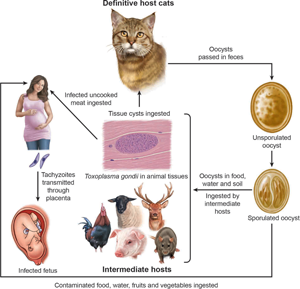
Introduction
Toxoplasmosis, caused by the protozoan parasite, Toxoplasma gondii, is a worldwide zoonosis, and infections are widely prevalent in humans and animals (Dubey and Beattie, Reference Dubey and Beattie1988; Dubey, Reference Dubey2010). Toxoplasmosis can cause serious illness in humans of all ages, and in particular immunosuppressed patients and neonates (Robert-Gangneux and Dardé, Reference Robert-Gangneux and Dardé2012; Torgerson and Mastroiacovo, Reference Torgerson and Mastroiacovo2013; Peyron et al., Reference Peyron, Wallon, Kieffer, Garweg, Wilson, Nizet, Maldonado, Remington and Klein2016; Dardé et al., Reference Dardé, Mercier, Su, Khan, Grigg, Weiss and Kim2020; McLeod et al., Reference McLeod, Cohen, Dovgin, Finkelstein, Boyer, Weiss and Kim2020). Although seroprevalence in Europe has declined in the past 2 decades, a very high prevalence is still prevalent in many countries (McLeod et al., Reference McLeod, Cohen, Dovgin, Finkelstein, Boyer, Weiss and Kim2020).
Sequelae in fetus resulting from T. gondii infections in women who become infected with this parasite during pregnancy can be devastating and enormous efforts are directed in some countries to prevent these consequences. Here an update on congenital toxoplasmosis in humans is provided, especially on the rate of congenital infections in humans worldwide.
Background of congenital toxoplasmosis
After the ingestion of food or water contaminated with T. gondii, there is parasitemia, and tachyzoites can invade the placenta if the woman is pregnant. Humans have haemochorial placenta. Nearly half of fetuses whose mothers become infected during pregnancy escape T. gondii infection. The global rate of transmission during pregnancy is 29% (Dunn et al., Reference Dunn, Wallon, Peyron, Petersen, Peckham and Gilbert1999). The stage of gestation at the time of mother's infection may determine the transmission of T. gondii to fetus. In general, the transmission of T. gondii is more efficient in the later half of gestation, mostly related to the anatomy and immune factors. For example, the thickness of the placenta varies with gestation; in early pregnancy, placental barrier of humans is 50–100 μ m thick and progressively decreases to 2.5–5 μ m at the end of pregnancy, allowing tachyzoites to more easily invade trophoblasts by the end of the gestational course (Blaszkowska and Góralska, Reference Blaszkowska and Góralska2014). Additionally, the internal cytotrophoblast layer is discontinuous, with its cells number decreasing during the gestational period. Infection early in gestation is clinically more severe, as reduced expression of Toll-like receptors in trophoblast cells during the first trimester of pregnancy may indicate a reduced ability of early placental cells to engage the immune response to intrauterine infection (Blaszkowska and Góralska, Reference Blaszkowska and Góralska2014).
Transplacental transmission of T. gondii infection generally occurs when a woman becomes infected during pregnancy. Rarely, congenital transmission occurs in women infected just before pregnancy or during chronic infection (Villena et al., Reference Villena, Chemla, Quereux, Dupouy, Leroux, Foudrinier and Pinon1998; Elbez-Rubinstein et al., Reference Elbez-Rubinstein, Ajzenberg, Dardé, Cohen, Dumètre, Yera, Gondon, Janaud and Thulliez2009). In addition, in immunosuppressed women, reactivation/reinfection of an infection acquired before pregnancy can lead to congenital toxoplasmosis but is rare (Peyron et al., Reference Peyron, Wallon, Kieffer, Garweg, Wilson, Nizet, Maldonado, Remington and Klein2016).
Transplacental infection can lead to a wide variety of manifestations in the fetus and infant including spontaneous abortion, stillbirth; it can also cause severe disease in live infant, but most children are asymptomatic at birth. Although T. gondii may sometimes cause sporadic abortion, there is no evidence that it causes habitual abortion. Most severe cases of prenatally acquired toxoplasmosis were reported first with the predominant manifestation of encephalomyelitis. Historically, the first confirmed case of congenital toxoplasmosis was in an infant girl who was delivered full term by Caesarean section on 23 May 1938 at Babies Hospital, New York (Wolf et al., Reference Wolf, Cowen and Paige1939). The girl developed convulsive seizures at 3 days of age and lesions were noted in the maculae of both eyes through an ophthalmoscope. She died of toxoplasmosis when 1-month-old and an autopsy was performed. At post-mortem, brain, spinal cord and right eye were removed for examination. Free and intracellular T. gondii were found in the lesions of encephalomyelitis and retinitis, and viable T. gondii was isolated in mice, rats and rabbits inoculated with tissues from the girl (Wolf et al., Reference Wolf, Cowen and Paige1939).
