Introduction
An ever-growing population has led to an increase in global demand for energy. At present, biodiesel is a vital substitute for prevalent diesel fuel, due to its renewable, non-toxic and biodegradable properties. Generally, consumption of fossil fuel has a number of less favourable outcomes compared with biofuels: (i) the current rate of consumption will cause the depletion of oil by the middle of this century, (ii) burning fossil fuels has led to an increased atmospheric CO2 concentration, (iii) fossil fuel usage has been responsible for global warming and climate change through greenhouse gas emission (Kraan, Reference Kraan2013). Biodiesel can be formed from a variety of terrestrial and marine agricultural commodities, commonly oilseeds with lipid content (such as soybeans, sunflower or palm seeds), micro- and macroalgae.
Although the cost of production of algal biodiesel may be higher than that of terrestrial crop plants, algae have been receiving attention as a potential biofuel feedstock as they have a number of advantages over traditional biofuel crops, including fast growth rates, high production biomass yields per unit area (i.e. no roots, leaves and stems when only seeds are required), no arable land nor fresh-water use (Chisti, Reference Chisti2007; Adams et al., Reference Adams, Toop, Donnison and Gallagher2011). In addition, growing terrestrial crops for biofuel may cause environmental impacts by reducing freshwater resources and food security (Shilton & Guieysse, Reference Shilton and Guieysse2010). Given these limitations, the interest in marine algae as alternative renewable feedstock has increased.
Although the major research, development and commercialization in respect of renewable energy production has focused on microalgae (Thomas, Reference Thomas2006; Talebi et al., Reference Talebi, Mohtashami, Tabatabaei, Tohidfar, Bagheri, Zeinalabedini, Mirzaei, Mirzajanzadeh, Shafaroudi and Bakhtiari2013; Rajkumar et al., Reference Rajkumar, Zahira and Mohd Sobri2014), there are significant technical challenges in the cost and complexity of cultivation at an industrial scale (Mata et al., Reference Mata, Martins and Caetano2010). Macroalgae are simpler to harvest, grow faster, can grow up to 60 m in length and are particularly suited to low technology cultivation methods (Rajkumar et al., Reference Rajkumar, Zahira and Mohd Sobri2014; Zarei Jeliani et al., Reference Zarei Jeliani, Yousefzadi, Sohrabi Pour and Toiserkani2017). Additionally, algal biomass can be grown in the unused coastal zone. In fact, utilization of seawater to produce algal biomass has great potential to alleviate the water crisis (Rajkumar et al., Reference Rajkumar, Zahira and Mohd Sobri2014). Therefore, a possible solution to the energy crisis is macroalgae cultivation at sea, which does not need arable land and fertilizer (Rajkumar et al., Reference Rajkumar, Zahira and Mohd Sobri2014).
Macroalgae have a high biomass content per unit of light and area and can contain large amounts of starch or oil, while not needing fresh water or agricultural land (Rajkumar et al., Reference Rajkumar, Zahira and Mohd Sobri2014). Algal biomolecules such as carbohydrates and lipids can also be converted to biofuel (Rajkumar et al., Reference Rajkumar, Zahira and Mohd Sobri2014). The carbohydrates of macroalgae such as Sargassum spp., Gracilaria spp., Laminaria spp., Prymnesium parvum, Gelidium amansii and Nizimuddinia zanardini are able to be converted to bioethanol by fermentation (Adams et al., Reference Adams, Gallagher and Donnison2009; Wi et al., Reference Wi, Kim, Mahadevan, Yang and Bae2009; Yazdani et al., Reference Yazdani, Zamani, Karimi and Taherzadeh2015). Maceiras et al. (Reference Maceiras, Rodriguez, Cancela, Urrejola and Sanchez2011) also reported that triglycerides from some macroalgae such as Ascophyllum nodosum, Codium tomentosum, Enteromorpha intestinalis, Fucus spiralis, Saccorhiza polyschides, Sargassum muticum, Ulva rigida and Pelvetia canaliculata are converted to biodiesel during the transesterification process.
Despite the use of macroalgae in food and feed supplements for both humans and animals, in medicine, and in the processing of phycolloids as thickening and gelling agents (Kelly & Dworjanyn, Reference Kelly and Dworjanyn2008), few studies have been conducted on bioenergy production using macroalgae (McDermid & Stuercke, Reference McDermid and Stuercke2003; Chen et al., Reference Chen, Zhou, Luo, Zhang and Chen2015). Aside from limitations of lipid content of < 5% dry matter, some species, in particular within the order Dictyotales, have a total lipid content above 11% dry matter (McDermid & Stuercke, Reference McDermid and Stuercke2003).
Fatty acid profiles of some of the macroalgae in this study have already been investigated, but environmental factors (e.g. temperature, humidity, location and sampling period), water resource and the presence of nutrients can affect total lipid and fatty acid content in algal biomass (Pereira et al., Reference Pereira, Barreira, Figueiredo, Custódio, Vizetto-Duarte, Polo, Rešek, Engelen and Varela2012; Abomohra et al., Reference Abomohra, El-Naggar and Baeshen2018). Although seaweed distribution on the coastline of the Persian Gulf in the south of Iran and their nutritional compounds, such as proteins, carbohydrates, minerals, dietary fibres, vitamins and polyunsaturated fatty acids (PUFAs) (Rohani-Ghadikolaei et al., Reference Rohani-Ghadikolaei, Abdulalian and Ng2012; Pirian et al., Reference Pirian, Zarei Jeliani, Sohrabipour, Arman, Faghihi and Yousefzadi2017, Reference Pirian, Zarei Jeliani, Arman, Sohrabipour and Yousefzadi2020), have already been studied, macroalgal exploitation as a source of biodiesel has not yet been fully addressed, and biodiesel indices of seaweeds from the Persian Gulf have not been determined.
In this work, as the first step to identify seaweed species for their potential use for biodiesel products, we simultaneously quantify the total lipid content and fatty acid profiles of the two main taxonomic groups belonging to green and brown tropical seaweed species from Persian Gulf shorelines of Iran. Then, we evaluate the relationship between total lipid content, fatty acid content and biodiesel indices, because these indices are directly influenced by the composition of fatty acid methyl esters (FAME). In brief, biodiesel indices defining biodiesel quality and properties, including cetane number (CN), iodine value (IV), cloud point (CP), saponification value (SV), degree of unsaturation (DU), long chain saturated factor (LCSF) and cold filter plugging point (CFPP) were estimated based on FAME profiling.
