Introduction
Damp or regularly submerged rocks are an important habitat for species of the genus Verrucaria. Orange et al. (Reference Orange, Hawksworth, McCarthy, Fletcher, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009) reported 16 Verrucaria species in freshwater habitats in Great Britain and Ireland (including three since transferred to Hydropunctaria or Placopyrenium), and Thüs & Schultz (Reference Thüs and Schultz2009) reported 17 species of Verrucaria (and 2 of Hydropunctaria) from Central Europe. The latter authors listed a further 25 published names as ‘uncertain species', reflecting the poor taxonomic knowledge of this group. Difficulties in studying Verrucaria arise from the simple structure and consequent scarcity of good morphological distinguishing features between species, the apparent variability of some taxa, and the large number of published names of doubtful application.
Three species below were first suspected as being undescribed taxa on morphological grounds, but DNA sequencing has provided greater confidence that they represent taxa distinct from those already known.
Materials and Methods
Fresh or herbarium material was sectioned by hand. Ascospores were measured in c. 5% KOH. Perithecial density was estimated for subjectively chosen areas of thallus by using a stereomicroscope fitted with a drawing tube, to allow simultaneous viewing of an area of thallus and of a square drawn on a sheet of paper, the square representing an area of 25 mm2 at the magnification in use. The number of perithecia which had their ostioles within the square was counted.
DNA extraction and sequencing
DNA was extracted from thallus or ascoma tissue of recently collected or frozen specimens, using the Qiagen DNeasy Plant Mini Kit; the manufacturer's instructions were followed except that warm water was used for the final elution. PCR amplification was carried out using Bioneer AccuPower PCR Premix in 20 µl tubes. The two internal transcribed spacer regions and the 5.8S region (ITS1–5.8S–ITS2) of the nuclear ribosomal genes, and the 5′ end of the nuclear ribosomal large subunit (LSU) were amplified, using the primers ITS1F, ITS4, LR3, nu-LSU-155-5′ and LR7 (Vilgalys & Hester Reference Vilgalys and Hester1990; Gardes & Bruns Reference Gardes and Bruns1993; Döring et al. Reference Döring, Clerc, Grube and Wedin2000). Part of the mitochondrial ribosomal small subunit (mtSSU) was amplified using the primers mrSSU1 and mrSSU3R (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999). The PCR thermal cycling parameters were: initial denaturation for 5 min at 94°C, followed by 5 cycles of 30 s at 94°C, 30 s at 55°C, and 1 min at 72°C, then 30 cycles of 30 s at 94°C, 30 s at 52°C and 1 min at 72°C. PCR products were visualized on agarose gels, stained with ethidium bromide, and purified using the Sigma GenElute PCR Clean-Up Kit. Sequencing was performed by Macrogen Inc.
Sequence editing and alignment
Sequences were assembled and edited using DNAstar Lasergene software (http://www.dnastar.com/products/lasergene.php). Alignment was carried out using BioEdit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html); ClustalW was used to create an initial alignment, which was edited manually. All newly generated sequences were deposited in GenBank (Table 1).
Table 1. Specimens used in the phylogenetic analyses. New sequences are in bold

Phylogenetic analysis
Three gene regions were analyzed, the ribosomal LSU (selected taxa), the ITS1+ITS2 region (all taxa treated below), and the mitochondrial SSU (selected taxa). Sequences used in analyses are listed in Table 1.
1. Ribosomal LSU analysis
Sixty sequences of Verrucariaceae from GenBank, and four sequences representing each of Verrucaria hydrophila, V. nodosa, V. placida, and V. rosula, were analyzed to assist in the recognition of taxa likely to be related to the three new species, and in the selection of suitable outgroups for the ITS analyses. Phylogenetic relationships and support values were investigated using Maximum Likelihood bootstrapping, as implemented in the RaxML Black Box, hosted by the Cipres Web Portal (http://www.phylo.org/sub_sections/portal/; Miller et al. Reference Miller, Pfeiffer and Schwartz2010), using the default settings.
2. ITS analysis
Two groups of taxa, suggested by the results of the LSU analysis, were analyzed separately, to allow a more successful alignment of ITS sequences:
-
a. Verrucaria hydrophila and V. placida, together with V. dolosa.
-
b. Verrucaria rosula, together with V. macrostoma, V. nigrescens, and V. viridula. Verrucaria nodosa (for which no LSU was available) was also included, due to strong similarities between the ITS of this species and V. rosula.
In both groups of taxa the 5.8S region was invariable and was omitted from the analysis. Analysis was undertaken using a Bayesian approach. Models of evolution for the ITS1 and ITS2 regions were selected using the Akaike Information Criterion in MrModeltest 2.2 (Nylander Reference Nylander2004). Gaps were treated as missing data. Using MrBayes 3.1.2 (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2005), two analyses of two parallel runs were carried out on a partitioned dataset for 1 000 000 generations, with trees sampled every 100 generations. Stationarity was considered to have been reached when the average standard deviation of split frequencies dropped to <0·01, and the values for the Potential Scale Reduction Factor were close to 1. A burn-in sample of 2500 trees was discarded from each run, respectively. Support values of ≥95% Bayesian posterior probabilities were regarded as significant.
3. mtSSU analysis
Mitochondrial SSU sequences from the first group of taxa of the ITS analysis were also analyzed using a Bayesian approach, using the same procedure as for the ITS.
Results
Phylogenetic analyses
The LSU analysis (not shown) recovered with good support a clade containing Verrucaria rosula, together with Endocarpon spp., V. macrostoma and V. nigrescens, V. polysticta and V. viridula (species belonging to the Endocarpon clade of Gueidan et al. Reference Gueidan, Roux and Lutzoni2007, Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss and Orange2009), and V. funckii (not included in the analysis by Gueidan et al.). Verrucaria dolosa, V. hydrophila and V. placida also formed a well-supported clade, but their position in relation to other sequences was unresolved.