In early 1950s, Dr Albert Sabin proposed a triad of signs: hydrocephalus or microcephalus, intracranial calcification and retinochoroiditis. This triad has been useful in drawing attention to prenatal toxoplasmosis. A better understanding came from the work of Eichenwald (Reference Eichenwald and Siim1960), who found asymptomatic and clinical toxoplasmosis in many children in 1950s in Austria. Although the study by Eichenwald (Reference Eichenwald and Siim1960) from selected cases before treatment (prenatal and postnatal) of infected children became a routine, it pointed that toxoplasmosis can cause serious illness in children including both generalized and neurological disease (Dubey and Beattie, Reference Dubey and Beattie1988). As stated earlier, the most common manifestation of prenatal toxoplasmosis is ocular disease, sometimes presented as microphthalmy, cataracts, strabismus or nystagmus and even total blindness.
Hydrocephalus is the most dramatic sign of congenital toxoplasmosis, and occurs in approximately 4% of symptomatic children (Hutson et al., Reference Hutson, Wheeler, McLone, Frim, Penn, Swisher, Heydemann, Boyer, Noble, Rabiah, Withers, Montoya, Wroblewski, Karrison, Grigg and McLeod2015). Initially, it was considered to be due to the blockage of aqueduct of Sylvius. Recently, 4 anatomical patterns of hydrocephalus were reported: (i) obstruction of aqueduct of Sylvius, occurring in 43% of cases, (ii) obstruction of foramina of Monroe occurring in 25% of cases, (iii) mixed aqueductal and foraminal obstruction, occurring in 11% of cases, and (iv) with no obstructive pathogenesis, and was seen in 21% of cases (Hutson et al., Reference Hutson, Wheeler, McLone, Frim, Penn, Swisher, Heydemann, Boyer, Noble, Rabiah, Withers, Montoya, Wroblewski, Karrison, Grigg and McLeod2015). Ocular symptoms are the most common signs of congenital toxoplasmosis.
Most prenatal infections are sub-clinical at birth. Disease, if present in the neonatal period, is likely to be severe, invariably with neurological signs and often with signs of generalized infection. Such patients rarely recover without serious sequelae. Disease appearing in the first few months of life is usually less severe and is manifested by nystagmus, convulsions, bulging fontanelle and abnormal increase in skull circumference. Such patients sometimes develop normally.
It is likely that many cases of prenatal toxoplasmosis are missed because of difficulty in diagnosis. Couvreur et al. (Reference Couvreur, Desmonts, Tournier and Szusterkac1984), who diagnosed prenatal toxoplasmosis in 210 babies aged 0–10 months, found premature birth, intrauterine growth retardation or both in 17%, hyperbilirubinemia in 10%, hydrocephaly or microcephaly in 9%, intracranial calcification in 11% and retinochoroiditis in 22%. Infection was fatal in 2 of the 210, severe in 10%, mild without neurological signs in 34% and subclinical in 55%. It is noteworthy that over half of the babies born with T. gondii infection had no clinical manifestations. As noted by Eichenwald (Reference Eichenwald and Siim1960) earlier, in congenitally infected children, virtually all organ systems may be affected (Peyron et al., Reference Peyron, Wallon, Kieffer, Garweg, Wilson, Nizet, Maldonado, Remington and Klein2016).
An important question concerns the subsequent fate of subclinically infected babies. Many of them develop retinochoroiditis, although it may not manifest until later in childhood, or even in adult life. In a follow-up of 11 congenitally infected children without symptoms at birth, 9 (82%) developed lesions within 20 years; 4 (36.6%) of them developed retinal scars that impaired vision, 5 (45.5%) developed scars without affecting vision (Koppe et al., Reference Koppe, Loewer-Siege and Roever-Bonnet1986). In a 14-year follow-up of 327 congenitally infected children in Lyon, France, 95 (29%) had lesions despite treatment for toxoplasmosis. At the final examination, 60 (18%) had lesions only in the eyes, 35 (11%) had CNS lesions (intracerebral calcification in 31, hydrocephalus in 6 and microcephalus in 1) (Wallon et al., Reference Wallon, Kodjikian, Binquet, Garweg, Fleury, Quantin and Peyron2004). In a study in the USA, 11 of 120 congenitally infected children (many with obvious symptoms) recruited in a treatment programme died within 4 years despite treatment (McLeod et al., Reference McLeod, Boyer, Karrison, Kasza, Swisher, Roizen, Jalbrzikowski, Remington, Heydemann, Noble, Mets, Holfels, Withers, Latkany and Meier2006a).
