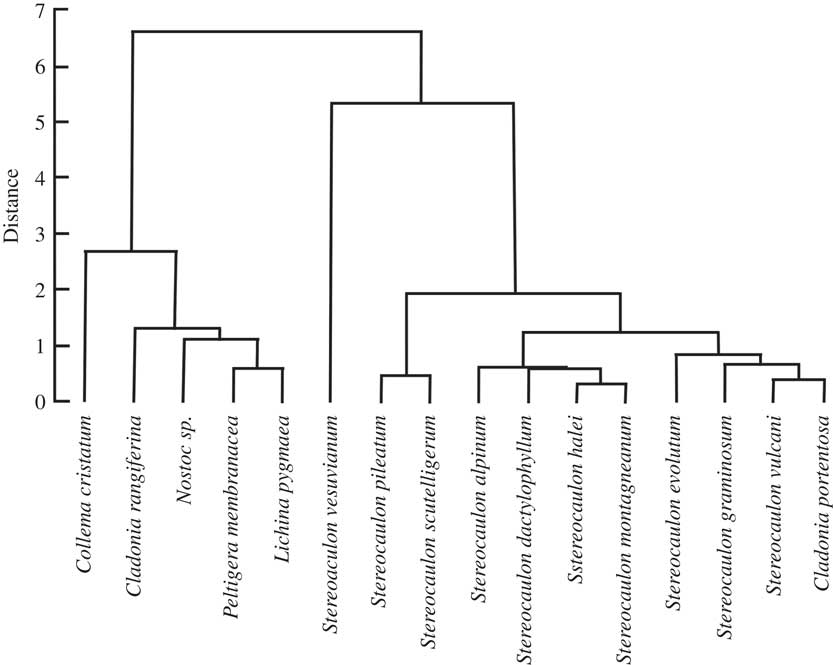
Nomenclature: i/ai-Cx : y n-z denotes a fatty acid containing x carbon atoms and y double bonds, where z indicates the position of the double bonds (i.e. the first double bond starting from the methyl terminal), i is an iso-fatty acid, and ai is an anteiso-fatty acid; Δ is a desaturase enzyme; Δ is an elongase enzyme.
Introduction
Total lipids, although hard to define, include lipids containing fatty acids (FAs), as well as fat-soluble vitamins (A, D, E, K), carotenoids and sterols (Cyberlipid Center; AOCS Lipid Library). FAs are found in lipids such as triglycerides, which are not only implicated in energy storage, but also in the phospholipids of membranes. The biosynthesis of FAs is via condensation of malonyl coenzyme A units by a FA synthase complex. They usually contain even numbers of carbon atoms in straight or branched chains, and may be saturated or unsaturated.
Thin-layer chromatography (TLC) and gas chromatography (GC) are the most common and most powerful analytical procedures for separating and analyzing lipids (Cyberlipid Center). TLC, followed by the isolation of simple lipids (e.g. sterols, carotenoids, total phospholipids, glycolipids), is the method usually applied to lichen lipid analysis (Nylander Reference Nylander1866; Culberson Reference Culberson1969; Hawksworth Reference Hawksworth1976; Solberg Reference Solberg1987; Tabacchi et al. Reference Tabacchi, Tsoupras and Huneck1987; Bachelor et al. Reference Bachelor, King and Richardson1990; Gonzalez et al. Reference Gonzalez, Rodriguez Pèrez, Hernandez Padron and Barrera1992; Sassaki et al. Reference Sassaki, Machado, Tischer, Gorin and Iacomini1999). As such, TLC has been a valuable tool for the systematics of lichens (Spribille et al. Reference Spribille, Klug and Mayrhofer2011). An alternative approach is GC, which is more sensitive and allows the separation and identification of individual fatty acids that are less abundant. However, intact lipids containing bound and free FAs are not volatile enough to be injected into the GC and are therefore usually first derivatized by saponification, followed by methylation to increase the volatility of the compounds, prior to analysis. After derivatization, the lipid’s skeleton (glycerol, for example, in triacylglycerol) and the volatile FA methyl esters (FAMEs) are obtained.
Since each phylum has a typical FA signature (Fig. 1), their FA profiles are increasingly being used as a chemotaxonomic tool for the identification and classification of fungi (Kock & Botha Reference Kock and Botha1998), bacteria (Kaneda Reference Kaneda1991), micro- and macroalgae (Kumari et al. Reference Kumari, Bijo, Mantri, Reddy and Jha2013; Sahu et al. Reference Sahu, Pancha, Jain, Paliwal, Ghosh, Patidar, Bhattacharya and Mishra2013) and octocorals (Imbs & Dautova Reference Imbs and Dautova2008). While FA profiles are generally reported as FAMEs in lichens (Dembitsky Reference Dembitsky1992; Rezanka & Dembitsky Reference Rezanka and Dembitsky1999; Dembitsky & Srebnik Reference Dembitsky and Srebnik2002), only one study has considered the relationship between FA profiles and lichen systematics (Sassaki et al. Reference Sassaki, Cruz, Gorin and Lacomini2001).