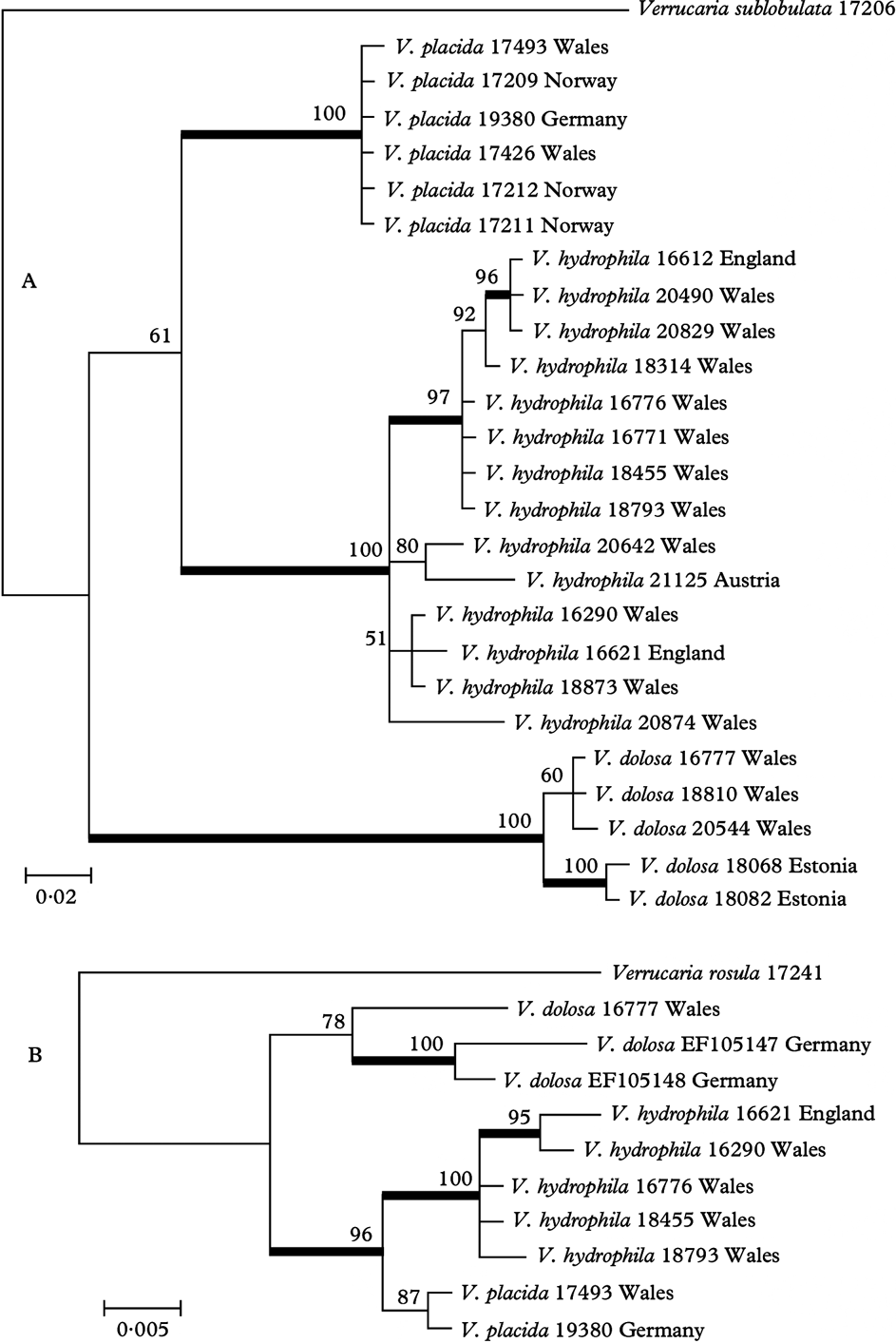
The ITS analysis of Verrucaria placida and related taxa recovered three well-supported clades, here regarded as corresponding to V. placida, V. hydrophila, and V. dolosa (Fig. 1A). Verrucaria placida sequences showed little variation, but within V. hydrophila and V. dolosa some subclades received good support. Analysis of available mtSSU sequences of the same taxa also recovered clades corresponding to V. placida, V. hydrophila and V. dolosa (Fig. 1B). The five sequences of V. hydrophila formed a well-supported clade. The two available sequences of V. placida formed a clade, but with low support. In V. dolosa, an mtSSU sequence was only available from one of the specimens used in the ITS analysis; this formed a poorly-supported clade with two mtSSU sequences from GenBank named as V. dolosa, suggesting that this species as understood by recent authors may be heterogeneous.

Fig. 1. Phylogenetic relationships of Verrucaria placida and related species, based on a Bayesian analysis. Branches in bold indicate a support of PP ≥95%. A, Bayesian analysis of the ITS1+ITS2 regions of the nuclear ribosomal DNA; the tree was rooted using Verrucaria sublobulata; B, Bayesian analysis of the mitochondrial SSU; the tree was rooted using Verrucaria rosula.
ITS1 of Verrucaria placida, V. hydrophila and V. dolosa also contains a short unalignable region, 8–12 bases long at approximately position 123–134 of the ITS. This region is distinctive for each species, though there is variation within V. hydrophila.
The ITS analysis of V. rosula and related taxa recovered three well-supported clades corresponding to V. rosula (seven sequences with little variation), V. macrostoma together with V. nigrescens and V. viridula, and V. nodosa (Fig. 2).