The morbidity of congenital toxoplasmosis in children is very high and true suffering may be underestimated (Havelaar et al., Reference Havelaar, Kemmeren and Kortbeek2007; Bénard et al., Reference Bénard, Petersen, Salamon, Chêne, Gilbert and Salmi2008; Stillwaggon et al., Reference Stillwaggon, Carrier, Sautter and McLeod2011; Torgerson and Mastroiacovo, Reference Torgerson and Mastroiacovo2013; El Bissati et al., Reference El Bissati, Levigne, Lykins, Adlaoui, Barkat, Berraho, Laboudi, El Mansouri, Ibrahimi, Rhajaoui, Quinn, Murugesan, Seghrouchni, Gómez-Marín, Peyron and McLeod2018; Binquet et al., Reference Binquet, Lejeune, Seror, Peyron, Bertaux, Scemama, Quantin, Béjean, Stillwaggon and Wallon2019; Picone et al., Reference Picone, Fuchs, Benoist, Binquet, Kieffer, Wallon, Wehbe, Mandelbrot and Villena2020). One study estimated 1.2 million disability-adjusted life years and an estimated 190 100 cases globally (Torgerson and Mastroiacovo, Reference Torgerson and Mastroiacovo2013).
The risk of congenital infection is lowest when mother becomes infected in the first trimester (10–15%) and highest when mother acquires infection during the third trimester (Table 1). If maternal infection occurs early in pregnancy, it results in fewer infected babies, but they are more severely affected than the greater number of infected babies born when infection is acquired later in pregnancy. The highest risk to the fetus is when infection is acquired at 10–24th week of gestation. As will be seen in Table 1, most of this information on the transmission of congenital toxoplasmosis is derived from studies in France. In their pioneering study, Desmonts and Couvreur (Reference Desmonts and Couvreur1974a, Reference Desmonts and Couvreurb) reported congenital toxoplasmosis in 210 children born at 1 hospital in Paris; approximately 26% were subclinically infected at birth. Only about 10% were clinically affected – 6% mildly and 4% severely, and up to 3% died in the neonatal period. In a subsequent study from the same hospital, Daffos et al. (Reference Daffos, Mirlesse, Hohlfeld, Jacquemard, Thulliez and Forestier1994) reported clinical outcome in 148 infected fetuses of 2030 mothers who seroconverted during pregnancy. Based on ultrasound examinations, they found that 48, 12 and 3% of fetuses had cerebral ventricular dilatations when mothers became infected in early (<16 weeks), middle (17–23 weeks) and late (after 24 weeks) gestation, respectively (Daffos et al., Reference Daffos, Mirlesse, Hohlfeld, Jacquemard, Thulliez and Forestier1994). In a multicentre European study of 255 congenitally infected children, gestational age at the time of seroconversion in mothers was correlated with cerebral lesions but not retinochoroiditis (Gras et al., Reference Gras, Wallon, Pollak, Cortina-Borja, Evengard, Hayde, Petersen and Gilbert2005). In this study, 51 of 255 infants had 1 or more lesions, and 9 had both intracranial and ocular lesions. Of these, 4 of 55 children died (at 13 months, 11 months, 3 months and 7 days of age). The mother of the baby that died at 1 week of age had seroconverted between 5 and 31 days of gestation and she had received spiramycin prophylaxis from 32nd week until delivery (Gras et al., Reference Gras, Wallon, Pollak, Cortina-Borja, Evengard, Hayde, Petersen and Gilbert2005). The frequency and severity of clinical disease in congenitally infected children in France and Austria has decreased dramatically in the last decade, perhaps because of improved early detection and treatment. Ultrasound sonography can aid the determination of severity of lesions in the fetus (Codaccioni et al., Reference Codaccioni, Picone, Lambert, Maurice, Pomar, Winer, Guibaud, Lavergne, Saliou, Quinio, Benachi, Noel, Ville, Cuillier, Pomares, Ferret, Filisetti, Weingertner, Vequeau-Goua, Cateau, Benoist, Wallon, Dommergues, Villena and Mandelbrot2020).
Table 1. Rate of congenital Toxoplasma gondii transmission according to the gestational age of maternal seroconversion in Francea

a Women who seroconverted during pregnancy were treated with anti-T. gondii drugs to prevent fetal transmission and damage to the fetus.
b No. of women who seroconverted during pregnancy.
A recent study in France retrospectively evaluated 88 cases of congenital toxoplasmosis with ultrasound anomalies diagnosed by fetal medicine experts, 45 (51.1%) had one or more cerebral lesions, the most common lesion being intracranial hyperechogenic cerebral nodular foci (Codaccioni et al., Reference Codaccioni, Picone, Lambert, Maurice, Pomar, Winer, Guibaud, Lavergne, Saliou, Quinio, Benachi, Noel, Ville, Cuillier, Pomares, Ferret, Filisetti, Weingertner, Vequeau-Goua, Cateau, Benoist, Wallon, Dommergues, Villena and Mandelbrot2020). In Table 2, initial data from screening studies are listed; at that time only a few follow-up studies on infected children were undertaken. Subsequently, many of these children were followed clinically for 4 or more years, and data included in the study were reported (The SYROCOT, 2007). Of 691 congenitally infected children from Europe, Brazil, Colombia and the USA, 24% had at least 1 clinical manifestation, 185 had ocular lesions and 13% had intracerebral calcification (The SYROCOT, 2007).