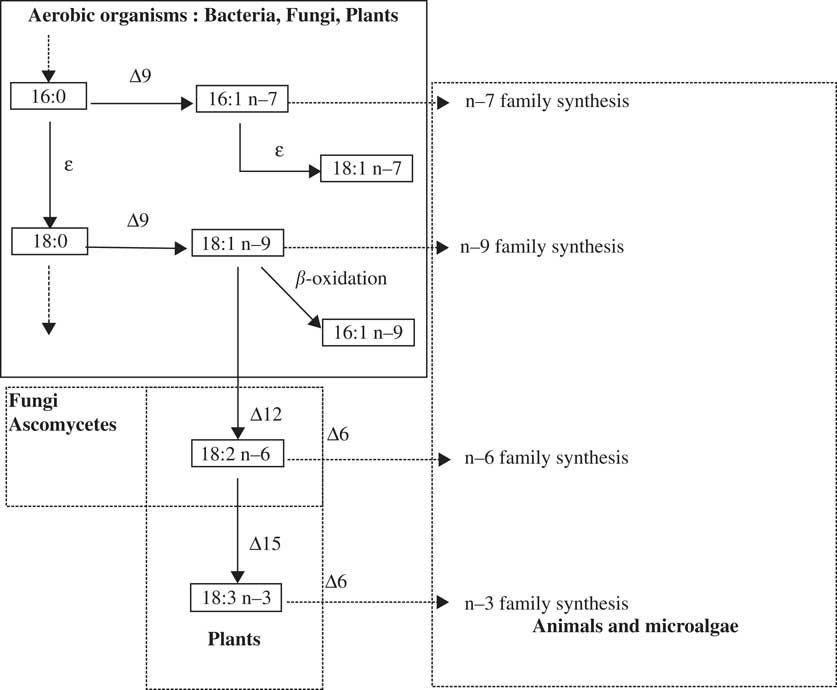
Fig. 1 Simplified biosynthetic pathways for major unsaturated fatty acids. Δx = desaturase where x indicates the position of the cis double bond introduced in the chain; ε = elongase; Cx:y n-z = a fatty acid containing x carbon atoms and y double bonds, where z indicates the position of the first double bond starting from the methyl terminal.
To extend these results, we focused on the lichen genus Stereocaulon, which is challenging because it contains a large number of species and relatively few distinguishing phenolic substance profiles. To address this we obtained, from different regions of the world, ten species of Stereocaulon, which represented four sections described by Lamb (Reference Lamb1951, Reference Lamb1977). Six other species were also included in this study to evaluate the discriminatory strength of FAME analysis. Two Cladonia species were included to allow a comparison with previously published results (Sassaki et al. Reference Sassaki, Cruz, Gorin and Lacomini2001). Three cyanolichens (Lichina pygmaea, Collema cristatum, Peltigera membranacea), along with a macroscopic form of a cyanobacterium (Nostoc sp.), corresponding to the photobiont of the Collema and Peltigera species, were also added for comparison.
Several reports on lichen FAs describe the composition of various genera (see Supplementary Material Text S1, available online). However, they are not easy to compare because a variety of lipid extraction protocols were used. We therefore first developed an optimization procedure for lipid extraction, using Stereocaulon scutelligerum. We compared the extraction duration, volume and composition of solvent mixture, extraction yields and diversity of FAMEs identified by gas chromatography coupled with flame ionization detection (GC-FID). Moreover, an internal standard was used to quantify the extraction yield for FAs in the fraction, to avoid considering other liposoluble compounds that were co-extracted. The selected method was then checked for reproducibility, using a second species submitted to the same protocol. Finally, we applied the selected method to the 16 species for a comparative analysis of the FAMEs profile.
After injection into the GC, the FAMEs are quantified by flame ionization and characterized by their retention time. They are then identified after an additional injection on a GC coupled to a mass spectrometric (MS) detector and compared with a database. The GC-FID-MS reveals the total FA profile, which includes the FA chain length (C8, C18, C23, etc.), the number and the position of double bonds of the unsaturated FAs (C18:2n-6, C22:6n-3). The analysis also distinguishes the iso- and anteiso-series for the branched-chain FAs (BCFAs). Thus the high sensitivity, selectivity and resolution of the GC-FID-MS method allowed an unambiguous determination of the FA composition and a quantification of total FAs, thanks to the internal standard heneicosanoic acid (C21:0).
Our goals were therefore to 1) analyze the FA content in the form of FAMEs of 15 lichens and one cyanobacterium, 2) compare the FA profiles of chlorolichens and cyanolichens and 3) determine, using hierarchical ascendant classification, if FA profiles are of taxonomic value for differentiating lichen genera, as well as Stereocaulon species, with regard to the classification scheme of Lamb (Reference Lamb1951, Reference Lamb1977). We applied the above, using a qualitative and quantitative approach based on FA analysis by GC-FID-MS, in order to elucidate lichen chemotaxonomy.
Materials and Methods
Lichen material
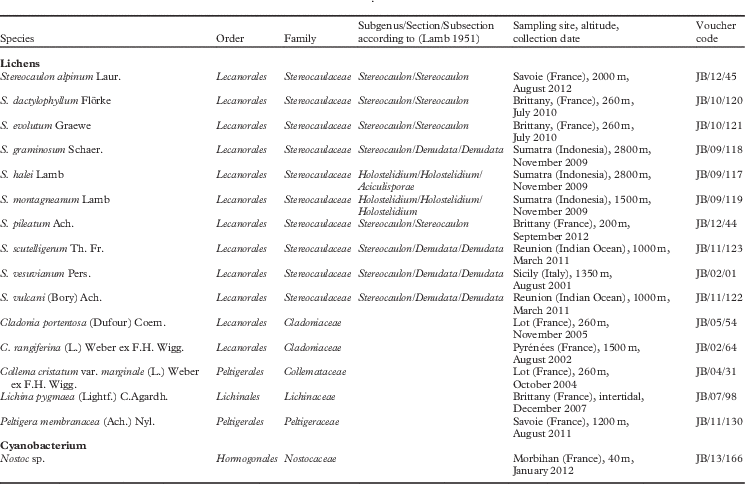
Fifteen lichens and one cyanobacterium were obtained from different locations (Table 1), representing a wide range of temperate and tropical climates. Three contained a chlorococcoid alga (Stereocaulon scutelligerum, Cladonia rangiferina, C. portentosa) and three contained a cyanobacterium only, two of these having Nostoc (Collema cristatum var. marginale, Peltigera membranacea) and one Calothrix (Lichina pygmaea). The nine remaining lichen species (Stereocaulon vulcani, S. vesuvianum, S. dactylophyllum, S. evolutum, S. pileatum, S. alpinum, S. graminosum, S. halei, S. montagneanum) contained both a chlorococcoid alga and an additional cyanobacterium in cephalodia. They are listed according to the taxonomic order, family, genus, section and subsection to which they belong. Stereocaulon species were identified and authenticated by HJM Sipman (Berlin Museum, Germany) and the other species (Cladonia, Peltigera, Collema, Lichina) by L. Massé (Rennes, France). Voucher specimens were deposited in the herbarium of Rennes1, as a code JB/year/number of species.