Fig. 2. Phylogenetic relationships of Verrucaria rosula and related species, based on a Bayesian analysis of the ITS1+ITS2 regions of the nuclear ribosomal DNA; the tree was rooted using Verrucaria elaeina. Branches in bold indicate a support of PP ≥95%.
The results support the integrity of the four species Verrucaria hydrophila, V. nodosa, V. placida and V. rosula, which are described below as new.
The Species
Verrucaria hydrophila Orange sp. nov.
MycoBank No.: MB802488
Verrucaria hydrela auct., non Ach., Syn. meth. lich.: 94 (1814).
Verrucaria denudata Zsch., Hedwigia 67: 74 (1927); nom. illeg., non Nyl. (1858), Mém. Soc. Acad. Maine-et-Loire 4: 49.
Thallus tenuis, 25–60 µm crassus, laevis, continuus. Perithecia prominentias conico-hemisphaericas formantia, prominentiae 240–400 µm latae, ab thallo pro parte maxima obtectae. Involucrellum conicum vel conico-hemisphaericum. Excipulum 145–225 µm latum. Ascosporae ellipsoideae, aseptatae, hyalinae, (15·0–)19·5–21·1–23·0(–26·0)×(6·5–)8·0–8·9–9·5(–11·0) µm. Pycnidia nulla.
Typus: Great Britain, Wales: V.C. 50, Denbighshire, Bontuchel, Coed y Fron-wyllt, Nant Melin-dŵr, 53·10°N, 3·37°W, national grid reference 33/0825.5713, on shaded stone by stream in woodland, 26 April 2007, A. Orange 16776 [NMW – C.2007.001.11; GenBank accession nos FJ664844 (ITS–LSU), JX848586 (mtSSU)].

Fig. 3. Verrucaria hydrophila (holotype). Scale=1 mm. In colour online.

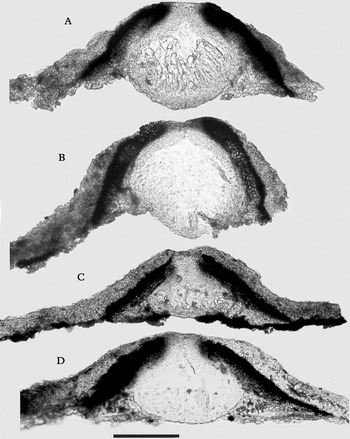
Fig. 4. Verrucaria hydrophila, sections of perithecia. A, holotype; B, Orange 18455; C, Orange 16612; D, Orange 16290. Scale=100 µm.
Thallus thin, 25–60 µm thick, smooth, continuous or sparsely cracked, grey-green to mid-brown, more or less translucent when fresh and wet; cells irregularly arranged, at most with a very weak vertical arrangement; cells coherent, with no air spaces between them; cortical pigment, when present, brown; adjacent conspecific thalli never separated by dark lines.
Perithecia forming conical-hemispherical mounds 240–400 µm wide, at first covered to the apex by a layer of thallus, later the black apex of the involucrellum sometimes exposed; 29–42–64 perithecia in an area of 25 mm2 (11 areas measured, on 5 sequenced specimens); ostiolar area inconspicuous, plane or convex, appearing as a pale dot 15–50 µm wide. Involucrellum conical, reaching to substratum, densely pigmented on upper side, often becoming almost colourless adjacent to base of exciple; pigment dark brown to dark reddish brown, K+darker brown to grey-brown. Exciple 145–225 µm diam., colourless except at apex. Ascospores (15·0–)19·5–21·1–23·0(–26·0)×(6·5–)8·0–8·9–9·5(–11·0) µm, (1·7–)2·2–2·4–2·6(–3·3) times as long as wide [473/34], perispore not detected.
Pycnidia not detected.
Ecology and distribution
On rocks and stones in streams, streamlets, and seepages. Associated species include Hydropunctaria rheitrophila, Verrucaria andesiatica, V. aquatilis, V. margacea, V. pachyderma, V. rosula and V. sublobulata. Throughout the British Isles. Widespread in Europe.
Notes
Distinguished by the smooth, thin thallus, small perithecia, the conical involucrellum which is covered by a thin layer of thallus, and rather small ascospores. Verrucaria placida and V. andesiatica (sensu Orange et al. Reference Orange, Hawksworth, McCarthy, Fletcher, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009) differ in the larger and more widely spaced perithecia and the larger ascospores (Table 2). Verrucaria dolosa differs in the distinctly smaller ascospores (15–18×6–8 µm), smaller, more crowded perithecia, and lack of a regular thalline covering to the mature perithecium. The young perithecium in V. hydrophila is typically covered to its apex by a layer of thallus, but the dark apex of the involucrellum may be exposed later. The exposure of the involucrellum is sometimes caused by abrasion in the stream environment, but can also be seen in specimens from tiny flushes where there is minimal sediment load and a very gentle current.
Table 2. Morphological differences between Verrucaria andesiatica, V. placida and V. hydrophila

* number of perithecia in an area 25 mm2.
† Verrucaria andesiatica sensu Orange et al. (Reference Orange, Hawksworth, McCarthy, Fletcher, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009)
This species has been widely known as Verrucaria hydrela (Santesson et al. Reference Santesson, Moberg, Nordin, Tønsberg and Vitikainen2004; Thüs & Schultz Reference Thüs and Schultz2009) but is not conspecific with the type of that species (H–ACH 682A!), which has a cracked thallus with slightly convex areoles, unlike any morph seen of the present species. The name Verrucaria denudata Zschacke has also been widely used for this species (e.g. Krzewicka Reference Krzewicka2012), but is unfortunately illegitimate.