Materials and methods
Sampling and preparation
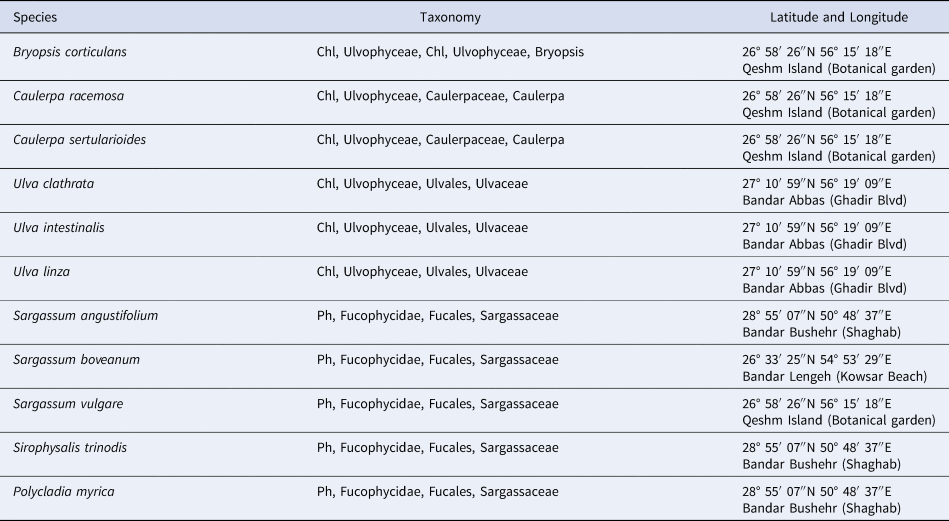
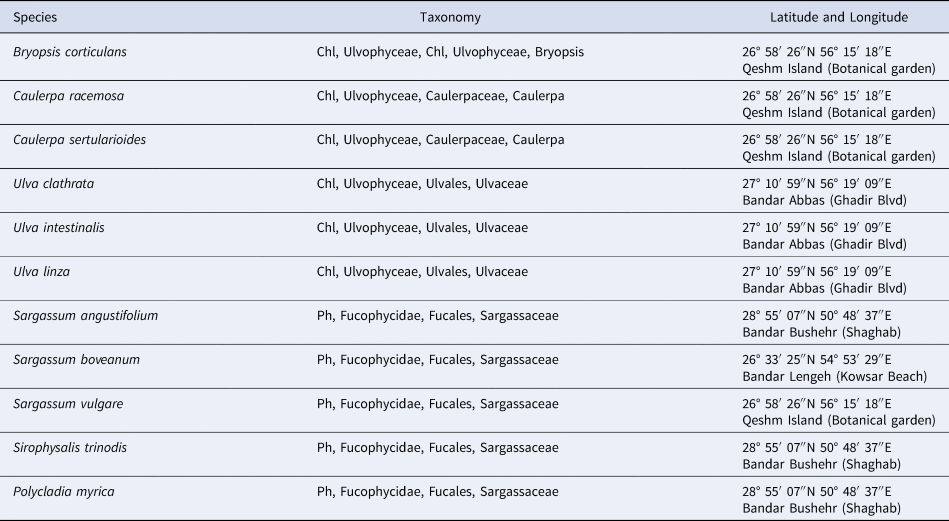
A total of 11 species of Chlorophyta (Caulerpa racemosa (Forsskål) J.Agardh, Caulerpa sertularioides (S.G.Gmelin) M.Howe, Bryopsis corticulans Setchell, Ulva linza Linnaeus, Ulva intestinalis Linnaeus and Ulva clathrata (Roth) C.Agardh), and Phaeophyta (Sargassum angustifolium C.Agardh, Sargassum vulgare C.Agardh, Sargassum boveanum J.Agardh, Polycladia myrica (S.G.Gmelin) C.Agardh and Sirophysalis trinodis (Forsskal) Kützing) were collected from the intertidal zone of Iranian coastlines, Persian Gulf, in April 2018 (Table 1). Identification of the seaweeds based on morphological characteristics was carried out with standard keys (Sohrabipour et al., Reference Sohrabipour, Nejadsatari, Assadi and Rabei2004; Sohrabipour & Rabiei, Reference Sohrabipour and Rabiei2007; Kokabi & Yousefzadi, Reference Kokabi and Yousefzadi2015). Samples were cleaned and washed with seawater to remove sand and epiphytes and transported to the laboratory under ambient conditions. Then the samples were rinsed with distilled water and dried at room temperature. The dried samples were kept in 4°C for further analysis.
Table 1. Seaweed species with collection details, and all sites are in the Persian Gulf, Iran
Analysis of total lipid content
The total lipid content was extracted according to Bligh & Dyer (Reference Bligh and Dyer1959). Briefly, dried seaweed powder was placed into a glass vial and a chloroform:methanol (2:1 v/v) mixture was added into it. The mixture was heated at 60°C for 1 h, followed by a filtration step (Whatman GF/A filter) to remove particles. The filtered extract was washed with 0.9% NaCl solution, mixed with a vortex spin and let to stand for 30 min until an upper and lower phase established. Then the upper phase was removed and the lower one, containing the lipids, was evaporated under a gentle stream of nitrogen. The lipid extract was then weighed and expressed as g of total lipids per 100 g dry weight of the sample (% DW) (Bligh & Dyer, Reference Bligh and Dyer1959).
Determination of fatty acid profile
Analysis of fFAME was done on a gas chromatography-flame ionization detector (GC-FID) according to the method of Miller & Berger (Reference Miller and Berger1985) with minor modifications. For FAME production, fatty acid samples were dissolved in KOH methanolic reagent and boiled for 45 min. Then, n-hexane solution was added and boiled for 5 min. After cooling, NaCl solution (0.85% w/w) was added. The upper phase was collected and was then diluted with n-hexane to provide the FAME. FAME samples were analysed using a gas chromatograph (Varian, 3800) equipped with a fused silica capillary column BPX 70 (25 m × 0.32 mm, film thickness 0.25 μm) and a flame ionization detector. The initial temperature of the polar column was 120°C then it was programmed to 190°C at 2°C min−1, and maintained at 190°C for 8 min. Split injection was conducted and nitrogen was used as a carrier gas, at flow rate of 0.85 ml min−1. The injector temperature was ramped from 50 to 250°C and the detector temperature was 250°C. The FAME peaks were detected by comparison of their retention times with the retention times of FAME standards (Miller & Berger, Reference Miller and Berger1985).
Biodiesel properties of FAME profile
The carbon chain sizes and the number of double bonds are factors that influence the parameters of biodiesel quality such as CN, IV, CFPP and the oxidation stability. The CN, SV and IV of samples were calculated using equations (1)–(3) (Fazelian et al., Reference Fazelian, Yousefzadi and Movafeghi2020):
Where D is the number of double bonds, M is the fatty acid molecular mass, and N is the percentage of each fatty acid component of oil. The LCSF can be estimated using equation (4). This factor was directly used to calculate CFPP in equation (5) (Talebi et al., Reference Talebi, Mohtashami, Tabatabaei, Tohidfar, Bagheri, Zeinalabedini, Mirzaei, Mirzajanzadeh, Shafaroudi and Bakhtiari2013):
DU was analysed based on equation (6), as the amount of monounsaturated (MUFAs) and polyunsaturated (PUFAs) fatty acids in the macroalgal oil.