Table 1 List of species, sampling locations, dates and altitudes for the lichens and other species used in the study

The material was air-dried for one day at room temperature, in the shade, and stored in darkness until used for experimentation.
Reagents
Boron trifluoride (BF3, 14% (by wt) in methanol), saturated, unsaturated, iso- and anteiso-FAs, the internal standard (heneicosanoic acid; C21:0), and other reagents and solvents, were purchased from Sigma-Aldrich (Saint-Quentin-Fallavier, France).
Sample preparation and optimization of lipid extraction
After cleaning, several whole thalli of air-dried lichens (200 mg) were ground with a mixer mill to ensure sufficient pulverization and homogenization, which allowed impregnation by the solvents during the extraction process. For the tripartite lichens, whole thalli included pseudopodetia, phyllocladia and cephalodia; cephalodia were unambiguously present and in large quantity for S. halei, S. montagneanum and S. graminosum. To optimize the extraction method, lichen thalli (S. scutelligerum) were tested using three systems: 1) a test tube rotator wheel (Labinco LD-79, Fisher Scientific); 2) a parallel synthesis apparatus (Heidolph Parallel Synthesis Apparatus®-Instruction Synthesis 1, France); 3) a Soxhlet Extraction Apparatus. Lipids were extracted according to the method described by Folch et al. (Reference Folch, Lees and Sloane Stanley1957), with a slight modification for the solvent system, as extraction was carried out in parallel using chloroform/methanol (2:1, v/v) and chloroform/methanol (2:1, v/v) + 5% acetic acid (v/v). The following five methods were tested in triplicate to evaluate the reproducibility of the extractions.
Method 1. A rotator wheel apparatus with a capacity to simultaneously extract 32 samples was used. Samples were extracted for 12 h with chloroform/methanol (2:1, v/v, 6 ml) at room temperature, under constant rotation at 10 rpm.
Methods 2 and 3. A Heidolph Parallel Synthesis apparatus with a capacity to simultaneously extract 24 samples was used. Samples were extracted three times, for 1 h each, with chloroform/methanol (2:1, v/v, 6 ml) with (Method 2) or without (Method 3) acetic acid, at 80 °C, under constant shaking at 700 rpm.
Methods 4 and 5. A Soxhlet apparatus was used, which allowed for only one extraction at a time. Samples were extracted once, at 60 °C, for 4 h, with chloroform/methanol (2:1, v/v, 250 ml) with (Method 4) or without (Method 5) acetic acid.
To standardize the method, we initially used 100 μg of margaric acid (C17:0), added at the beginning of the extraction process. However, after we detected endogenous C17:0 in the 16 samples, we replaced this standard with 100 μg of heneicosanoic acid (C21:0), thus removing any doubts about the performance of this quantitative analysis.
Samples treated with Methods 1 to 3 were centrifuged at low speed (470 g) for 20 min at room temperature to recover the organic phase containing the total lipids, whereas solvents of samples treated with Methods 4 and 5 were first reduced under pressure. All were then evaporated to dryness under a nitrogen flow. The final extracts were stored at −20 °C under nitrogen until derivatization.
After selection of the most accurate method (as described in the Results), reproducibility was again tested using thalli of another Stereocaulon species, S. vesuvianum.
Sample derivatization and GC-FID/GC-MS analysis
Total extracts were derivatized by saponification, using sodium hydroxide (0·5 M) in MeOH for 30 min at 70 °C, then methanolyzed in 14% BF3 in MeOH for 15 min at the same temperature. After hydrolysis, the resulting FAMEs were extracted from this reaction mixture with n-hexane. They were then analyzed by GC (Agilent 6890N, Toulouse, France) using a split injector (10:1) at 250 °C, a bonded silica capillary column (BPX 70, 60 m × 0·25 mm, 0·25 µm film thickness; SGE, Villeneuve-St Georges, France) and helium as the carrier gas (constant flow: 1·5 ml/min). The column temperature program started at 150 °C, increased by 1·3 °C/min to 220 °C and held at 220 °C for 10 min. The FID temperature was 250 °C (air: 450 ml/min; hydrogen: 40 ml/min). Identification of FAMEs was based on retention times obtained for FAMEs, prepared from FA standards and detected by FID.
For calculations based on identification of FAMEs by GC-FID, peak areas were determined using ChemStation software (Agilent, version B04.02) and results were expressed as % of total identified FAs (Equation 1):
where % i represents the percentage of total identified FAs, AUCi is the FAME peak area, and ∑AUC is the sum of FA peak areas on the chromatogram. The quantity of total FAs was calculated using the internal standard and expressed as mgg−1 dry weight of lichen (Equation 2):
where M represents the total FA content expressed as mgg−1 dry weight of lichen; mC21:0, as an FA, is the weight of the internal standard in mg; AUCC21:0 is the area of the peak of the internal standard on the chromatogram; and m is the weight of the lichen from which the lipids were extracted (in g).
The unambiguous identification for most of the FAMEs was performed by comparing their retention times with those prepared from standard FAs, including iso- and anteiso-FAs (11-methyldodecanoic acid (iso-C13:0)), 12-methyltetradecanoic acid (anteiso-C15:0), 13-methyltetradecanoic acid (iso-C15:0), 14-methylhexadecanoic acid (anteiso-C17:0) and 19-methyleicosanoic acid (iso C21:0). When ambiguous identifications were obtained by GC-FID, samples were analyzed by GC-MS using a 7890N chromatograph (Agilent) fitted with the same column as above and connected to a 5975C MSD working in the EI mode (70 eV). Mass spectra of FAMEs were compared to those in the NIST database (v2.0, 2008).
Data analysis
For reproducibility among experiments, values of extraction yields are expressed as means ± 1SD. Statistical significance of the differences was assessed, using GraphPad Prism (GraphPad Software Inc., La Jolla, CA), by analysis of variance followed by the Student-Newman-Keuls post-hoc test.