The type specimen of Verrucaria subhydrela Serv. [(Czech Republic) Bohemia, (montes Krušné hory), Rudohoří, Hassenbach s.v. ad Volyne, 650 m, 1950, Servít (PRM 758626—holotype!)] is similar in external appearance to this species, and the single available section shows a spreading, conical involucrellum. Despite this, Servít (Reference Servít1951) distinguished V. subhydrela from V. denudata by the involucrellum not spreading sideways (and by the presence of a thalline covering to the perithecium). Servít's drawing shows a thin, appressed involucrellum unlike any examples seen in V. hydrophila. This disparity between the type specimen and protologue provides a poor basis for a name, and there is the possibility that the description is based on discordant elements: although the two small fragments of the holotype appear to be uniform, other parts of the collection may exist, as Servít cites a duplicate sent to A. H. Magnusson.
The opportunity is taken here to describe this common and widespread but unnamed taxon as a new species, based on a type specimen for which ITS, and partial LSU and mtSSU sequences are available. The available ITS sequences for the species show some variation, and it is possible that a more intensive survey over a wider geographical area would delimit additional taxa within this group. In this case, sequenced specimens would be invaluable in order to fix the application of the new name with confidence.
Selected specimens examined. Great Britain: Wales: V.C. 41, Glamorgan: St Fagans, Museum of Welsh Life, 31/1133.7711, 2009, Orange 18314 (NMW – C.2010.001.123). V.C. 42, Breconshire: 2·5 km north-west of Erwood, near Tregaer, River Wye, 32/0795.4416, 2010, Orange 18793 (NMW – C.2010.001.128). V.C. 43, Radnorshire: Llanstephan, Bach Howey, 32/105.428, 1993, Orange 9746 (NMW – C.2003.002.44]). V.C. 44, Carmarthenshire: near Rhandirmwyn, Nant y Gelynen, 22/7484.4577, 2012, Orange 20874 (NMW – C.2012.002.58). V.C. 46, Cardiganshire: Devil's Bridge, west side of Mynach, 22/741.772, 1998, Orange 12515 (NMW – C.2003.002.40); south of Ponterwyd, Ystumtuen, south of Penrhiw, Afon Tuen, 22/7381.7793, 2010, Orange 18873 (NMW – C.2010.001.117). V.C. 48, Merioneth: Talsarnau, Bryn Bwbach, 23/6393.3685, 2005, Orange 16290 (NMW – C.2005.001.347); Dolgellau, near Hendre-gefeilliaid, 23/7582.1815, 2009, Orange 18455 (NMW – C.2009.002.66); Ganllwyd, Rhaeadr Mawddach, 23/7360.2757, 2011, Orange 20642 (NMW.C.2011.014.97); Ganllwyd, Dolmelynllyn Hall, 23/7264.2976, 2011, Orange 20490 (NMW – C.2011.014.27); west of Dolgellau, Coedydd Abergwynant, 23/6799.1695, 2012, Orange 20829 (NMW – C.2012.002.57). V.C. 50, Denbighshire: Bontuchel, Coed y Fron-wyllt, Nant Melin-dŵr, 33/0825.5713, 2007, Orange 116771 (NMW – C.2010.001.225). England: V.C. 70, Cumberland: Keswick, Threlkeld, 35/2994.2548, 2006, Orange 16612 (NMW – C.2005.001.553); Keswick, Threlkeld, Glenderaterra Beck, 35/296.251, 2006, Orange 16621 (NMW – C.2005.001.559). Scotland: V.C. 97, Westerness: near Fort William, Loch Linnhe, 27/0507.6715, 2004, Orange 15018 (NMW – C.2004.002.399). – Austria: Tyrol: Kühtai, Finstertal, 47°12·46′N, 11°01·44′E, alt. 2025 m, 2012, Orange 21125 (NMW – C.2012-002.131).
Verrucaria nodosa Orange sp. nov.
MycoBank No.: MB802489
Thallus epilithicus, cinereo-viridis vel brunneus, inaequalis, ex areolis imbricatis constans. Perithecia prominentias formans, raro immersa, prominentiae 220–460 µm latae, nuda vel inferne ab thallo obtectae. Involucrellum anguste conicum vel ad excipulum adpressum. Excipulum 190–310 µm latum. Asci 8 sporae continens. Ascosporae aseptatae, hyalinae, (17–)20·5–22·2–24·0(–28)×(8·0–)9·0–9·7–10·5(–11·5) µm.
Typus: Great Britain, Wales: V.C. 48, Merioneth, north-west of Llanuwchllyn, Afon Lliw, 52·88°N, 3·76°W, national grid reference 23/8153.3268, alt. 350 m, on unshaded rock in stream, 28 September 2011, A. Orange 20660 (NMW – C.2012.002.127—holotypus; GenBank accession no JX848561).
(Figs 5–7)

Fig. 5. Verrucaria nodosa (holotype), part of thallus lightly shaded by moss. Scale=1 mm. In colour online.

Fig. 6. Verrucaria nodosa. A, Orange 20458; B, Orange 20648. Scales=1 mm. In colour online.

Fig. 7. Verrucaria nodosa, sections of perithecia. A, holotype; B, Orange 20455; C & D, Orange 20458; E, Orange 20648. Scale=300 µm.
Prothallus not detected. Thallus superficial, grey-green (in shade) to dark brown, 70–500 µm thick, initially comprising discrete, dorsiventrally flattened units which become crowded and overlapping, forming an uneven crust with sparse secondary cracks, sometimes overtopping each other to make a thick uneven crust. Surface of thallus uneven; thallus in section divided into units of variable size, 30–325 µm thick, paraplectenchymatous; cells isodiametric to shortly oblong, c. 5–9×4–8 µm, polygonal and mostly coherent, or of somewhat rounded outline, and with numerous air spaces between the cells; upper (and sometimes also lower) surface of units with brown-pigmented walls. Photobiont cells 5·5–9·0×4·0–6·5 µm.