Equation (7) was used to estimate the CP value based from the C16:0 content in fatty acid profiles, because Fazelian and coworkers reported a strong correlation between CP and palmitic acid (C16:0) amount (Fazelian et al., Reference Fazelian, Yousefzadi and Movafeghi2020).
Statistical analysis
The total lipid content of seaweeds was performed in three replicates and the data were shown as mean ± standard deviation (SD) (N = 3). The means were compared by Duncan's test and different letters denote a significant difference (P < 0.05). The data for the fatty acid profile were analysed using a single sample (N = 1).
Result and discussion
Total lipid
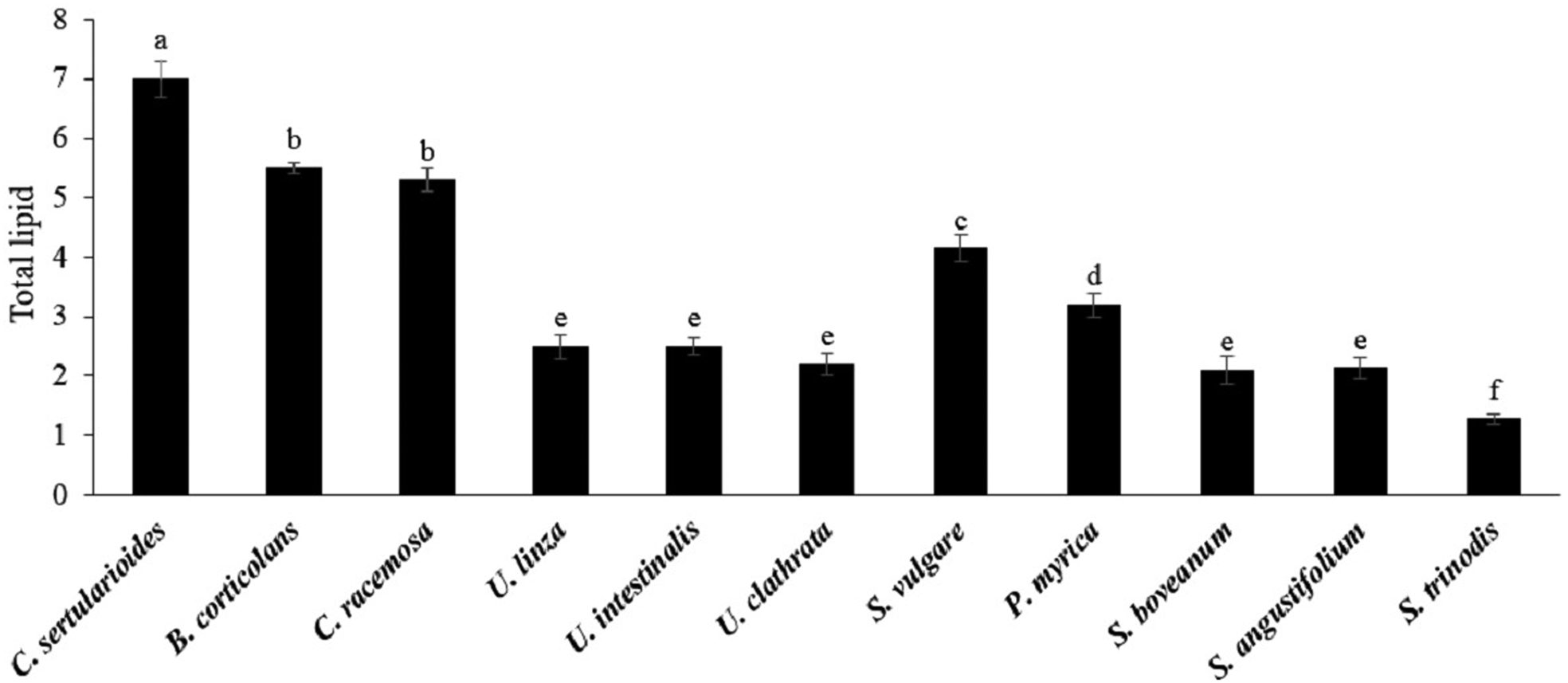
Total lipid content is one of the potential indicators of the suitability of a feedstock for biodiesel production. Overall lipid content of the investigated seaweeds was below 10% DW (Figure 1). Significant differences were found between samples (P < 0.05), with the maximum value being obtained in green seaweeds. The highest total lipid content was recorded in C. sertularioides (7.0 ± 0.3%) followed by B. corticulans and C. rasimosa (5.5 and 5.3%, respectively). In contrast, the lowest content of total lipid was observed in S. trinodis from brown seaweeds (1.25%). Further, no significant differences were found between Ulva spp., S. boveanum and S. angustifolium (~2%). Pirian et al. (Reference Pirian, Zarei Jeliani, Arman, Sohrabipour and Yousefzadi2020) also reported that the lipid content of eight macroalgae species, S. boveanum, S. trinodis, H. caroides, P. perforata, G. rugosa, C. racemosa, C. sertularioides and B. corticulans from the Persian Gulf was about 1.27–9.13%. Similar to our results, Gosch et al. (Reference Gosch, Magnusson, Paul and de Nys2012) showed the orders of Bryopsidale (68.9 mg g−1 DW ± 10.5 SE) and Cladophorale (57.5 mg g−1 DW ± 12.4 SE) had the highest total lipid content among the green seaweeds. Furthermore, C. sertularioides (130.4 mg g−1 DW ± 8.4 SE) and Derbesia tenuissima (121.4 mg g−1 DW ± 3.4 SE) had the highest total lipid content among the Bryopsidales (Gosch et al., Reference Gosch, Magnusson, Paul and de Nys2012). Other authors have described relatively low amounts of total lipids (below 7% DW) in most investigated seaweeds, while some species had a total lipid content of more than 10% DW (Abomohra et al., Reference Abomohra, El-sheekh and Dieter2017). Based on the results, there was a remarkable variation between green and brown seaweeds in terms of total lipid content, while no significant differences were observed between various species of a genus. However, there was significant variation in lipid contents in the same species. For example, Abomohra et al. (Reference Abomohra, El-sheekh and Dieter2017) found that the total lipid content of U. linza and U. intestinalis was ~8.30 ± 0.70% DW and 11.63 ± 0.78% DW, respectively, and these are interesting candidates for biodiesel production. However, the total lipid content of these seaweeds in the present study was ~2% DW. Generally, total lipid content is influenced by environmental conditions and the intrinsic potential of each alga. Macroalgae grown in full light have lower total lipid content compared with those grown in the shade (Hotimchenko, Reference Hotimchenko2002). Nitrogen starvation is another environmental factor which can increase the lipid synthesis in seaweeds (Hotimchenko, Reference Hotimchenko2002; Gosch et al., Reference Gosch, Magnusson, Paul and de Nys2012). Abomohra et al. (Reference Abomohra, El-sheekh and Dieter2017) demonstrated that coastal seawater in Jeddah contains sufficient main nutrients including nitrogen and phosphorus for algal growth. Relatively little data are available for the genetic ability of algae for lipid synthesis (Gosch et al., Reference Gosch, Magnusson, Paul and de Nys2012; Talebi et al., Reference Talebi, Mohtashami, Tabatabaei, Tohidfar, Bagheri, Zeinalabedini, Mirzaei, Mirzajanzadeh, Shafaroudi and Bakhtiari2013). For example, microalgal species such as Chlorella sp., Dunaliella salina, Botryococcus braunii and Nannochloropsis sp. presented potential to produce biofuel due to their high lipid content, but C. vulgaris was shown to have the highest biomass and lipid productivity (Talebi et al., Reference Talebi, Mohtashami, Tabatabaei, Tohidfar, Bagheri, Zeinalabedini, Mirzaei, Mirzajanzadeh, Shafaroudi and Bakhtiari2013).
