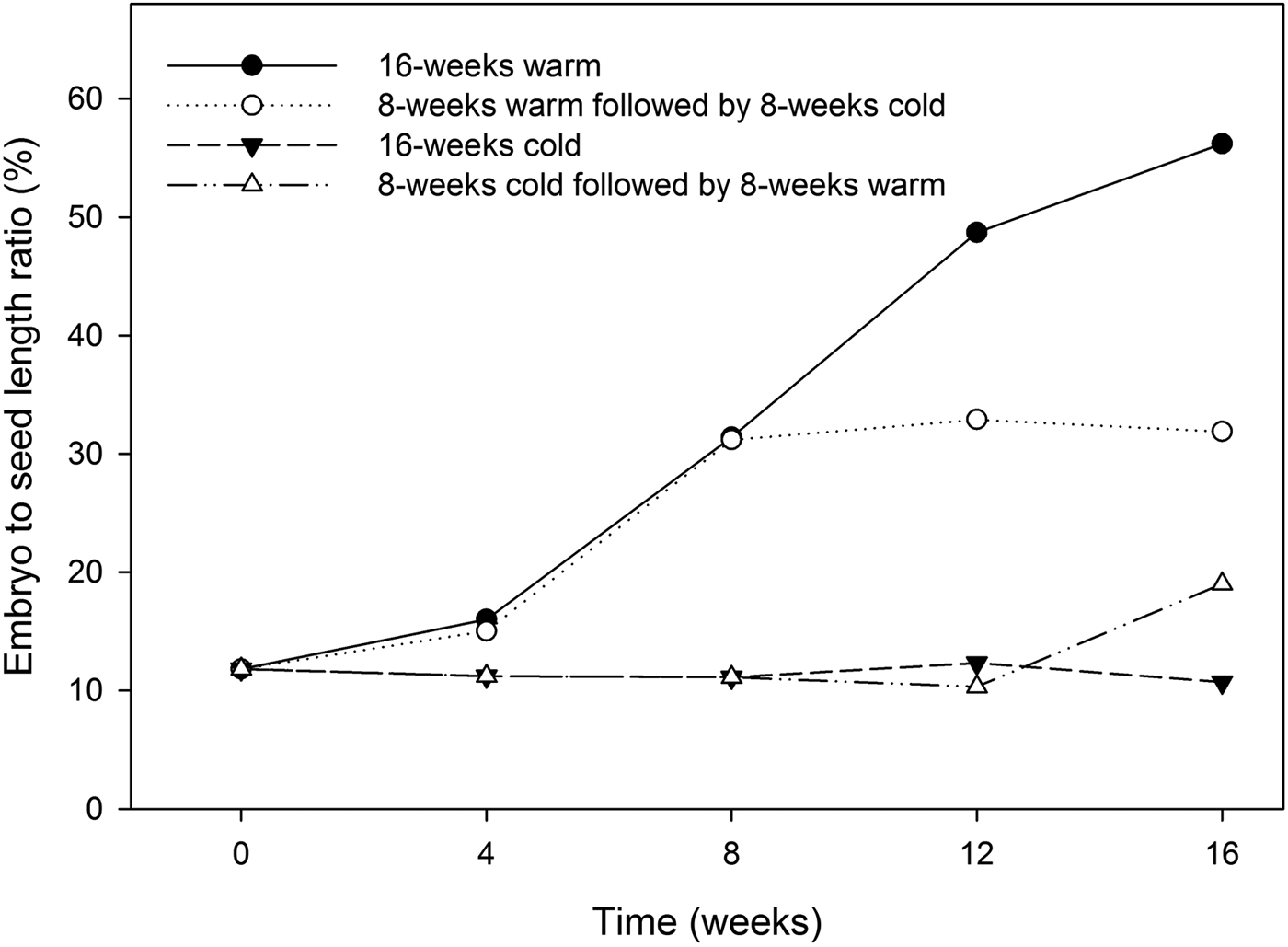
Figure 4. Embryo to seed length ratio in seeds treated by 16 weeks warm (20°C) stratification, 16 weeks cold (5°C) stratification, 8 weeks warm followed by 8 weeks cold stratification, or 8 weeks cold followed by 8 weeks warm.