Perithecia forming low to moderately convex conical-hemispherical projections 220–460 µm diam., sometimes only the lower 0·2–0·3 of perithecium height is immersed in the thallus, the projecting part naked or with an irregular thalline cover below, or sometimes perithecium partly or almost completely overtopped by thallus units. Ostiole inconspicuous or appearing as a pale dot 20–40 µm wide, plane or slightly projecting. Involucrellum appressed to exciple above, usually becoming broader below, rather steeply conical-hemispherical in outline, densely pigmented throughout, or with a pale area adjacent to base of exciple; pigment dark brown to dark reddish brown, ostiolar area often with dark green pigment. Exciple 190–310 µm diam., colourless or the outer layer pigmented throughout. Periphyses c. 25–40 µm long (measured in situ). Ascospores colourless, ellipsoid, simple, (17–)20·5–22·2–24·0(–28)×(8·0–)9·0–9·7–10·5(–11·5) µm, (2–)2·1–2·3–2·5(–3) times as long as wide [75/4].
Pycnidia not detected.
Ecology and distribution
On rocks beside streams, unshaded or lightly shaded, in open woodland or unimproved grassland; associated species include Ionaspis lacustris, Rhizocarpon lavatum, Porpidia hydrophila, Sporodictyon cruentum, Trapelia coarctata, and the bryophytes Racomitrium aciculare and Scapania undulata. So far recorded only from three streams in North Wales.
Notes
This species resembles Verrucaria rosula in the uneven thallus composed of initially discrete units which coalesce and overlap with age. The external appearance varies considerably, probably due to differences in moisture and exposure of the different collecting sites. The initially discrete units are less finely crenulate than in V. rosula, and the surface is more coarsely uneven. In section, the thallus of V. nodosa is less clearly divided into small units than in V. rosula, and the cells frequently have air spaces between them. The involucrellum in V. nodosa is more uniform in thickness than in V. rosula. However, it is possible that some of these differences are due in part to habitat differences: the available specimens of V. nodosa are mostly from sunny rocks which are not permanently damp. It is likely that poorly developed specimens of V. nodosa and V. rosula will be difficult to distinguish by morphology.
Selected specimens examined. Great Britain: Wales: V.C. 48, Merioneth: Ganllwyd, Rhaeadr Mawddach, 23/7358.2756, alt. 140 m, 2011, Orange 20648 (NMW – C.2012.002.128). V.C. 49, Caernarvonshire: east of Beddgelert, stream below Llyn Llagi, 23/6396.4886, alt. 200 m, 2011, Orange 20455 (NMW – C.2011.014.23); ibid., 23/6436.4870, alt. 315 m, 2011, Orange 20458 (NMW – C.2011.014.24).
Verrucaria placida Orange sp. nov.
MycoBank No.: MB802490
Thallus tenuis, 40–65 µm crassus, laevis, continuus. Perithecia prominentias conico-hemisphaericas formantia, prominentiae 400–600 µm latae, ab thallo pro parte maxima obtectae. Involucrellum conicum. Excipulum 250–310 µm latum. Ascosporae ellipsoideae, aseptatae, hyalinae, (19·0–)21·5–24·0–26·5(–30·5)×(8·0–)9·0–9·9–10·5(–12·0) µm. Pycnidia nulla.
Typus: Norway, Hordaland, Modalen, S of Moelvi river, by Bjørkehaugbekken, 60°50·69′N, 05°58·34′E, national grid 32 V LN 35342 49329, alt. 120 m, on stone in shaded streamlet in woodland, with Verrucaria sublobulata, 27 June 2007, A. Orange 17212 & T. Tønsberg (NMW – C.2010.001.116—holotypus; GenBank accession no JX848573).
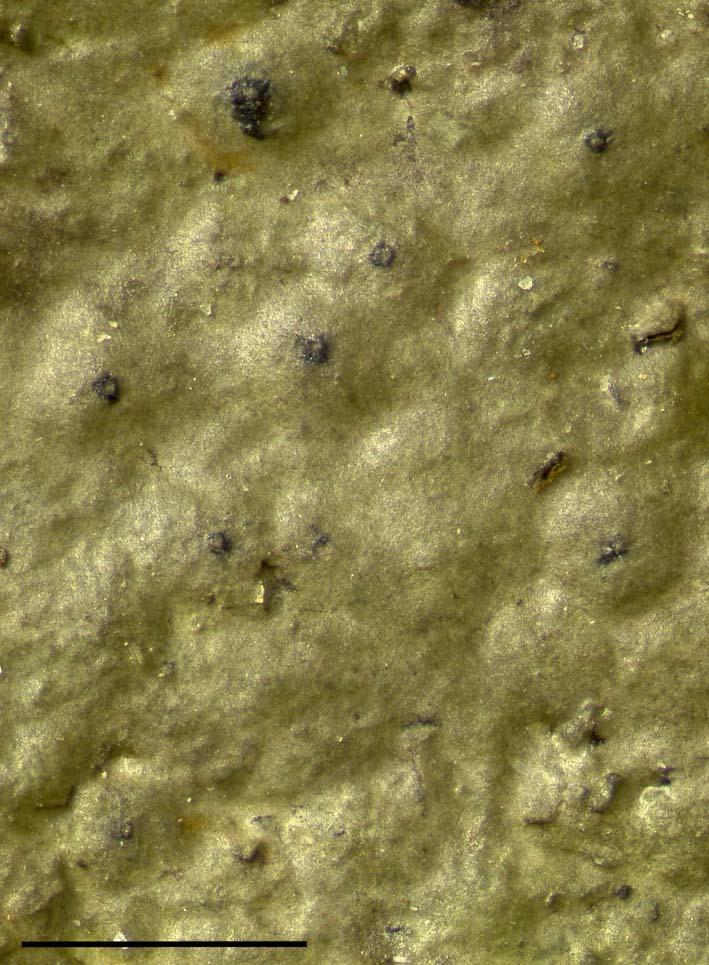
Fig. 8. Verrucaria placida (holotype). Scale=1 mm. In colour online.