For the Hierarchical Ascendant Classification (HAC) analysis (package stats, function hclust), normality of data was firstly assessed on the FA composition using the multivariate Shapiro-Francia test (package mvsf; Delmail et al. Reference Delmail, Labrousse and Botineau2011), with software R 2.13.0. The HAC analysis, the biplot analysis (package stats, function biplot), and the explained-discrimination assessment were performed on these data to highlight the variance between total and saturated FA contents. Similar analyses were then carried out on the iso- and anteiso-FA compositions of these lichens.
HAC is used here, instead of a Principal Component Analysis (PCA), to better visualize distances between species, especially because close relationships occur between several species, thus rendering PCA less informative. The explained-discrimination assessment is calculated using the function prcomp (package stats) on the data matrix to obtain all explained variances and the subfunction $rotation is used to obtain the weights of parameters on the factorial axis.
Results
Optimization of the extraction method
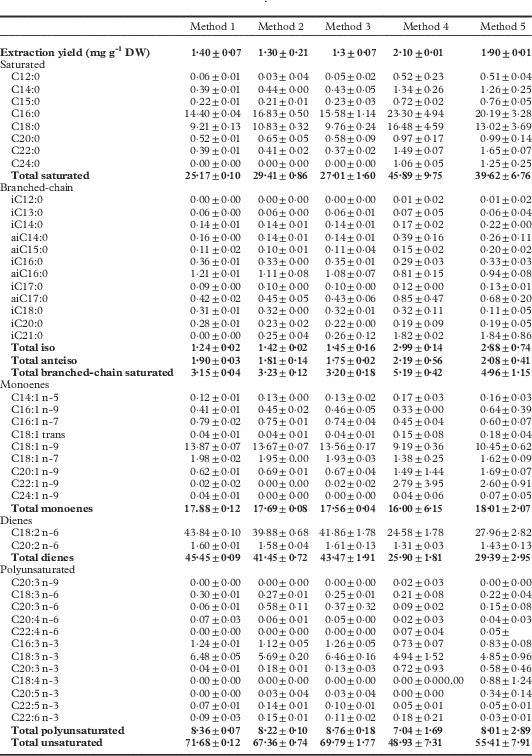
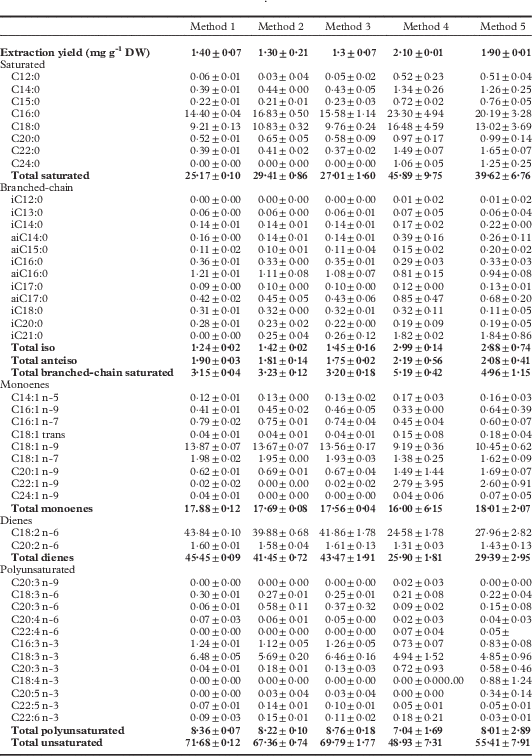
Five extraction methods were applied to 200 mg of dried thallus powder from Stereocaulon scutelligerum in order to select the fastest, lowest-cost method that would result in a complete extraction, reproducible results and a large FA diversity. Extractions were carried out using a rotating mixing wheel, a Soxhlet apparatus and a parallel synthesis robot. Extraction solvents included the usual lipid solvent (chloroform/methanol; 2:1, v/v) with and without acetic acid (5%, v/v); an exception was the rotating wheel, where only the acid-free lipid solvent was used. Statistical analyses were performed to compare the results of each method. The various extraction methods resulted in different yields and FA (in the form of FAMEs) profiles (Table 2). The highest extraction yields were obtained with Methods 4 and 5 (2·1% and 1·9%, respectively), but they were nevertheless excluded because of: 1) a lack of reproducibility; 2) a low unsaturated FAs extraction rate; and 3) an unacceptably long extraction time. Method 1 was also discarded because of a lower content of minor polyunsaturated FAs (PUFAs). Method 3 was preferred over Method 2, as acetic acid did not substantially improve extraction yields. Thus, Method 3 was selected for all analyses on the 16 species, namely extraction using the Heidolph Parallel Synthesis Apparatus, three extractions for 1 h each, with chloroform/methanol (2:1, v/v, 6 ml), at 80 °C, under constant shaking at 700 rpm. The selected method, applied to two lichen species (S. scutelligerum and S. vesuvianum), gave good reproducibility for extraction yields (0·77 ± 0·03 mgg−1 dried lichen and 0·82 ± 0·02 mgg−1 dried lichen, respectively) and FA profiles (see Supplementary Materials Table S1, available online). A fourth extraction with the same protocol did not result in any additional FAs (data not shown), indicating a complete lipid extraction.