Impact of gibberellic acid on dormancy release
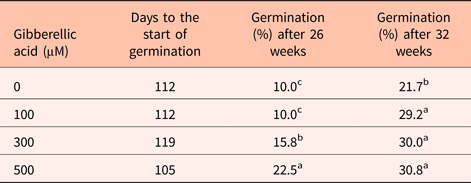
Seeds treated with gibberellic acid (GA) only partially satisfied the need for warm stratification (Table 2). Seeds treated with GA germinated at ~30% compared with 22% for untreated seeds at 20°C. The more dramatic influence of GA treatment was the reduced time to the start of germination that was between 7 and 35 days sooner in seeds treated with 300 and 500 µM GA, respectively (Table 2). The fact that GA only partially substituted for warm stratification could be explained by limited GA uptake through the intact pericarp. In an attempt to increase GA uptake, part of the pericarp was removed prior to GA treatment. However, these seeds deteriorated during germination.
Impact of temperature on dormancy release and germination
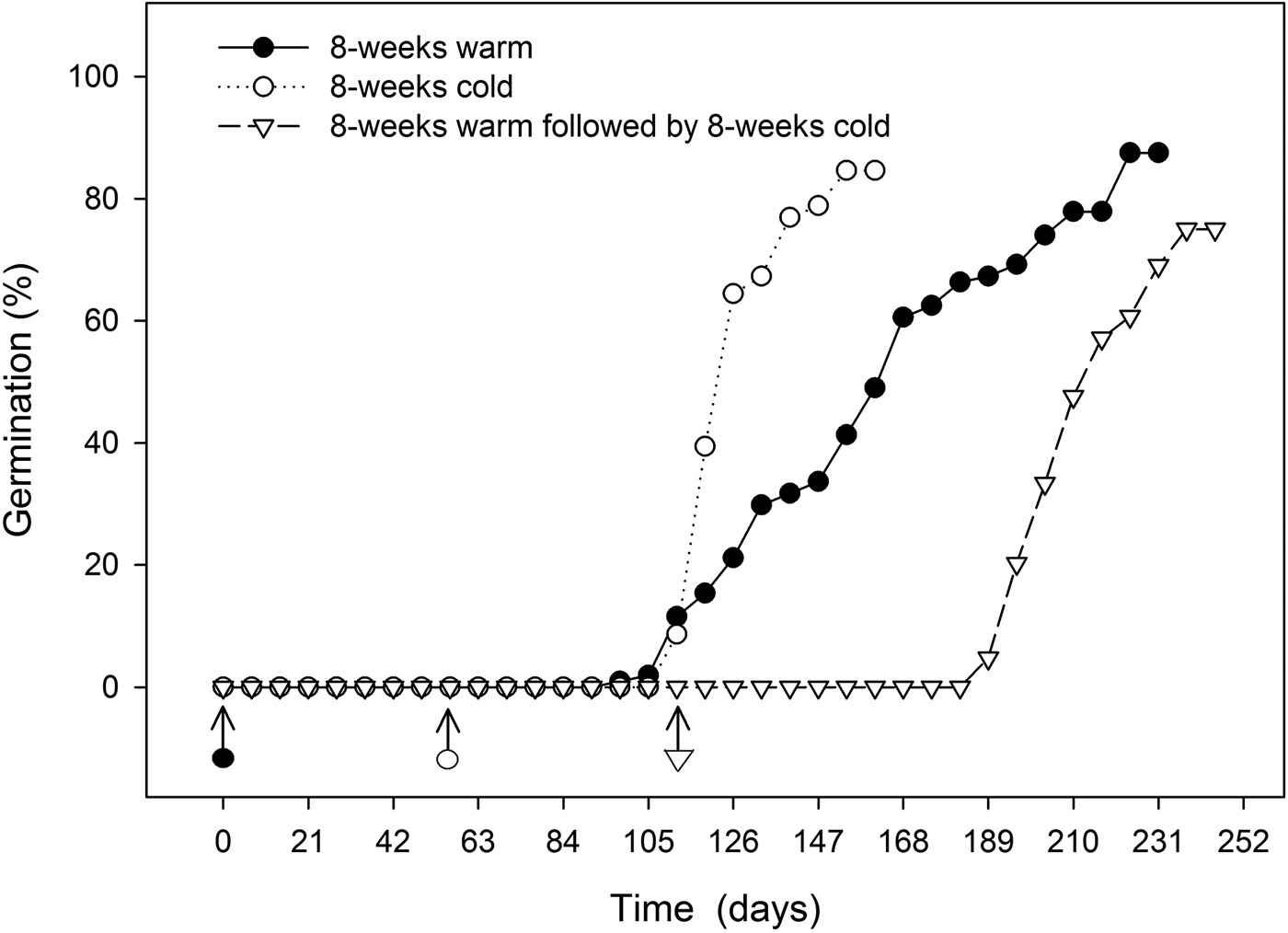
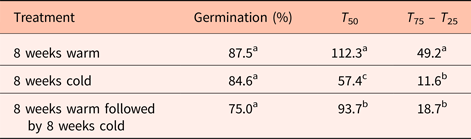
Final germination percentage was not affected by stratification temperature (Table 1), but embryo growth and germination only took place at warm (20°C) temperatures (Fig. 4). Seeds exposed to warm temperatures initiated germination after about 12 weeks but did not reach maximal germination until 30 weeks (Fig. 5). These seeds took the longest time to reach 50% germination and had the greatest spread in the time between 25 and 75% germination (Table 1). Seeds exposed to cold stratification either after warm stratification or prior to warm germination temperatures showed significant increases in germination