Table 2. Congenital T. gondii infection in humans based on prenatal or postnatal screeninga

a Modified from Dubey (Reference Dubey2010).
b CNS, central nervous system signs; ICC, intracerebral calcification; NS, not stated; RC, retinochoroiditis.
c Of 3708 mothers, 7 seroconverted during pregnancy. One infected fetus with abnormal prenatal ultrasound identified by prenatal screening, and infections in 3 babies were found by cord blood screening.
d Of 2786 screened, 19 mothers seroconverted during pregnancy and 14 children were born infected. Three children were found infected by screening of 532 newborns; 8 of these infected children were selected for The SYROCOT analysis because diagnosis of cerebral lesion was done with tomography; those studies based on ultrasound were discarded (personnel communication from Gomez-Marin to J.P.D-February 25, 2021).
e In 2007, there were 818 700 live births in France through the national screening programme; 272 congenital T. gondii-infections were recognized; 160 infections were diagnosed postnatally (130 at the age of 2 months, 22 between 2 months and 1 year). Of 235 cases, infection was acquired in first trimester in 17 (7%), 83 (35%) in the second trimester and 135 (58%) in third trimester.
f Of 1624 mothers who seroconverted during pregnancy. Lesions in utero in 5, 20 at birth, 21 after birth. RC lesions discovered at birth in 2, in the first year in 20 and third year in 5.
g In a multicentre retrospective study of 16 362 women in Barcelona in 1999, seroprevalence was 28.6% with primary infection of 1.02 per 1000 susceptible women; 9 of 12 susceptible women seroconverted during pregnancy. Out of 5 infected children, 4 were asymptomatic at birth; outcome of fifth infant was not stated.
As stated earlier, 60–70% of babies born from infected mothers escape infection. The severity of toxoplasmosis in the fetus or the infant is not related to the degree of symptoms of T. gondii infection in the mother. In 1 study that retrospectively examined risk factors among women who gave birth to infected children, 52% could not recall being sick (Boyer et al., Reference Boyer, Holfels, Roizen, Swisher, Mack, Remington, Withers, Meier and McLeod2005). In another study from a hospital in Lyon, France, of 603 women who seroconverted during pregnancy, only 36 (5%) had clinical symptoms (Dunn et al., Reference Dunn, Wallon, Peyron, Petersen, Peckham and Gilbert1999). In 161 of these 603 women (504 were treated for toxoplasmosis), infection was transmitted to their fetuses; 5 of them aborted, 3 were stillborn. Most of the 153 live born children were followed for 54 months after birth; 41 (27%) developed clinical signs (33 had retinochoroiditis, 14 had intracerebral calcification, 8 had combinations of signs, 1 child died when 8 days old) (Dunn et al., Reference Dunn, Wallon, Peyron, Petersen, Peckham and Gilbert1999).
The severity of symptoms is primarily related to the trimester of pregnancy when the mother becomes infected with T. gondii (Table 1). However, T. gondii genotype might be another factor, and this topic was reviewed recently (Dardé et al., Reference Dardé, Mercier, Su, Khan, Grigg, Weiss and Kim2020; Mcleod et al., Reference McLeod, Cohen, Dovgin, Finkelstein, Boyer, Weiss and Kim2020). Toxoplasma gondii strains are grouped into clonal Type I, II, III and atypical, based on different systems of genotyping (Dardé et al., Reference Dardé, Mercier, Su, Khan, Grigg, Weiss and Kim2020). Type II and III T. gondii strains predominate in Europe and North America, and Type I is rare. A different situation prevails in South America. The T. gondii strains from Brazil are mostly atypical and clonal strains are rare. In France, most strains isolated from congenital infections are Type II and the severity of congenital toxoplasmosis is related to the trimester of pregnancy when mother becomes infected (Dardé et al., Reference Dardé, Mercier, Su, Khan, Grigg, Weiss and Kim2020). However, in Brazil, women who became infected during the third trimester of pregnancy during oocyst-associated outbreaks of T. gondii had congenitally infected children with severe toxoplasmosis (Conceição et al., Reference Conceição, Belucik, Missio, Gustavo Brenner, Henrique Monteiro, Ribeiro, Costa, Valadão, Commodaro, de Oliveira Dias and Belfort2021). The severity of congenital toxoplasmosis in Brazil in comparison with France is thought to be associated with atypical T. gondii genotypes (Dubey et al., Reference Dubey, Lago, Gennari, Su and Jones2012). However, compared with France, relatively few strains from congenitally infected children in Brazil have been isolated and fully genotyped; 14 genotypes were reported (Carneiro et al., Reference Carneiro, Andrade, Costa, Pinheiro, Vasconcelos-Santos, Ferreira, Su, Januário and Vitor2013). There was no association of genotype with the severity of ocular lesions. However, in the USA, by using a serotyping assay, ocular toxoplasmosis and different anatomical patterns of hydrocephalus were associated with T. gondii Type II than in non-Type II (McLeod et al., Reference McLeod, Boyer, Lee, Mui, Wroblewski, Karrison, Noble, Withers, Swisher, Heydemann, Sautter, Babiarz, Rabiah, Meier and Grigg2012). It should be noted that serotyping has limited efficiency in distinguishing genotypes.