Table 2 Comparison of five fatty acid extraction methods, based on the extraction yield (mg g -1 dry weight) and FA profiles (% of total) in the lichen Stereocaulon scutelligerum (mean ± 1 SD, n = 3). Total values are given in bold
Fatty acid profile of the lichens
Fatty acids of the 16 species
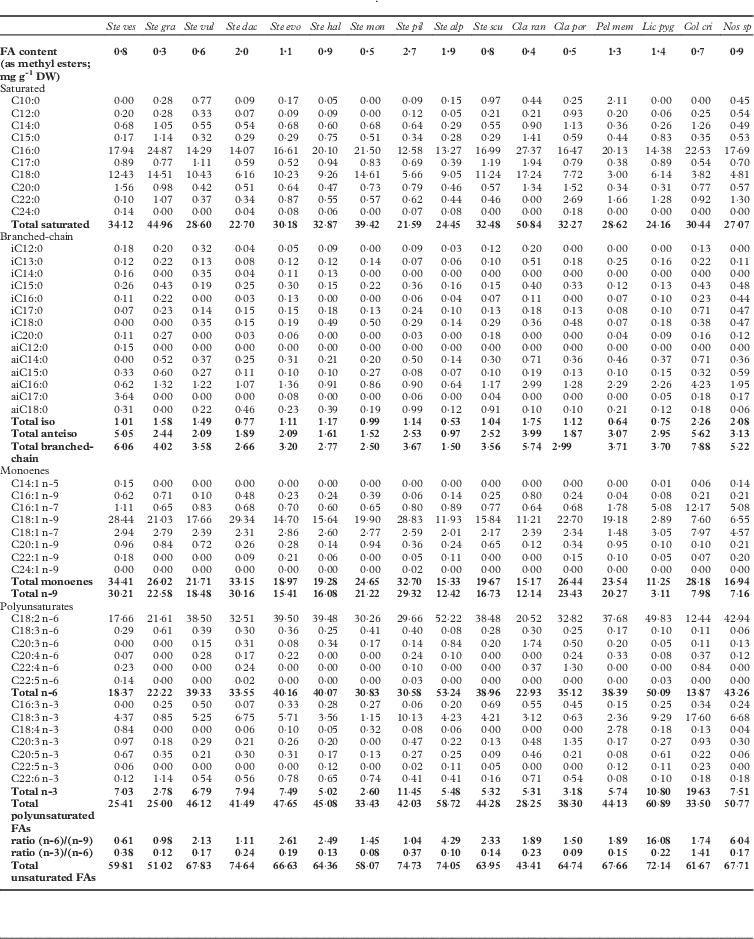
The FA composition of the 16 species examined by capillary GC-FID is shown in Table 3. Total FAs varied from 0·3 to 2·7 mgg−1 of dried lichen. The higher content of FAs was observed in S. pileatum (2·7 mgg−1) and S. dactylophyllum (2 mgg−1), compared to S. graminosum (0·3 mgg−1), S. montagneanum (0·5 mgg−1) and both Cladonia species (0·4 and 0·5 mgg−1, respectively).
Table 3 Total fatty acid (as FAMEs) composition (% of total FAs) of 15 species of lichen in the genera Stereocaulon, Cladonia, Peltigera, Lichina, Collema, and one cyanobacterium (Nostoc sp.). Total values are given in bold.

Key to lichen species abbreviations: Ste ves, Stereocaulon vesuvianum; Ste gra, S. graminosum; Ste vul, S. vulcani; Ste dac, S. dactylophyllum; Ste evo, S. evolutum; Ste hal, S. halei; Ste mon, S. montagneanum; Ste pil, S. pileatum; Ste alp, S. alpinum; Ste scu, S. scutelligerum; Cla ran, Cladonia rangiferina; Cla por, C. portentosa; Pel mem, Peltigera membranacea; Lic pyg, Lichina pygmaea; Col cri, Collema cristatum var. marginale; Nos sp, Nostoc sp.
Forty-five FAs were identified, including 24 saturated FAs, corresponding to a 30% ratio, except for S. graminosum (45·0%) and Cladonia rangiferina (50·8%). The major saturated acids were palmitic acid (C16:0; 12·6–27·4%) and stearic acid (C18:0; 3·0–17·2%). However, the content of C18:0 was low (<8%) for Stereocaulon dactylophyllum, S. pileatum, Peltigera membranacea, Lichina pygmaea, Nostoc sp. and Collema cristatum var. marginale. The proportion of BCFAs was highest for C. cristatum (7·9%), S. vesuvianum (6·1%), C. rangiferina (5·7%) and Nostoc (5·2%). These were distributed between iso-acids (C10:0, C12:0, C13:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0) and anteiso-acids (C14:0, C15:0, C16:0, C17:0, C18:0) in a ratio of 1:2.
Each lichen species contained significant levels of unsaturated FAs. Total monoene FAs varied from 11·2 to 34·4%; the major one was C18:1(n-9) (28·8%), although levels of C16:1(n-7) and C18:1(n-7) were also significant for some lichen species, such as C. cristatum (12·2% and 8·0%), Nostoc (5·1% and 4·6%) and L. pygmaea (5·1% and 3·1%). Other monounsaturated FAs were also detected, but at trace levels, for example C14:1(n-5), C16:1(n-9), C20:1(n-9), C22:1(n-9), C24:1(n-9). The unique diene C18:2(n-6) varied between 12·4% (C. cristatum var. marginale and S. vesuvianum) and 52·2% (S. alpinum and L. pygmaea). The triene FAs found belong to the families n-3 (C16:3(n-3), C18:3(n-3), C20:3(n-3)) and w-6 (C18:3(n-6), C20:3(n-6)). FAs with four, five and six double bonds were identified, but at low levels (0·3–3·4%). The n-3 FAs were present at high levels (10–19%) for C. cristatum, L. pygmaea and S. pileatum, but in low quantities for S. graminosum (2·8%) and S. montagneanum (2·6%). A high content of n-6 FAs was found in L. pygmaea (50·1%) and S. alpinum (53·2%), whereas they were at lower levels in C. cristatum (13·9%) and S. vesuvianum (18·4%). The n-9 FA levels were low for L. pygmaea (3·1%), but higher (c 30%) for S. vesuvianum, S. dactylophyllum and S. pileatum.