speed and uniformity (Table 1). Comparing seeds exposed to 8 weeks warm to 8 weeks cold stratification prior to germination showed that cold-stratified seeds reached 50% final germination approximately 55 days sooner than warm-stratified seeds (Table 1 and Fig. 5). Therefore, even though the embryos in cold-stratified seeds did not grow until transfer to warm temperature, they apparently responded to the cold temperature by increasing their growth potential for faster and more uniform germination. Abelia is closely related to Heptacodium with similar fruit and seed morphology (Jacobs et al., Reference Jacobs, Lens and Smets2009). Abelia seeds (fruits) showed a similar response to cold stratification where there were no differences in final germination percentages due to either warm or cold stratification, but seeds exposed to 8 weeks cold stratification showed faster, more uniform germination (Scheiber and Robacker, Reference Scheiber and Robacker2003).
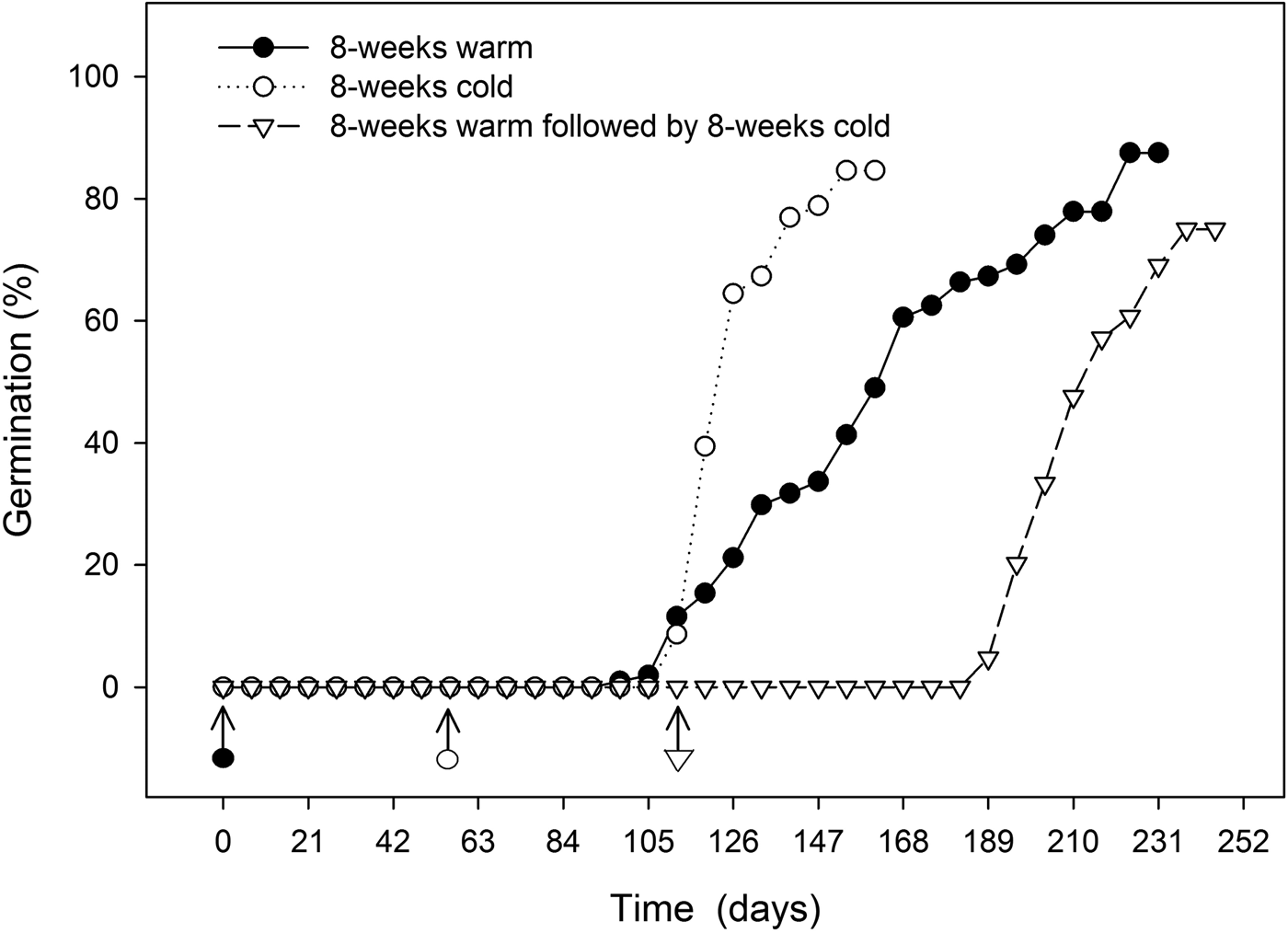
Figure 5. Time course for germination in seeds treated by 8 weeks warm (20°C) stratification, 8 weeks cold (5°C) stratification, or 8 weeks warm followed by 8 weeks cold prior to germination at 20°C in the light. Arrows indicate the beginning of warm treatment.
Table 1. Germination percentage, speed and uniformity in Heptacodium seeds exposed to warm (20°C) and cold (5°C) stratification prior to germination at 20°C
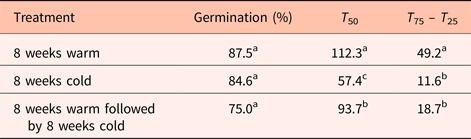