Fig. 9. Verrucaria placida, Orange 19830. Scale=1 mm. In colour online.

Fig. 10. Verrucaria placida, sections of perithecia. A, holotype; B, Orange 19830. Scale=100 µm.
Thallus thin, 26–65 µm thick, subgelatinous, translucent when fresh and wet; smooth, more or less matt or slightly glossy, continuous, grey-green to mid brown; contiguous conspecific thalli not separated by dark lines; cells irregularly arranged or in weakly defined columns, coherent, without air spaces between cells; cortex poorly differentiated, comprising a thin layer with few or no photobiont cells (a pseudocortex sensu Gueidan et al. Reference Gueidan, Roux and Lutzoni2007), cortical pigment, when present, brown; thallus without a dark basal layer.
Perithecia forming conical-hemispherical mounds 400–600 µm wide, at first covered by thallus up to apex, later sometimes eroded to expose black apex, 13–21–27 in an area of 25 mm2 (18 areas measured, on 6 specimens). Exciple 250–310 µm wide. Involucrellum conical, reaching to substratum, pigment dark brown, K+dark grey. Periphyses 20–45 µm long. Asci 8-spored. Ascospores simple, hyaline, ellipsoid, (19·0–)21·5–24·0–26·5(–30·5)×(8·0–)9·0–9·9–10·5(–12·0) µm, length/breadth ratio (2·0–)2·2–2·4–2·7(–3·0) [133/8], perispore apparently absent in mature spores.
Pycnidia not detected.
Etymology
The epithet (from Latin ‘placida': quiet, peaceful) was suggested by the smooth, unbroken thallus and the characteristic but unstriking appearance of this lichen.
Ecology and distribution
On shaded siliceous rocks and stones in small streams in woodland. Associated species include Ionaspis lacustris, Verrucaria rosula and V. sublobulata. In Great Britain, so far detected in a semi-natural Quercus petraea woodland (Coed yr Allt-gôch) in Mid Wales at an altitude of 300–350 m; also known from streams in forest in Norway and southern Germany.
Notes
This species is related to Verrucaria hydrophila, but the ITS sequence shows considerable differences. Verrucaria hydrophila differs in the smaller, more crowded perithecia and smaller ascospores. However, V. placida is more likely to be confused with V. andesiatica (sensu Orange et al. Reference Orange, Hawksworth, McCarthy, Fletcher, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009), which is morphologically similar but not closely related (it is more closely related to V. funckii). Verrucaria andesiatica sensu Orange et al. (Reference Orange, Hawksworth, McCarthy, Fletcher, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009) differs in the wider ascospores; in addition, pycnidia are sometimes present, adjacent thalli may be separated by dark lines, the thallus is typically even smoother and more glossy, and the perithecia are usually a little less densely spaced, 12–16–26 in an area of 25 mm2 (19 areas measured, on 5 specimens) (Table 2).
Additional specimens examined. Great Britain: Wales: V.C. 43, Radnorshire: Elan Valley, Penygarreg Reservoir, Coed yr Allt-gôch, 22/902.680, 1991, Orange 9118 (NMW – C.2003.002.42); ibid., 1991, Orange 9122 (NMW – C.2004.002.314); ibid., 22/9027.6809, 2008, Orange 17426 (NMW – C.2007.001.266); ibid., 2008, Orange 17493 (NMW – C.2007.001.269).—Norway: Hordaland: Modalen, S of Moelvi river, by Bjørkehaugbekken stream, 60°50·69′N, 05°58·34′E, national grid 32 V LN 35342 49329, alt. 120 m, 2007, Orange 17209 & Tønsberg (NMW – C.2010.001.114); same locality and date, Orange 17211 & Tønsberg (NMW – C.2010.001.115).—Germany: Baden-Württemberg: Schwarzwald, west of Bärental, 47°52·00′N, 08°03·67′E, alt. 980 m, 2010, Orange 19380 (NMW – C.2010.001.111).
Verrucaria rosula Orange sp. nov.
MycoBank No.: MB802491
Thallus epilithicus, cinereo-viridis vel brunneus, inaequalis, ex granulis parvulis similis goniocystis constans. Perithecia prominentias formans, prominentiae 240–400 µm latae, inferne ab thallo obtectae, superne nudae. Involucrellum conicum vel ad excipulum adpressum. Excipulum 180–305 µm latum. Asci 8 sporae continens. Ascosporae aseptatae, hyalinae, (20·5–)22·5–24·8–27·0(–32·0)×(7·5–)9·0–10·0–11·0(–14·0) µm.
Typus: Great Britain, Wales: V.C. 42, Breconshire, Brecon Beacons, Cwm Dringarth, Nant y Gwair, 51·87°N, 3·52°W, national grid ref. 22/9539.2042, alt. 470 m, on unshaded rock in flush, 4 April 2007, A. Orange 16753 (NMW – C.2007.001.4—holotypus; GenBank accession no FJ664883).

Fig. 11. Verrucaria rosula (holotype). Scale=1 mm. In colour online.