Estimates of congenital transmission
An estimate of the incidence of clinically manifest prenatal toxoplasmosis may be obtained in 3 ways: first, from reports of observed cases and, second, from calculations based on the infection rate during pregnancy, and third, from screening of babies at birth. Representative examples of estimates of congenital infections based primarily on screening of mothers during pregnancy are given in Table 2. Many countries have some surveillance programmes (van der Giessen et al., Reference van der Giessen, Deksne, Gómez-Morales, Troell, Gomes, Sotiraki, Rozycki, Kucsera, Djurković-Djaković and Robertson2021). Congenital infections noted during acute outbreaks of toxoplasmosis summarized recently (Dubey, Reference Dubey2021) were excluded from Table 2. Data from the National Collaborative Chicago-based Toxoplasmosis Study in the USA (Boyer et al., Reference Boyer, Holfels, Roizen, Swisher, Mack, Remington, Withers, Meier and McLeod2005, Reference Boyer, Hill, Mui, Wroblewski, Karrison, Dubey, Sautter, Noble, Withers, Swisher, Heydemann, Hosten, Babiarz, Lee, Meier and McLeod2011; McLeod et al., Reference McLeod, Boyer, Karrison, Kasza, Swisher, Roizen, Jalbrzikowski, Remington, Heydemann, Noble, Mets, Holfels, Withers, Latkany and Meier2006a, Reference McLeod, Khan, Noble, Latkany, Jalbrzikowski and Boyerb, Reference McLeod, Kieffer, Sautter, Hosten and Pelloux2009, Reference McLeod, Cohen, Dovgin, Finkelstein, Boyer, Weiss and Kim2020) are not included in Table 2.
More information is available from countries that have screening (prenatal or postnatal) programmes (Tables 2 and 3).
Table 3. Congenital toxoplasmosis (CT) in children, based on cases reported at national level in the surveillance system (Toxosurv/National Reference Centre on Toxoplasmosis)a

a Courtesy of Toxosurv/National Reference Centre on Toxoplasmosis.
Most of the estimates of congenital infections are from studies that are 10–40 years old, but they are listed in Table 2 to provide perspective. Only Austria and France have compulsory screening of pregnant women for T. gondii infection. Rates of congenital transmission are difficult to compare among countries because of the different methodology used. In some studies, only seroconversion during pregnancy was reported (Sagel et al., Reference Sagel, Krämer and Mikolajczyk2011). One could guess/estimate congenital infection based on the assumption of 50% rate of transmission from mother to fetus. Although there is no national screening for toxoplasmosis in Brazil, valuable information has been obtained from testing of children at few hospitals; these studies were reviewed in detail previously (Dubey et al., Reference Dubey, Lago, Gennari, Su and Jones2012) and summarized here in Table 2.
France
Much of the information on congenital toxoplasmosis is derived from studies in France (Tables 1–3). Mass screening of women during pregnancy was initiated by Georges Desmonts in Paris, France in the 1960s looking at seroconversion in women during pregnancy and the transmission of T. gondii to the fetus (Desmonts and Couvreur, Reference Desmonts and Couvreur1974a, Reference Desmonts and Couvreurb); the screening programme became mandatory in France in 1992. France has a population around 65 million, with less than 900 000 pregnancies. All women are screened for T. gondii antibodies at their first prenatal visit and those with IgG antibodies are not tested further. Seroconversion data are sought through monthly screening and seroconverted women are followed clinically by ultrasound examinations and treated with anti-T. gondii therapy to prevent transmission to the fetus or fetal damage. In Lyon, in a cohort of 603 pregnant women with confirmed toxoplasmosis, the overall maternal–fetal transmission rate was 29% (95% CI 25–33), which marked a sharp increase in risk with the duration of gestation from 6% at 13 weeks to 72% at 36 weeks (Dunn et al., Reference Dunn, Wallon, Peyron, Petersen, Peckham and Gilbert1999).