Means within a column followed by the same letter were not different at α = 0.05 using Tukey's test.
Conclusion
Heptacodium displays the characteristics of seeds with non-deep simple morphophysiological dormancy as warm or cold stratification was effective for dormancy release (Table 1), embryo growth prior to germination only occurred at warm temperatures (Fig. 4) and gibberellic acid partially substituted for cold stratification (Table 2). The current suggested protocol for nursery seed propagation is 5 months warm stratification followed by 3 months cold stratification (Dirr and Heuser, Reference Dirr and Heuser2006). Based on the current study, it is evident that seeds should be cold stratified for at least 2 months followed by warm conditions for germination. This treatment led to the most rapid and uniform germination (Fig. 5). Alternatively, dormancy release and germination will occur in seeds held only under warm conditions, but time to final germination can be delayed by up to 80 days compared with cold-stratified seeds (Fig. 5).
Table 2. The impact of gibberellic acid on Heptacodium seed germination at 20°C
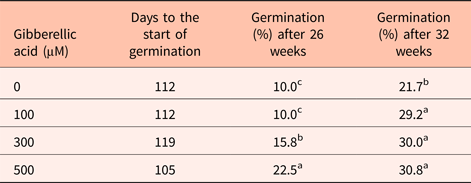
Means within a column followed by the same letter were not different at α = 0.05 using Tukey's test.
Acknowledgements
This is publication no. 17-11-057 of the Kentucky Agricultural Experiment Station and is published with the approval of the Director. This project was supported by the USDA National Institute of Food and Agriculture, hatch project number KY011042.
Conflicts of interest
None.