Fig. 12. Verrucaria rosula. A, rosette-like area of growth composed of small subunits (holotype); B, Orange 17241. Scales: A=500 µm; B=1 mm. In colour online.
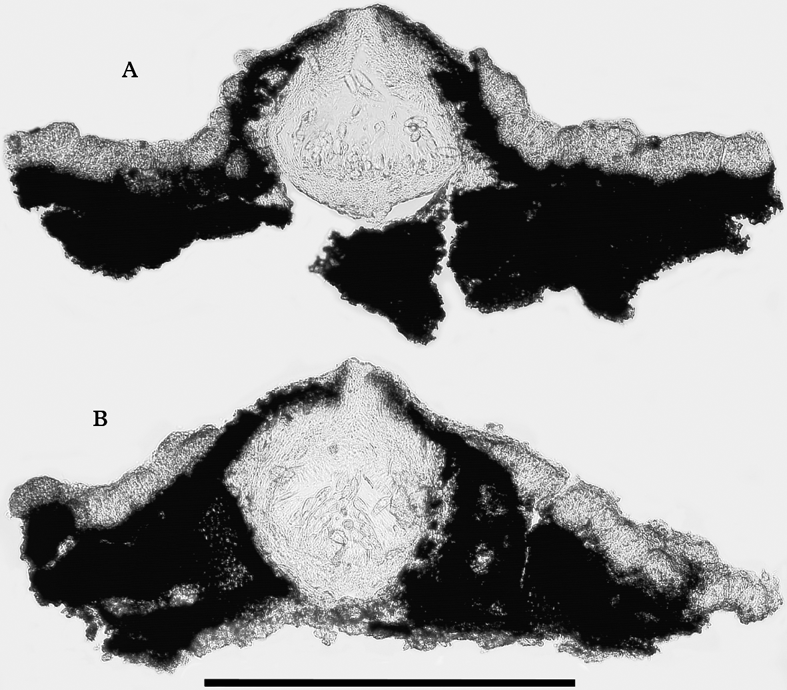
Fig. 13. Verrucaria rosula, sections of perithecia. A, holotype; B, Orange 17489. Scale=300 µm.
Prothallus absent. Thallus superficial, grey-green (in shade) to brown, 40–200 µm thick, initiated by very small granules, first visible as isodiametric structures c. 20–60 µm wide, these increasing in size to form dorsiventrally flattened, more or less circular, rosette-like patches with a minutely crenulate margin; patches coalescing with neighbouring ones to form a diffuse crust which is uncracked or has sparse secondary cracks; healthy layers of thallus often overlying older layers, which are partly dead or which contain abundant brown pigment. Surface of thallus minutely uneven at a scale of c. 20–50 µm; in section usually divided into units c. 30–80 µm wide; paraplectenchymatous, fungal cells more or less isodiametric, 4–10×3–6 µm, walls thin or slightly thickened, c. 0·5–0·8 µm thick, cells at surface (except in shade) with brown-pigmented walls. Photobiont cells 4–10×3·5–7·5 µm.
Perithecia forming projections 240–400 µm diam., often with an irregular covering of thallus below, but naked above, ostiolar region often conspicuous, visible as a pale brown or whitish plane or convex dot 40–80 µm wide. Exciple 180–305 µm diam., unpigmented except for dilute brown pigment near ostiole. Involucrellum variable, thin to well developed, often appressed to exciple, or more or less conical and reaching to the substratum. Asci 8-spored. Ascospores simple, colourless, oblong-ellipsoid, mostly widest above the middle, (20·5–)22·5–24·8–27·0(–32·0)×(7·5–)9·0–10·0–11·0(–14·0) µm, length/breadth ratio (2·0–)2·3–2·5–2·7(–3·1) [159/10], perispore absent.
Pycnidia rare or overlooked (detected on two specimens); conidia c. 5·0×1·2 µm.
Etymology
From the Latin ‘rosula’, suggested by the rosette-like areas of new thallus growth.
Ecology and distribution
On damp siliceous rocks and stones in or near streams, or on flushed ground. Associated species include Ionaspis lacustris, Thelidium pluvium, Verrucaria cernaensis, V. hydrophila, V. sublobulata and V. margacea. Recorded up to an altitude of 470 m in Great Britain (SW England, Wales, Scotland), and at an altitude of c. 640 m in France.
Notes
The mature thallus is an aggregation of dorsiventrally flattened units, expanding at their margins, which coalesce and overlap each other. In turn these are composed of much smaller units, c. 30–80 µm wide, which usually appear as more or less discrete, slightly convex regions in section, and which are responsible for the minutely uneven surface of the thallus (often clearly seen under the dissecting microscope, sometimes more obscure). The perithecia are often overlain by patches of thallus below, but lack a regular and complete thalline covering. The ostiolar region is often rather conspicuous as a pale convex dot; this is a minor but rather characteristic feature of this species.
Although this is a locally frequent species in damp habitats, it appears to have been overlooked, and no published names for this taxon have been found. Verrucaria consociata Serv. [Czech Republic, Rudohoří, Suniperk, 1950, coll. M. Servít (PRM 756809—holotype!)] also has a thallus composed of goniocysts, but the perithecia and ascospores are smaller, and the thallus is weakly developed. Only one specimen of V. rosula has been detected in a herbarium (Watson, BM), originally identified as Verrucaria margacea.