Most complete data on the estimates of congenital toxoplasmosis were recently made available from France; 2 or 3 infants were infected per 10 000 live births (Table 3). Based on 12-year data, the number of congenitally infected children decreased from 272 (0.031%) in 2007 to 151 (0.018%) in 2018, including the severe congenital infections (Table 3).
Austria
Austria has a population of around 9 million and is one of the first countries to establish a screening programme for T. gondii infection during pregnancy (Thalhammer, Reference Thalhammer1973, Reference Thalhammer, Thalhammer, Baumgarten and Pollak1978). During 1992–2008, 1 387 689 women/infants were tested for T. gondii infection by serology, molecular tests and cord blood screening (Prusa et al., Reference Prusa, Kasper, Olischar, Husslein, Pollak and Hayde2013, Reference Prusa, Kasper, Pollak, Gleiss, Waldhoer and Hayde2015a, Reference Prusa, Kasper, Pollak, Olischar, Gleiss and Haydeb, Reference Prusa, Kasper, Sawers, Walter, Hayde and Stillwaggon2017). Annually 8.5% per 10 000 women acquired T. gondii infection during pregnancy with an estimated 1 congenitally infected infant per 10 000 pregnancies (Prusa et al., Reference Prusa, Kasper, Pollak, Olischar, Gleiss and Hayde2015b). Data regarding clinical outcome are summarized in Table 2. Rationale was provided by the Austrian Government concerning the cost savings because of prenatal screening (Prusa et al., Reference Prusa, Kasper, Sawers, Walter, Hayde and Stillwaggon2017).
Other European countries
There are no recent data on congenital transmission. Data in the past 2 decades are summarized in Table 2. The national screening programme in Denmark was discontinued because of low rate of congenital transmission and the cost-effectiveness (Table 2).
Africa
Little information is available from Africa. A review paper stated 21 cases of congenital toxoplasmosis from 6 hospitals in 3 geographic areas of Morocco (El Bissati et al., Reference El Bissati, Levigne, Lykins, Adlaoui, Barkat, Berraho, Laboudi, El Mansouri, Ibrahimi, Rhajaoui, Quinn, Murugesan, Seghrouchni, Gómez-Marín, Peyron and McLeod2018). Based on the number of live births annually in these 6 centres, 4–8 congenitally infected children were born per 10 000 births but details are lacking (El Bissati et al., Reference El Bissati, Levigne, Lykins, Adlaoui, Barkat, Berraho, Laboudi, El Mansouri, Ibrahimi, Rhajaoui, Quinn, Murugesan, Seghrouchni, Gómez-Marín, Peyron and McLeod2018). There are many unconfirmed reports of congenital toxoplasmosis and spontaneous abortion associated with toxoplasmosis in Egypt; these were recently reviewed by Abbas et al. (Reference Abbas, Villena and Dubey2020). Most of these reports are based on serological results on single samples from pregnant women, and detection of DNA in the placenta.
USA
A neonatal screening was initiated in Massachusetts, USA in 1980s (Guerina et al., Reference Guerina, Hsu, Meissner, Maguire, Lynfield, Stechenberg, Abroms, Pasternack, Hoff, Eaton and Grady1994). Prenatal screening is based on testing mothers for T. gondii seroconversion during pregnancy and postnatal screening is often sampling of cord blood or heel pricks of the newborn combined with phenylketonuria testing (Guthrie cards). However, most of these data were based on prevalence at birth without a follow-up. The 1994 study revealed a low rate of congenital transmission (Table 2).
As stated earlier, through the National Collaborative Chicago-based Toxoplasmosis Study, information has been gained concerning epidemiology, clinical presentation, treatment of congenitally infected children in the USA (Boyer et al., Reference Boyer, Holfels, Roizen, Swisher, Mack, Remington, Withers, Meier and McLeod2005, Reference Boyer, Hill, Mui, Wroblewski, Karrison, Dubey, Sautter, Noble, Withers, Swisher, Heydemann, Hosten, Babiarz, Lee, Meier and McLeod2011; McLeod et al., Reference McLeod, Boyer, Karrison, Kasza, Swisher, Roizen, Jalbrzikowski, Remington, Heydemann, Noble, Mets, Holfels, Withers, Latkany and Meier2006a, Reference McLeod, Kieffer, Sautter, Hosten and Pelloux2009, Reference McLeod, Cohen, Dovgin, Finkelstein, Boyer, Weiss and Kim2020).
Brazil
Brazil has a population of around 213 million. Although there is no national screening of T. gondii in pregnant women or children in Brazil, several centres are performing screening based on convenience and affordability. Data summarized by Dubey et al. (Reference Dubey, Lago, Gennari, Su and Jones2012), up to 2011, indicated 5–23 congenital infections per 10 000 births. Often the sampling was based on who could pay for the tests, and under these circumstances, there will be lower representation of samples from low economic groups. There is also the possibility of false negativity based on IgM testing because many infants with congenital toxoplasmosis are negative for IgM antibodies at birth. There is no national reference laboratory for the confirmation of T. gondii serological testing in Brazil.