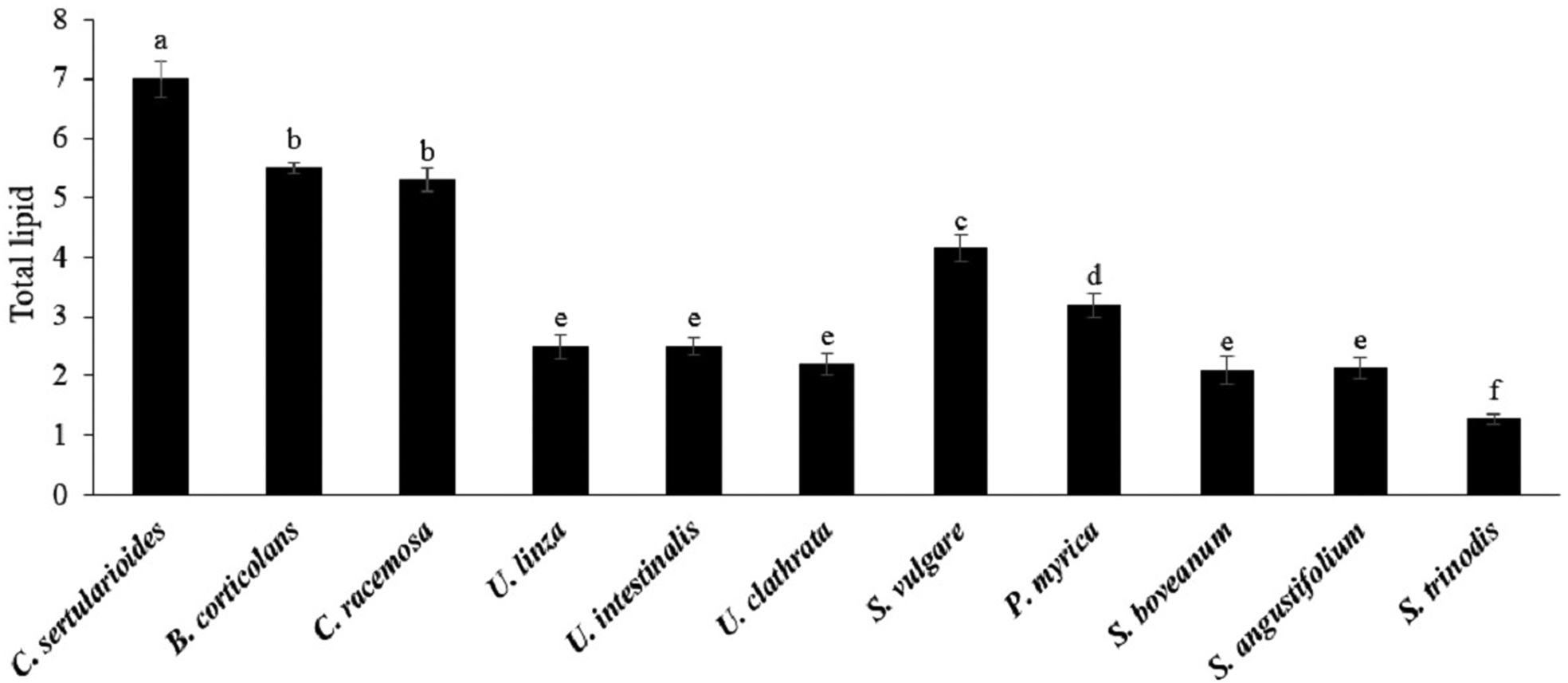
Fig. 1. Total lipid contents of green and brown seaweeds (% DW). Data are means ± standard deviation (SD), and different letters denote significant difference at P < 0.05.