Multivariate analysis
Four HAC analyses were carried out to compare the 16 species with respect to total FAs, saturated FAs, unsaturated FAs (see Supplementary Material Figure S1, available online) and BCFAs (Fig. 2). The correspondence observed between the total-, saturated- and unsaturated-FA distributions in the chlorolichens and the cyanolichens, as well as in the Stereocaulon species, was poor (see Supplementary Material Figure S1, available online), except when BCFAs were selected for HAC analysis. No experiment was conducted on a tripartite Stereocaulon to observe if a pseudopodetium without cephalodia would give a different quantitative profile to a pseudopodetium with a cephalodium attached to it, but preliminary results do not support this. Cyanobacteria of S. montagneanum and S. halei were very much alike (Scytonema type, even if the type of cephalodium is different: sacculate for S. montagneanum and protosacculate for S. halei). Despite this, dendrograms derived from total-, saturated- and unsaturated-FA data failed to correlate with either species: S. montagneanum seems to be closer to S. graminosum (Nostoc type in the cephalodia) than to S. halei. These preliminary results have to be confirmed by other studies. Both anteiso-C16:0 and C17:0 contributed in a large part (26% and 45% on factorial axis PC1, and 47% and 29% on factorial axis PC2) to the discrimination between the cyanolichens and the genus Stereocaulon (Fig. 3). Stereocaulon vesuvianum was very distinct on the biplot because it contained a high value of aiC17:0 (3·5%). Moreover, the Stereocaulon species S. halei and S. montagneanum, as well as S. graminosum and S. vulcani, had similar profiles.
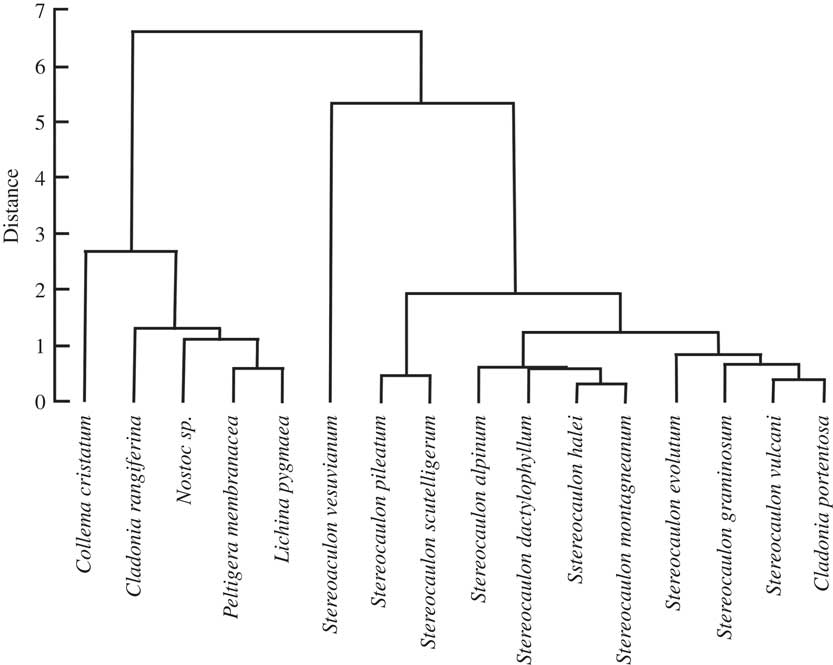
Fig. 2 Dendrogram obtained by hierarchical cluster analysis of 15 species of lichens in the genera Stereocaulon, Cladonia, Peltigera, Collema, and one cyanobacterium (Nostoc sp.), based on iso- and anteiso-fatty acids (FAs).
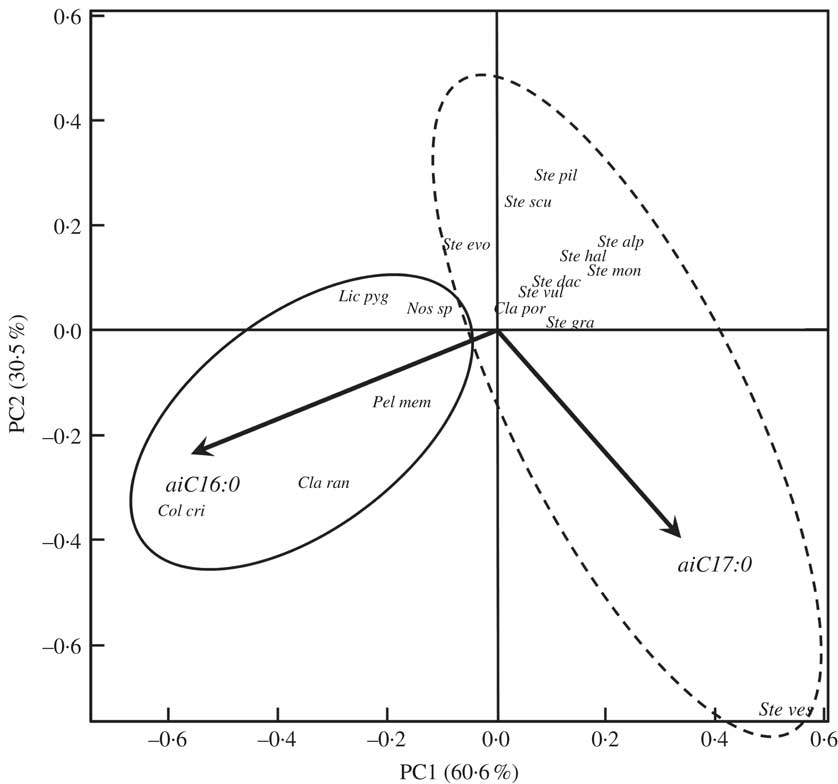
Fig. 3 Biplot of principal component analysis of the 15 species of lichen and one cyanobacterium (Nostoc sp.), based on iso- and anteiso-FAs. When vectors are too close, they are merged and so unreadable; they are insignificant in the discrimination. The explained variances of factorial axes (PC1 and PC2) are indicated in parentheses near the respective axes. Fatty acid abbreviations: ai = anteiso-fatty acid; Cx : y where x = number of carbon atoms and y = number of double bonds. Key to lichen species abbreviations: Ste ves, Stereocaulon vesuvianum; Ste gra, S. graminosum; Ste vul, S. vulcani; Ste dac, S. dactylophyllum; Ste evo, S. evolutum; Ste hal, S. halei; Ste mon, S. montagneanum; Ste pil, S. pileatum; Ste alp, S. alpinum; Ste scu, S. scutelligerum; Cla ran, Cladonia rangiferina; Cla por, C. portentosa; Pel mem, Peltigera membranacea; Lic pyg, Lichina pygmaea; Col cri, Collema cristatum var. marginale; Nos sp, Nostoc sp.