The most comprehensive study on congenital toxoplasmosis in children is from the State of Minas Gerais, Brazil (Vasconcelos-Santos et al., Reference Vasconcelos-Santos, Azevedo, Campos, Oréfice, Queiroz-Andrade, Carellos, Romanelli, Januário, Resende, Martins-Filho, Carneiro, Vitor and Caiaffa2009; de Resende et al., Reference de Resende, de Andrade, de Azevedo, Perissinoto and Vieira2010; Carneiro et al., Reference Carneiro, Andrade, Costa, Pinheiro, Vasconcelos-Santos, Ferreira, Su, Januário and Vitor2013). In this study, blood samples were collected from 146 307 newborns at 1560 public health care centres in 853 cities in the state of Minas Gerais. All serological testing was performed in one laboratory initially using an IgM-ELISA capture test kit (Toxo IgMQ-Preven, Symbiosis, Leme, Brazil) and results were confirmed on further testing for IgA antibodies by ELISA, and IgG and IgM anti-T. gondii (enzyme-linked fluorometric assay, VIDAS, BioMérrieux SA, Lyon, France), using blood samples from infants and their mothers. Additionally, infected children were followed clinically months after delivery (Vasconcelos-Santos et al., Reference Vasconcelos-Santos, Azevedo, Campos, Oréfice, Queiroz-Andrade, Carellos, Romanelli, Januário, Resende, Martins-Filho, Carneiro, Vitor and Caiaffa2009; de Resende et al., Reference de Resende, de Andrade, de Azevedo, Perissinoto and Vieira2010). Congenital toxoplasmosis was suspected in 235 infants (1 in 622), and confirmed in 190 children (1 in 770 live births); this figure of 1 per 770 live births does not include in utero mortality due to toxoplasmosis nor infants negative for IgM antibodies at birth (Dubey et al., Reference Dubey, Lago, Gennari, Su and Jones2012).
Of the 106 infected children identified early in screening programme, 46 (43.4%) had hearing loss; 4 of these had sensorineural hearing loss and 13 had conductive hearing loss (de Resende et al., Reference de Resende, de Andrade, de Azevedo, Perissinoto and Vieira2010). Most of these children had ophthalmic lesions (Vasconcelos-Santos et al., Reference Vasconcelos-Santos, Azevedo, Campos, Oréfice, Queiroz-Andrade, Carellos, Romanelli, Januário, Resende, Martins-Filho, Carneiro, Vitor and Caiaffa2009). One hundred and forty-two (79.8%) out of 178 children that underwent ophthalmic examination at 2 months of age had ocular lesions. In 113 of these children, lesions were bilateral; 46.3% of them had macular lesions (de Resende et al., Reference de Resende, de Andrade, de Azevedo, Perissinoto and Vieira2010). Viable T. gondii was isolated by mouse bioassay from peripheral blood in 27 (15.2%) out of 178 children when they were 4 months or older (Carneiro et al., Reference Carneiro, Andrade, Costa, Pinheiro, Vasconcelos-Santos, Ferreira, Su, Januário and Vitor2013). To our knowledge, this is the highest rate of parasitemia demonstrated in congenitally infected children. Genetically, 14 of the 24 isolates tested by 10 PCR-RFLP markers revealed 14 genotypes, distinct from those in Europe (Dardé et al., Reference Dardé, Mercier, Su, Khan, Grigg, Weiss and Kim2020).
A recent study from a hospital in Porto Alegre, Brazil reported long-term follow-up of 77 congenitally infected children from a retrospective investigation of patients 1996–2017 (Lago et al., Reference Lago, Endres, Scheeren and Fiori2021). The children were followed for 2–25 years (Table 2). Most children had ocular lesions (55 children) and 44 had intracerebral calcification, a hallmark of congenital toxoplasmosis. Fewer ocular lesions were detected in children who were treated before they were 4 months old (35.2%) vs those treated after they were 12 months old (77.8%), clearly revealing the benefit of early treatment. Two peaks of retinochoroiditis were detected between 4–5 and 9–14 years (Lago et al., Reference Lago, Endres, Scheeren and Fiori2021). Other lesions in these children were hydrocephalus in 4, microcephalus in 9 and hearing loss in 3 (Lago et al., Reference Lago, Endres, Scheeren and Fiori2021).