Analysis of fatty acid profile
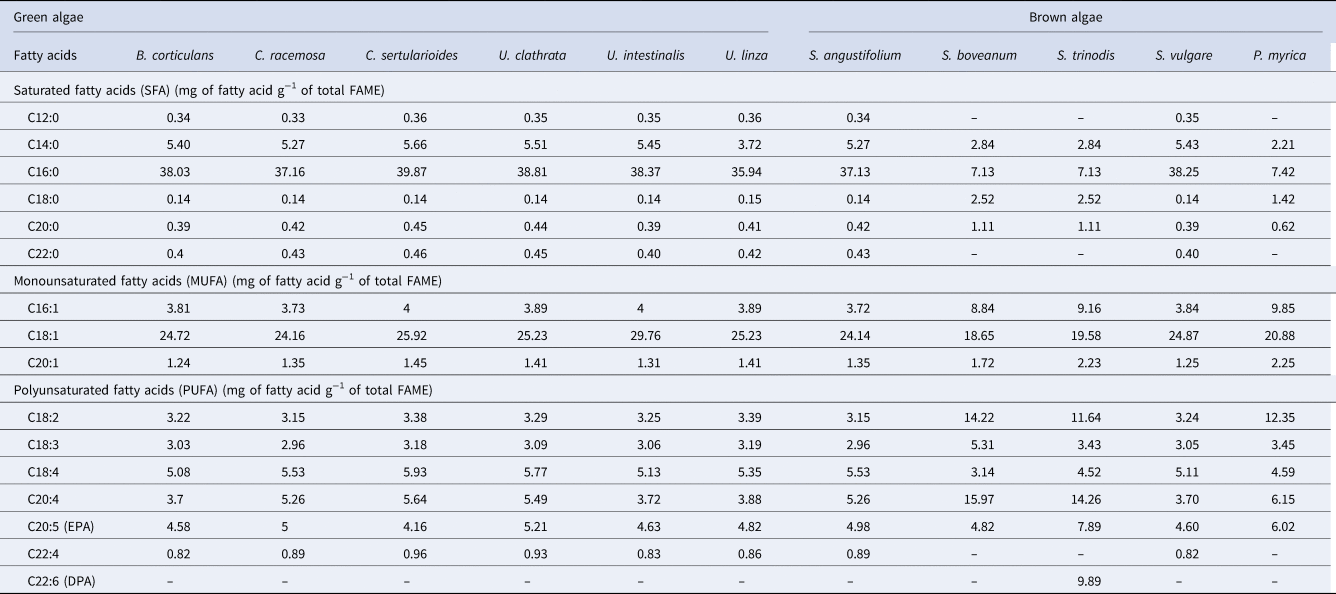
Fatty acids from seaweeds followed the same pattern across all the seaweeds tested with unsaturated fatty acids (USFA) > saturated fatty acids (SFA). The fatty acid profile of green seaweeds and some of the brown seaweeds (S. angustifolium and S. vulgare) had the following distribution: SFAs were the most abundant (41–46.94 mg g−1), followed by MUFAs (29.21–35.07 mg g−1) and PUFAs (20.43–23.78 mg g−1) (Table 2). In contrast, the highest content of PUFAs was obtained in S. boveanum (54.59 mg g−1), and the lowest of SFAs was observed in S. trinodis (11.67 mg g−1) (Table 2). The highest ratio of SFAs/USFAs was recorded in S. vulgare, B. corticulans and U. intestinalis equalling ~0.89 (Table 2). In all samples the most abundant SFA was palmitic acid (C16:0; 7.13–39.78 mg g−1) with minor quantities of behenic acid (C22:0; ~0.4 mg g−1), and stearic acid (C18:0; 0.14–2.52 mg g−1) (Table 2). Palmitic acid was higher predominantly in the green seaweed C. sertularioides (39.78 mg g−1) than the brown seaweeds S. boveanum and S. trinodis (~7 mg g−1) (Table 2). Similarly, Rohani-Ghadikolaei et al. (Reference Rohani-Ghadikolaei, Abdulalian and Ng2012), found that C14:0 (4.5–12.4%) and C16:0 (38.0–59.8%) were the major SFAs in green and brown seaweeds from the Persian Gulf of Iran. Other researchers have reported the high level of C16:0 in Phaeophyceae (Khotimchenko et al., Reference Khotimchenko, Vaskovsky and Titlyanova2002; Belattmania et al., Reference Belattmania, Engelen, Pereira, Serrão, Custódio, Varela, Zrid, Reani and Sabour2018).
Table 2. The composition of fatty acid methyl esters in 11 different algal species from the Persian Gulf
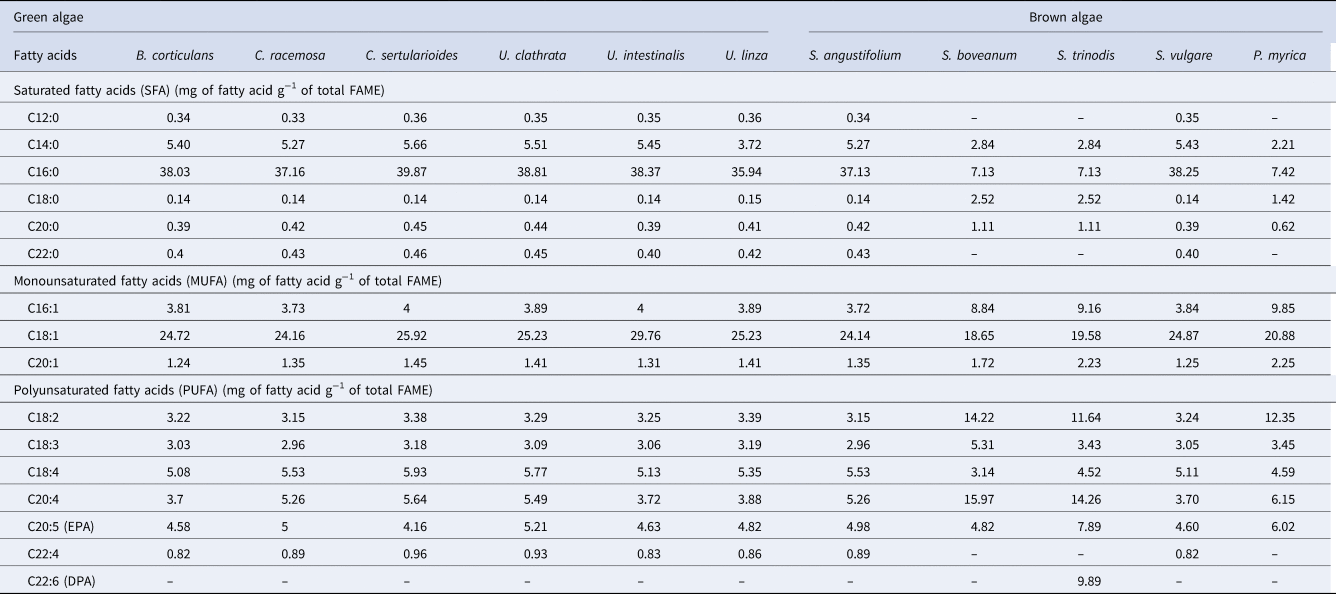
The brown seaweeds, S. boveanum and S. trinodis had the highest USFA concentration (~83.2 mg g−1) compared with green seaweeds, with the lowest amount of USFAs observed in B. corticulans (50.2 mg g−1). The MUFA content in green seaweeds (U. linza, 35.07 mg g−1) was higher than brown seaweeds, while the PUFA content was high in the brown seaweeds S. boveanum (54.59 mg g−1) and S. trinodis (51.63 mg g−1) (Table 2). Oleic acid (C18:1) was the main MUFA in the seaweeds in particular U. linza (29.76 mg g−1) and C. sertularioides (25.92 mg g−1), but high content of C16:1 (9.85 mg g−1) and C20:1 (2.25 mg g−1) were observed in P. myrica (Table 2). Similarly, PUFAs were the most abundant fatty acids in brown seaweeds collected from Hakodate coasts (Terasaki et al., Reference Terasaki, Hirose, Narayan, Baba, Kawagoe, Yasui, Saga, Hosokawa and Miyashita2009). The data of Pereira et al. (Reference Pereira, Barreira, Figueiredo, Custódio, Vizetto-Duarte, Polo, Rešek, Engelen and Varela2012) also showed that Rhodophytes and Phaeophytes have higher ratios of PUFAs/SFAs than Chlorophytes, but as an exception, Ulva presented high concentrations of α-linolenic acid (C18:3n-3, 16.51 ± 0.23 mg g−1). In contrast, Gosch et al. (Reference Gosch, Magnusson, Paul and de Nys2012) found that the green seaweeds are rich in PUFA (n-3) and in particular a high level of C18:3 (n-3), whereas some brown seaweeds have high levels of SFAs, in particular C14:0.