Selected specimens examined. Great Britain: Wales: V.C. 42, Breconshire: north of Fan Nedd, Blaen Senni, 22/9143.1982, alt. 320 m, 2007, Orange 17241 (NMW – C.2007.001.102); Llanwrtyd Wells, Irfon, 22/8673.4726, alt. 215 m, 2011, Orange 20542 (NMW – C.2012.002.129). V.C. 43, Radnorshire: Penygarreg Reservoir, Coed yr Allt-gôch, 22/905.676, 1991, Orange 9131 (NMW – C.2005.001.62, M); ibid., 22/9060.6770, alt. 350 m, 2008, Orange 17489 (NMW – C.2007.001.283). V.C. 46, Cardiganshire: south of Ponterwyd, Ystumtuen, south of Penrhiw, Afon Tuen, 22/7381.7793, alt. 220 m, 2010, Orange 18876 (NMW.C.2010.001.118). V.C. 48, Merioneth: west of Penmaenpool, Coedydd Afon Gwynant, 23/681.166, 1991, Orange 8570 (NMW – C.2005.001.66); Talsarnau, Afon y Glyn, 23/6239.3583, 2002, Orange 13919c (NMW – C.2005.001.701); Ganllwyd, Afon Wen, 23/739.230, 1998, Orange 11988 (NMW – C.2005.001.699); Talsarnau, Bryn Bwbach, Coed Caerwych, 23/6418.3683, alt. 150 m, 2005, Orange 16285 (NMW – C.2005.001.700). V.C. 50, Denbighshire: Llanrhaeadr-ym-Mochnant, Afon Iwrch, 33/124.280, 1998, Orange 12119 (NMW – C.2005.001.697). England: V.C. 5, South Somerset: Treborough, Cold Harbour, 1916, W. Watson (BM); 5 km SW of Porlock, Wilmersham Common, 21/859.426, 1993, Orange 9680 (NMW – C.2005.001.63). Scotland: V.C. 97, Westerness: Achnacarry, River Arkaig, 27/17602.88118, 2011, A. Acton (hb. A. Acton).—France: Cantal: 5 km SE of Mur-de-Barrez, 0·4 km W of Truyère, 44°49′N, 2°43′E, 1990, Orange 7969 (NMW – C90.13.90).
Names considered inapplicable to the taxa treated above
Verrucaria hydrela Ach.
Syn. meth. lich.: 94 (1814); type: [Sweden] ‘Svecia Vestmannia' (H–ACH 682 A!).
The herbarium specimen H–ACH 682 comprises two fragments of rock pasted onto a slip of paper, and bearing different species. The left fragment (H–ACH 682 A, labelled ‘Svecia') has a Verrucaria species with a pale brown thallus which is cracked into somewhat convex areoles; the ascospores are (17·5–)18·5–20·4–22·0(–25·5)×7·5–8·2–9·0(–10·0) µm, length/breadth ratio (2·1–)2·3–2·5–2·7(–3·1) [35 measured]. No other lichens or bryophytes are present on this fragment of rock. The right fragment (H–ACH 682 B, without locality) carries a lichen with a thin, faintly cracked thallus, with prominent black perithecia 80–160 µm diam.; in the one perithecium examined microscopically, no hymenium was present. It is possible that the perithecia belong to a lichenicolous fungus.
The description in the protologue is not detailed enough to determine with certainty which specimen was the basis of Acharius' description; however, H–ACH 682 A is labelled ‘Svecia', which is in accordance with the protologue, and the description of the thallus as ‘verruculosa' also fits this fragment better than fragment B, which furthermore is without locality. The lichen on fragment A does not correspond to any taxon known to the writer, but it is not the same taxon to which the name V. hydrela has been applied by recent authors. Probably this species is known only from the type locality in Sweden.
On the other hand, the type material of V. hydrela (H–ACH 682A!) strongly resembles the alleged type material of V. laevata (H–ACH 683A!), a taxon which was described four years earlier (see below).
Verrucaria laevata Ach.
Lichenogr. univ.: 284 (1810); type: [Germany] ‘ad saxa fluminis Queis Lusatiae' [Lusatia] [Mosig] [material apparently lost].
A packet in Acharius' herbarium in H (H–ACH 683) contains a slip of paper to which two fragments of rock are pasted, and which is annotated ‘Verrucaria laevata' by Acharius. The left fragment (H–ACH 683 A) is annotated ‘Lusatia', a locality that agrees with the protologue. However, it is very likely that this is not the fragment originally attached here. The current fragment bears a lichen very similar in appearance to H–ACH 682 A (the type of Verrucaria hydrela). In addition, the paper slip to which H–ACH 682 A is attached bears a glue mark which corresponds in size to the fragment currently in the position of H–ACH 683 A, suggesting that this mark indicates the true position of the fragment. Vainio also believed that the fragment currently labelled H–ACH 683 A was a misplaced piece of V. hydrela, attached in error (specimen annotated ‘V. hydrela Ach. errore haec positam' by Vainio). Vainio was not determining a type of V. laevata, as supposed by Swinscow (Reference Swinscow1968). Thus the original specimen of V. laevata appears to have been detached and lost.
The right-hand fragment in H–ACH 683 (H–ACH 683 B) is labelled ‘Anglia' and ‘Harriman', and carries an undetermined Verrucaria species, growing with Eiglera flavida. It is likely that the specimen is not from a truly freshwater habitat. This fragment was incorrectly ascribed to V. aethiobola by Thüs (Reference Thüs2002). Thüs designated this fragment as a lectotype of V. laevata, but this is not a suitable choice, as there is no evidence that it is conspecific with the type of V. laevata.
The description of V. laevata in the protologue is too vague to suggest which species might have been intended. As the original material is lost, Verrucaria laevata should be regarded as a nomen dubium.
I am grateful to Andy Acton for submitting a specimen of Verrucaria rosula, to Tor Tønsberg for making fieldwork possible in Norway in 2007, the curators of H and PRM for granting loans, and to two anonymous reviewers for their helpful comments.