Discussion
Before evaluating if FAs have a taxonomic value in discriminating among lichen species, we evaluated five methods for extracting the highest yield and diversity of FAs. Of the several solvent systems used for extracting lipids from lichens, we chose to optimize the classical method developed by Folch et al. (Reference Folch, Lees and Sloane Stanley1957). The method was modified by the use of acetic acid, as it ensures phospholipid extraction (Hanus et al. Reference Hanus, Temina and Dembitsky2008; Temina et al. Reference Temina, Levitsky and Dembitsky2010). However, the FA profiles and extraction yields were unchanged, whether acetic acid was used or not. Although lichen lipids are most commonly extracted, after maceration, using a Soxhlet apparatus, we chose to use the Parallel Synthesis Apparatus (Method 3: three extractions, for 1 h each, with chloroform/methanol (2:1, v/v, 6 ml), at 80 °C, under constant shaking at 700 rpm). Historically, the ratio of lichen powder weight to solvent volume for lipid extraction has ranged between 3 mgml−1 (Reis et al. Reference Reis, Iacomini, Gorin, Mera de Souza, Grube, Cordeiro and Sassaki2005) and 100 mgml−1 (Sassaki et al. Reference Sassaki, Cruz, Gorin and Lacomini2001). In this case, the ratio was c 30 mgml−1 for Methods 1, 2 and 3, and 1 mgml−1 for Methods 4 and 5, which could explain our better extraction yields. Total FAs are composed of lipids from cellular membranes and energy storage deposits. Thus their content can vary widely, making comparisons with literature values difficult. In this study we therefore targeted total FAs derived only from phospholipids and glycolipids, which also had a better chance of being conserved between species. These were successfully quantified by using an internal standard (heneicosanoic acid; C21:0). The alternative was to calculate the weights of total extracts without derivatizing the samples. However, this would include fatty acids covering lipids (triglycerids and phospholipids), other lipids without FAs (sterols, liposoluble vitamins) but also depsides and depsidones (Dertien et al. Reference Dertien, De Kok and Kuiper1977; Dembitsky et al. Reference Dembitsky, Bychek, Shustov and Rozentsvet1991, Reference Dembitsky, Rezanka and Bychek1992a ).
Stereocaulon species collected in tropical or hot climates, such as S. graminosum, S. montagneanum and S. vulcani (Table 1), contained lower levels of FAs (<1 mgg−1 dry weight) than those from temperate locations. Stress conditions, such as a decrease in temperature of only a few degrees, nitrogen starvation and increased light intensity, are known to favour FA accumulation (Dembitsky et al. Reference Dembitsky, Rezanka and Bychek1994; Reis et al. Reference Reis, Iacomini, Gorin, Mera de Souza, Grube, Cordeiro and Sassaki2005; Goss & Wilhelm Reference Goss and Wilhelm2009). This would be consistent with the higher FA content of the temperate S. pileatum and S. alpinum. However, S. evolutum and S. dactylophyllum were both collected from the same temperate location (Brittany, France; Table 1), yet there is a twofold difference in FA content between these two species (Table 3). Thus, growth at a given temperature is not necessarily a unique parameter that determines FA content, but rather genetics, combined with environmental conditions (e.g. altitude, air pollution, seasonal effects), must also be considered (Temina et al. Reference Temina, Levitsky and Dembitsky2010).
The use of gas chromatography, with a capillary column and flame ionization detection, allowed a complete and detailed FA record. This approach complements and contributes to completing the classical analyses (e.g. TLC) performed on lichen terpenoids and steroids. The FA profiles retrieved for the lichens are in agreement with the literature for the major FAs, that is, a high content of C16:0, C18:0, C18:1(n-9) and C18:2(n-6). This is supported in the literature by FAME analyses of 69 species of lichens (see Supplementary Material Text S1, available online). The total FA profile was reported for four Collema species (Temina et al. Reference Temina, Levitsky and Dembitsky2010): both C. fuscovirens and C. flaccidum exhibited a profile different to that of C. cristatum and C. callopismum. These differences were attributed to their growth substratum: epiphytic for C. fuscovirens and C. flaccidum, and lithophilic for C. cristatum and C. callopismum. Our C. cristatum var. marginale sample was collected on rocks and reinforces this observation, as its FA profile is closer to that of C. cristatum and C. callopismum. The FA profile of Peltigera membranacea is different to that of P. didactyla (syn. P. spuria) and P. canina, both of which contain the cyanobacterium Nostoc as the main photobiont. The main difference is the high content of the diene C18:2(n-6) and the low levels of polyene in P. membranacea, as previously observed (Dembitsky et al. Reference Dembitsky, Rezanka and Bychek1992b ; Rezanka & Dembitsky Reference Rezanka and Dembitsky1999). Three other samples of Cladonia rangiferina were previously analyzed (Yamamoto & Watanabe Reference Yamamoto and Watanabe1974; Dembitsky et al. Reference Dembitsky, Bychek, Shustov and Rozentsvet1991; Rezanka & Dembitsky Reference Rezanka and Dembitsky1999; Sassaki et al. Reference Sassaki, Cruz, Gorin and Lacomini2001), and their profiles are similar to our current data. An exception is Yamamoto & Watanabe (Reference Yamamoto and Watanabe1974), who reported a lower diversity of saturated FAs. However, this could be attributed to the use of a low-resolution column. Guschina et al. (Reference Guschina, Dobson and Harwood2003) found that those lichen species that harbour Nostoc contain more C18:3(n-3) than those that contained a green lga. Furthermore, they did not produce FAs with chains longer than C18 (Ratledge & WilkinsonReference Ratledge and Wilkinson1988). In our case, all the cyanolichens produced minor quantities of unsaturated FAs longer than C18.