In the present study, arachidonic (C20:4; 15.97 mg g−1), linoleic (C18:2; 14.22 mg g−1) and docosahexaenoic (C22:6 omega 3; 11.13 mg g−1) acid were the major PUFAs in S. boveanum, while the high content of stearidonic acid (C18:4; 5.93 mg g−1) and C20:4 (5.64 mg g−1) were observed in green seaweeds (especially C. sertularioides) (Table 2). The C16 and C18 fatty acids with a high degree of unsaturation have been identified in previous studies (Thompson, Reference Thompson1996; Kendel et al., Reference Kendel, Wielgosz-Collin, Bertrand, Roussakis, Bourgougnon and Bedoux2015). The green seaweeds are also characterized by high levels of C18 PUFAs and C18/C20 PUFAs (Khotimchenko et al., Reference Khotimchenko, Vaskovsky and Titlyanova2002). The PUFA data of green seaweeds showed differences with the results of some researchers who described green seaweeds containing high levels of 18:2 and 18:3, similar to terrestrial plants, and linoleic acid (18:2 n-6) is the main PUFA of most Chlorophytes as well as α-linolenic acid (18:3 n-3) being characteristic of the Ulvales (Khotimchenko et al., Reference Khotimchenko, Vaskovsky and Titlyanova2002; Li et al., Reference Li, Fan, Han and Lou2002; Kendel et al., Reference Kendel, Wielgosz-Collin, Bertrand, Roussakis, Bourgougnon and Bedoux2015). However, the fatty acid profile of S. boveanum was in agreement with those reported in previous studies, where C20:4 and C18:2 were the most abundant fatty acids in brown seaweeds (Najdek et al., Reference Najdek, Iveša, Paliaga, Blažina and Čelig2014; McCauley et al., Reference McCauley, Meyer, Winberg, Ranson and Skropeta2015; Belattmania et al., Reference Belattmania, Engelen, Pereira, Serrão, Custódio, Varela, Zrid, Reani and Sabour2018).
The eicosapentaenoic acid (EPA; C20:5) was observed in all species. The highest amount was 7.89 mg g−1 in S. trinodis and the lowest amount was 4.16 mg g−1 in C. sertularioides (Table 2). Interestingly, docosahexaenoic acid (DHA; C22:6 omega 3) was only found in high concentrations in the brown seaweeds, S. boveanum (11.13 mg g−1) and S. trinodis (9.89 mg g−1) (Table 2). The content of EPA and DHA in the present study were higher than in the data presented by Rohani-Ghadikolaei et al. (Reference Rohani-Ghadikolaei, Abdulalian and Ng2012), where the EPA level was 1.9–5.4% and DHA level was 0.2% in brown seaweeds (S. ilicifolium and Colpomenia sinuosa) from the Persian Gulf of Iran (Rohani-Ghadikolaei et al., Reference Rohani-Ghadikolaei, Abdulalian and Ng2012).
According to our findings, there are some variations in the FAME profile of the green (containing a higher SFA level) and brown seaweeds (containing a higher USFA level) from the Persian Gulf of Iran. The observed differences are probably due to differences in environmental conditions (e.g. temperature, salinity, pH) and the intrinsic genetic ability of different species. It has also been reported that biodiesel quality is determined usually by the composition of fatty acids accumulated in the seaweed biomass, which is affected by environmental conditions, lipid extraction method and algal type (Radakovits et al., Reference Radakovits, Jinkerson, Darzins and Posewitz2010; Ho et al., Reference Ho, Chen and Chang2012; Talebi et al., Reference Talebi, Mohtashami, Tabatabaei, Tohidfar, Bagheri, Zeinalabedini, Mirzaei, Mirzajanzadeh, Shafaroudi and Bakhtiari2013).
Estimation of biodiesel properties
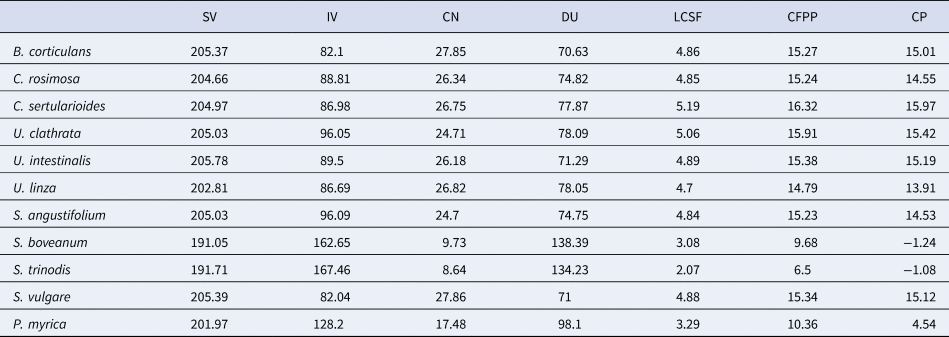
Seaweed oil can easily be transformed into biodiesel during the transesterification process, originating FAMEs. High biomass content, rapid growth and lipid accumulation of a variety of macroalgae has led to their use in the biodiesel industry (Li et al., Reference Li, Han, Sommerfeld and Hu2011). Biodiesel quality is determined usually by the composition of fatty acids accumulated in the algal biomass and biodiesel properties. The analysis of biodiesel properties has been shown in Table 3. The CN value was measured using SV and IV. The samples of S. angustifolium, S. vulgare, U. intestinalis, U. clathrata, B. corticulans showed a proximately narrow range for SV with an average of 205, but S. boveanum and S. trinodis had the lowest value (~191). The highest proportion of CN and SV was obtained in S. vulgare at 27.86 and 205.39 and in B. corticulans at 27.85 and 205.37, respectively. The IV level that is directly related to the DU level showed the highest amount in S. trinodis (167.46) and S. boveanum (162.65). Such fatty acid profile resulted in DU levels equal to 138.39 for S. boveanum and 134.23 for S. trinodis indicating two ends of an extreme. The contents of IV and DU in the brown seaweeds were higher than in the green.
Table 3. Biodiesel indicators of 11 different algal species from the Persian Gulf
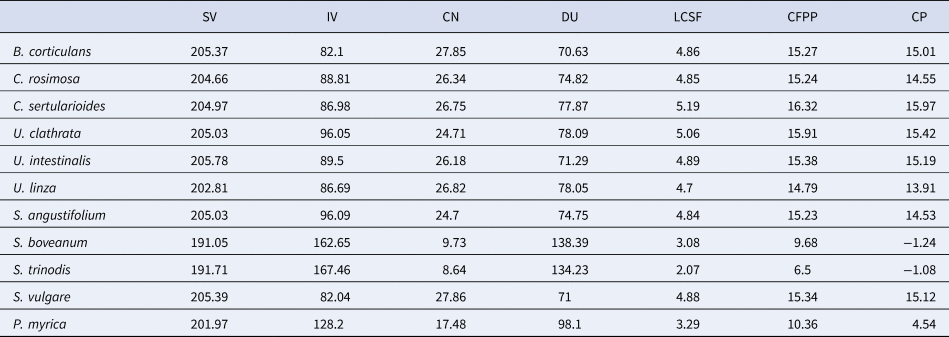
The oxidative instability indexes analysed were DU and IV, while cold flow indicators were LCSF, CFPP and CP. The Brown seaweeds, S. boveanum and S. trinodis showed the highest quantities of USFAs, DU and IV while the green seaweed B. corticulans had the lowest amount of these fatty acids. The contents of DU and IV is affected by the level of USFAs. Among the total fatty acids, USFAs (C18:1 and C18:2) are more reactive towards oxidation and the first step in oxidation is the absorption of oxygen by methylene groups near to the double bonds. Therefore, the stability of biodiesel is reduced by increasing the number of double bonds (C = C) (Zuleta et al., Reference Zuleta, Rios and Benjumea2012). Nevertheless, PUFA content can also be important in controlling the oxidation of biodiesel compounds and high content of these fatty acids reduces the final stability of biodiesel (Demirbaş, Reference Demirbaş2008; Talebi et al., Reference Talebi, Mohtashami, Tabatabaei, Tohidfar, Bagheri, Zeinalabedini, Mirzaei, Mirzajanzadeh, Shafaroudi and Bakhtiari2013; He et al., Reference He, Yan, Pei, Wu, Gebreluel, Zou and Wang2017).
The analysis of cold flow indicators showed that C. sertularioides had the highest level of LCSF equalling 5.19, while U. linza had the lowest level (4.7). In brown seaweeds, S. vulgare showed the highest level of LCSF (4.88%), and S. trinodis had the lowest content of LCSF (2.07%). Nevertheless, the LCSF level in green seaweeds was higher than brown species. This parameter is related to the CFPP factor and is an important element in the oxidative stability of the produced biodiesel. The level of CFPP, similar to LCSF, was higher in C. sertularioides ( + 16.32°C) and S. vulgare ( + 15.34°C). The other cold flow indicator studied was CP content. The highest level of CP was found in C. sertularioides ( + 15.97°C) and S. vulgare ( + 15.12°C). It should be noted that these two species had the highest SFA, LCSF and CFPP values, but the content in C. sertularioides was higher than S. vulgare.
Introduction of macroalgal strains for biodiesel production
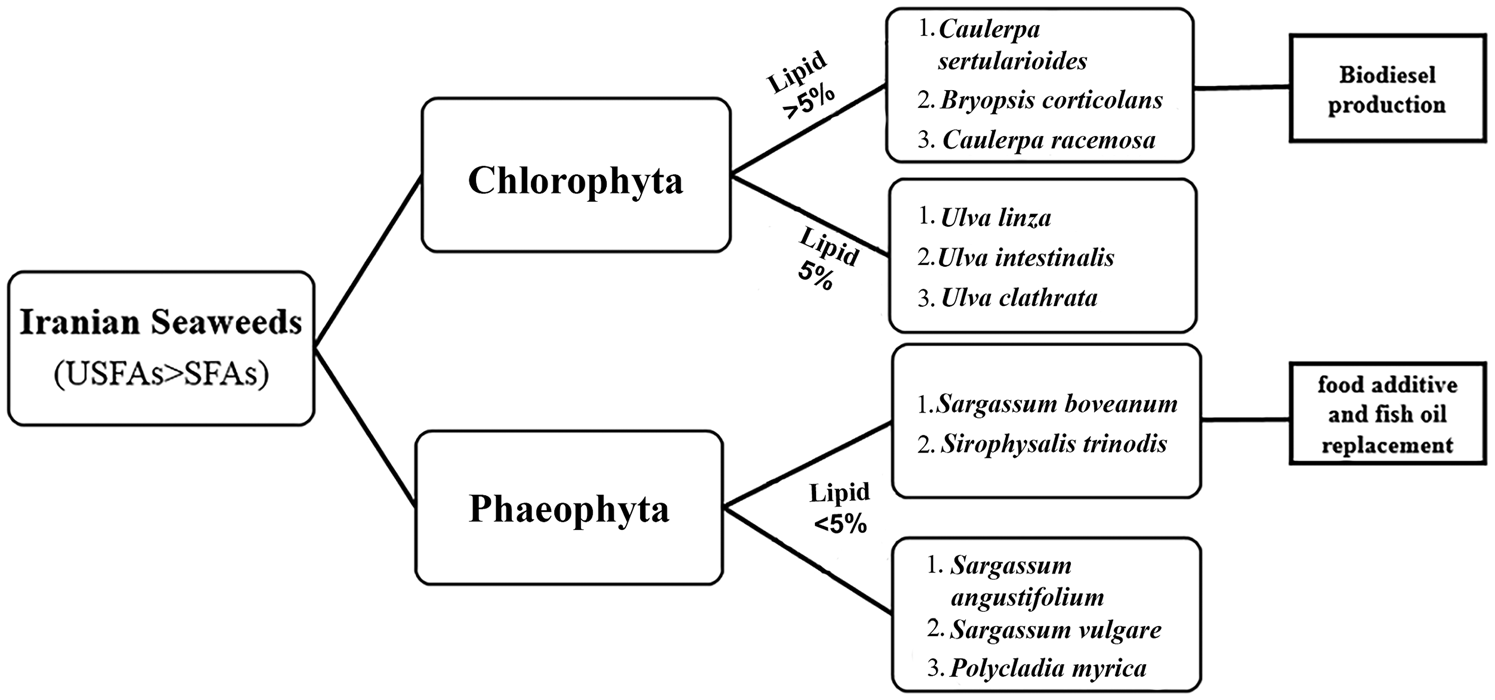
The total lipid content, fatty acid composition and biodiesel indicators can help to identify suitable macroalgae for biodiesel production (Gosch et al., Reference Gosch, Magnusson, Paul and de Nys2012; Talebi et al., Reference Talebi, Mohtashami, Tabatabaei, Tohidfar, Bagheri, Zeinalabedini, Mirzaei, Mirzajanzadeh, Shafaroudi and Bakhtiari2013). Biomass productivity and volumetric lipid productivity are other factors to be considered for the selection of algae for oil-based products (Rodolfi et al., Reference Rodolfi, Chini Zittelli, Bassi, Padovani, Biondi, Bonini and Tredici2009; Talebi et al., Reference Talebi, Mohtashami, Tabatabaei, Tohidfar, Bagheri, Zeinalabedini, Mirzaei, Mirzajanzadeh, Shafaroudi and Bakhtiari2013). Chemical analysis of seaweeds collected from the Persian Gulf of Iran showed that C. sertularioides, C. racemosa, B. corticulans, U. clathrata and U. intestinalis, S. vulgare and S. angustifolium had the highest proportion of SFAs, SFAs/USFAs and cold flow indicators. These species have the maximum value of CN and the minimum value of IV. Furthermore, the highest value of CN but the lowest value of IV was observed in S. vulgare and B. corticulans. The CN and SV level of these samples were similar to the results of Talebi et al. (Reference Talebi, Mohtashami, Tabatabaei, Tohidfar, Bagheri, Zeinalabedini, Mirzaei, Mirzajanzadeh, Shafaroudi and Bakhtiari2013), and Fazelian et al. (Reference Fazelian, Yousefzadi and Movafeghi2020), who identified that the CN and SV have a direct relation with the LCSF, and the higher the LCSF, the larger the CFPP will be. Green seaweeds, C. sertularioides, C. racemosa and B. corticulans also accumulated a high total lipid content (> 5% DW), but the low total lipid content was a limiting factor for biodiesel production from the other seaweeds, in particular U. clathrata and U. intestinalis. Most studies found that macroalgae with low total lipid contents (< 5% DW) are unsuitable for biodiesel production (Montgomery & Gerking, Reference Montgomery and Gerking1980; McDermid & Stuercke, Reference McDermid and Stuercke2003). Furthermore, some macroalgae are suitable for oil-based products due to their high total lipid and fatty acid content (Gosch et al., Reference Gosch, Magnusson, Paul and de Nys2012). On the other hand, macroalgae could be introduced as an important source for biodiesel production due to the rapid growth, easy culture, low cost and high biomass values (Chisti, Reference Chisti2007; Adams et al., Reference Adams, Toop, Donnison and Gallagher2011; Zarei Jeliani et al., Reference Zarei Jeliani, Yousefzadi, Sohrabi Pour and Toiserkani2017).