The biosynthetic pathway for FAs uses two types of enzyme: elongases (ε), for adding two carbons to the FA at the carboxyl end, and desaturases (Δx), for adding cis-double bonds at specified positions (x). These desaturases are rate-limiting enzymes. Two major desaturases, Δ12 and Δ15, are involved in the biosynthesis of the (n-6) and (n-3) families, respectively. The ratios (n-6)/(n-9) and (n-3)/(n-6) reflect the activity of each enzyme (Fig. 1); the higher the ratio, the higher the enzyme activity (Table 3). Most lichens have a high (n-6)/(n-9) ratio (i.e. >1·5), especially the cyanolichen L. pygmaea (16·1), the chlorolichen S. alpinum (4·3), and also the cyanobacterium Nostoc sp. (6·1). This could be attributed to their very low content of (n-9) monoenes and their high content of C18:2(n-6), indicating a possible high activity of the Δ12 desaturase. However, even if the four cyanolichens and the cyanobacterium do not share a similar unsaturated FA profile, certain parts of their metabolic pathways appear to be particularly active, especially for C. cristatum var. marginale. Its high (n-3)/(n-6) ratio, combined with a lower (n-6)/(n-9) ratio, is in agreement with its high content of C18:3(n-3), C20:3(n-3) and C20:5(n-3), and its lower content of C18:2(n-6). For this species, the biosynthetic pathway upstream of C18:3(n-3) is enhanced due to the activity of the Δ15 desaturase, which results in a larger diversity and content of trienes. These metabolic pathways are also used by the cyanolichens L. pygmaea and C. cristatum var. marginale, and the cyanobacterium Nostoc sp. These species also contain a high level of (n-7) monoene FAs (>5%), in equal quantity to C18:1(n-9), which is the common precursor for unsaturated FAs. The biosynthetic pathway for FAs in the cyanolichens is different to that in the chlorolichens, as the former had a higher activity of desaturases. These results are in agreement with the FAME profiles reported for the cyanolichens Peltigera didactyla (syn. P. spuria) and Leptogium saturninum (Rezanka & Dembitsky Reference Rezanka and Dembitsky1999) and the tripartite lichen Peltigera aphthosa (Dembitsky et al. Reference Dembitsky, Rezanka and Bychek1992b ). However, they differ to those for the Collema species, which were harvested from rocks or bark (Temina et al. Reference Temina, Levitsky and Dembitsky2010).
Dendrograms derived from total-, saturated- and unsaturated-FA data failed to separate Stereocaulon, Cladonia and the cyanolichens into distinct groups. Despite the small amounts of iso- and anteiso-FAs in lichens and in Nostoc sp. (0·5–2·3% and 1·0–5·6%, respectively), their presence appears to be informative for Stereocaulon species, given that our method distinguished this genus from the cyanolichens. Excluding S. vesuvianum from the PCA and HCA had no influence on the results. Nevertheless, this profile did not consistently separate the close genera Cladonia and Stereocaulon, nor the Stereocaulon species in sect. Denudata. Thus it would be premature to conclude that the value of FA profiles for lichen taxonomy is as high as it is for well-documented bacteria. The chemotyping study of Sassaki et al. (Reference Sassaki, Cruz, Gorin and Lacomini2001) had previously shown that total FAME data were of limited use for elucidating the taxonomy of Cladonia species. For example, C. miniata was positioned outside the group, whereas Parmotrema delicatum was included. Separate analyses of phospholipids (membrane structure) and triglycerides, including glycolipids (energy storage), should be considered to improve and confirm the value of FA profiles in the chemotaxonomy of lichens.
BCFAs are found in many bacterial strains (Zelles Reference Zelles1999). These are synthesized in bacteria by the deamination of valine, leucine or isoleucine, giving 2-methylpropionyl-CoA, 3-methylbutyryl-CoA and 2-methylbutyryl-CoA, followed by elongation to yield iso- and anteiso-FAs (Kaneda Reference Kaneda1963, Reference Kaneda1977, Reference Kaneda1991). Interestingly, bacterial-fungal interactions occur in many contexts (e.g. in plankton, mixed biofilms, intrahyphal colonization) as ectosymbiotic and endosymbiotic relationships (Frey-Klett et al. Reference Frey-Klett, Burlinson, Deveau, Barret, Tarkka and Sarniguet2011). Lichens also exhibit this relationship with bacteria (Finegold et al. Reference Finegold, Singer, Federle and Vestal1990; Dembitsky et al. Reference Dembitsky, Rezanka and Bychek1992a ; Matsumoto et al. Reference Matsumoto, Nienow, Friedmann, Sekiya and Ocampo-Friedmann2004; González et al. Reference González, Ayuso-Sacido, Anderson and Genilloud2005). Indeed, the non-photoautotrophic bacteria of the lichen microbiome are increasingly regarded as significant components of lichen thalli (Grube et al. Reference Grube, Cardinale, de Castro, Müller and Berg2009). Alphaproteobacteria are believed to dominate the bacterial communities of the chlorolichens (Hodkinson et al. Reference Hodkinson, Gottel, Schadt and Lutzoni2012). Bacteria from this class were likewise found on the thalli of C. rangiferina (Cardinale et al. Reference Cardinale, Puglia and Grube2006) and L. pygmaea (Parrot et al. Reference Parrot, Intertaglia, Grübe, Suzuki and Tomasi2014), along with dominant populations of Firmicutes and Actinobacteria, respectively. However, their biomass contribution within the lichen thallus appears to be too low (Anderson Reference Anderson2014) to be correlated with the percentage of BCFAs obtained. This suggests that such a symbiosis between multiple partners (e.g. fungi, algae, bacteria) could modify the FA synthesis and distribution pattern via a complex molecular communication that has yet to be investigated thoroughly.
In memory of Dr Louis Jean-Claude Massé, who introduced us to the world of lichens.
We thank Marion Millot, Marylène Chollet, Philippe Uriac, Nina Corlay and Friardi Ismed for providing Stereocaulon alpinum, Peltigera membranacea, S. pileatum, S.vulcani and S. scutelligerum, S. graminosum, S. halei, and S. montagneanum, respectively. We are also grateful to Lydie Sparfel for performing the variance analysis and to Sophie Tomasi for valuable comments to improve the manuscript and Stephen S. Bates for proof reading. The authors thank the Vietnamese government for the grant that supported Vu Thi Huyen.
Supplementary Material
For supplementary material accompanying this paper http://dx.doi.org/10.1017/S0024282916000141