According to our findings, the best seaweed for biodiesel production in hot regions of the Persian Gulf was C. sertularioides, because this species showed the highest value of SFAs and cold flow indicators as well as the lowest quantities of DU. Cold flow indices of biodiesel are affected by the SFA content, and the high levels of these fatty acids produce biodiesel with higher oxidative stability and higher ignition quality (cetane number) (Knothe, Reference Knothe2008; Gosch et al., Reference Gosch, Magnusson, Paul and de Nys2012). Rising CFPP and CP to near 12°C makes the biodiesel inappropriate for use in cold regions (Pinzi et al., Reference Pinzi, Leiva, Arzamendi, Gandia and Dorado2011; Talebi et al., Reference Talebi, Mohtashami, Tabatabaei, Tohidfar, Bagheri, Zeinalabedini, Mirzaei, Mirzajanzadeh, Shafaroudi and Bakhtiari2013).
The type of fatty acids can also affect the quality of the produced biodiesel. Nascimento et al. (Reference Nascimento, Marques, Dominguez Cabanelas, Pereira, Druzian, De Souza, Vital Vich, De Carvalho and Nascimento2013) have detected that the composition of algal fatty acids has an important role in the quality of biofuels, and the ratio of SFAs/USFAs determined the final quality of biodiesel. High levels of SFAs increased the biodiesel stability to oxidation in tropical environments, while USFAs increased biodiesel fluidity. Therefore, the proper combination of SFAs and USFAs produced a higher quality of biodiesel (Altun et al., Reference Altun, Yaşar and Öner2010). In this study, the main fatty acids observed in seaweeds, especially in C. sertularioides and S. vulgare was palmitic acid (C16:0) and stearic acid (C18:0). The contents of C16:0 and C18:0 have the greatest impact on the CP value (Imahara et al., Reference Imahara, Minami and Saka2006; Talebi et al., Reference Talebi, Mohtashami, Tabatabaei, Tohidfar, Bagheri, Zeinalabedini, Mirzaei, Mirzajanzadeh, Shafaroudi and Bakhtiari2013). The C16 and CP value determined in this study are in agreement with their results. High levels of palmitoleic acid (C16:1) and oleic acid (C18:1) can also increase biodiesel quality through improving the low-temperature properties and kinematic viscosity, and reducing the emissions of hydrocarbon and CO2, while high levels of PUFAs decrease the stability of biofuel (Knothe, Reference Knothe2008; Nascimento et al., Reference Nascimento, Marques, Dominguez Cabanelas, Pereira, Druzian, De Souza, Vital Vich, De Carvalho and Nascimento2013).
The greatest value of C16:1 and C18:1 was observed in P. myrica and U. linza respectively. Ulva linza had a high content of SFAs, SFAs/USFAs and biodiesel indicators, but the low lipid content (< 5% DW) is a limiting factor for utilization in biodiesel production. The combination of U. linza and P. myrica with C. sertularioides, C. rosimosa, and B. corticulans would lead to an improvement of the biodiesel properties. Talebi et al. (Reference Talebi, Mohtashami, Tabatabaei, Tohidfar, Bagheri, Zeinalabedini, Mirzaei, Mirzajanzadeh, Shafaroudi and Bakhtiari2013) also described that a mixed culture or mixed biomass of Amorpha sp. (containing high levels of C16:1, SFAs, CP and CFPP) with Chlorella sp. and Dunaliella sp. (containing high levels of C18:1 and IV) would increase the stability of the biodiesel owing to reduced DU.
In the other strains, S. boveanum and S. trinodis had the greatest content of USFAs (especially C18:2 and C18:3), IV and DU. In contrast, the lowest level of USFAs, IV and DU was observed in B. corticulans, which had a high content of cold flow indicators. IV and DU have a direct relationship with USFAs, while the CFPP decreases with increasing unsaturation (Talebi et al., Reference Talebi, Mohtashami, Tabatabaei, Tohidfar, Bagheri, Zeinalabedini, Mirzaei, Mirzajanzadeh, Shafaroudi and Bakhtiari2013). Also, higher USFAs, DU and IV, results in lower oxidative stability. Interestingly, S. boveanum and S. trinodis had the highest content of n-3 PUFA and these seaweeds could potentially be used as a food or feed additives.
Conclusion
These results suggest that the biodiesel from C. sertularioides can be used in hot regions, (i) because this seaweed was found to be a rich source of lipids, namely, SFAs and (ii) due to its high cold flow indices (LCSF, CFPP and CP). In contrast, S. boveanum and S. trinodis had the highest content of n-3 PUFA, IV and DU, which implies that these seaweeds could potentially be used as a food additive and fish oil replacement in Iran (Figure 2).

Fig. 2. Schematic diagram of different seaweed species from the Persian Gulf of Iran.
Acknowledgements
This research was financially supported by University of Hormozgan.
Author contributions
Zahra Zarei Jeliani and Morteza Yosefzadi carried out the experiment, Nasrin Fazelian analysed the data, Zahra Zarei Jeliani and Nasrin Fazelian wrote the manuscript and Nasrin Fazelian supervised and advised the entire research work. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.