Based on limited studies, both the rate of congenital infection and the severity of disease in congenitally infected children are higher in Brazil than in Europe. This topic was discussed by Dubey et al. (Reference Dubey, Lago, Gennari, Su and Jones2012) and is repeated here. This conclusion was based on a comparison of ocular lesions in 30 children in Brazil with 281 children in Europe using a similar methodology. In these 30 Brazilian children, the ocular lesions were more extensive and more likely to involve the area of the retina affecting the central vision than in the European children, despite the fact that most of the Brazilian children had been treated for toxoplasmosis for 12 months (Gilbert et al., Reference Gilbert, Freeman, Lago, Bahia-Oliveira, Tan, Wallon, Buffolano, Stanford and Petersen2008). This study also concluded that the Brazilian children had a 5 times higher risk of severe toxoplasmosis than children in Europe. In another report, the risk of intracranial lesions detected by computed tomography scan was much higher in Brazilian children than in children in Europe (The SYROCOT, 2007). Some of these differences are thought to be related to the genetic makeup of the T. gondii strains in humans in Brazil. Indeed, the T. gondii strains from the Minas Gerais study had atypical genotype compared with most of the strains from congenitally infected children in France that were mostly Type II (Ajzenberg et al., Reference Ajzenberg, Cogné, Paris, Bessières, Thulliez, Filisetti, Pelloux, Marty and Dardé2002; Dardé et al., Reference Dardé, Mercier, Su, Khan, Grigg, Weiss and Kim2020). In addition to genotype, several other factors should not be ignored including the host genetics, the environment, cultural and economic factors.
Other South American and Central American countries
More data have been reported from Colombia, and the pattern of clinical manifestations and prevalence is like from Brazil (Table 2). In a selected survey, 15 congenital infections were identified among 15 333 women sampled (Gómez-Marin et al., Reference Gómez-Marin, de-la-Torre, Angel-Muller, Rubio, Arenas, Osorio, Nuñez, Pinzon, Mendez-Cordoba, Bustos, de-la-Hoz, Silva, Beltran, Chacon, Marrugo, Manjarres, Baquero, Lora, Torres, Zuluaga, Estrada, Moscote, Silva, Rivera, Molina, Najera, Sanabria, Ramirez, Alarcon, Restrepo, Falla, Rodriguez and Castaño2011). No information is available from other countries in this region.
China, India and other Asian countries
There is little or no information on the rates of congenital transmission of T. gondii in these countries.
Prophylactic treatment during pregnancy and prenatal screening
Prevention of infection of the fetus by prophylactic treatment of the mother depends on the delay between maternal infection and its transmission to the fetus. It is also hoped that if infection is already present in the fetus, treatment may limit its ill effects. Treatment is begun as soon as possible during the prenatal period. In Austria and France, it is by spiramycin before the 20th week of pregnancy and thereafter by pyrimethamine and sulfonamide (Picone et al., Reference Picone, Fuchs, Benoist, Binquet, Kieffer, Wallon, Wehbe, Mandelbrot and Villena2020).
A large European multicentre cohort study found no evidence that pre-natal treatment with either spiramycin or sulphonamide combined with pyrimethamine influenced maternal transmission (Gilbert et al., Reference Gilbert, Gras, Wallon, Peyron, Ades and Dunn2001). The relative risk of mother-to-child transmission of T. gondii compared to Lyon, France (women treated) was 1.24 in Austria (women treated), 0.59 in Denmark (no treatment) and 0.65 in the Netherlands (50% treated, 50% not treated) (Gilbert et al., Reference Gilbert, Gras, Wallon, Peyron, Ades and Dunn2001). A meta-analysis of 22 European cohorts found weak evidence that treatment started within 3 weeks of seroconversion reduced mother-to-child transmission compared with treatment started after 8 weeks or more (The SYROCOTT, 2007). On the other hand, the number of congenital infections, based on the mothers testing screening in Lyon, France, decreased after 1992 (46.6% after 1992 vs 59.4% from 1987–1991) when screening of susceptible women became mandatory and antenatal treatment was initiated as soon as the diagnosis was made (Wallon et al., Reference Wallon, Peyron, Cornu, Vinault, Abrahamowicz, Kopp and Binquet2013). In a recent randomized multicentre trial of sulfadiazine plus pyrimethamine (SzP), and spiramycin (Sp) in 143 mothers (73 SzP group, 70 Sp group) who seroconverted during pregnancy, the transmission rate of congenital toxoplasmosis was 2-fold lower in the SzP group (18.5%) than the Sp group (30%) (Mandelbrot et al., Reference Mandelbrot, Kieffer, Sitta, Laurichesse-Delmas, Winer, Mesnard, Berrebi, Le Bouar, Bory, Cordier, Ville, Perrotin, Jouannic, Biquard, d'Ercole, Houfflin-Debarge, Villena and Thiébaut2018). Cerebral lesions were noted in 0/73 in the SzP group vs 6 of 70 in the Sp group, indicating the effectiveness of therapy in reducing damage to fetal tissues.
One current practice is to start treatment with spiramycin if the woman becomes infected in the first or early second trimester of pregnancy, and then perform amniocentesis to detect fetal infection (Montoya, Reference Montoya2018). If fetal infection is detected, then therapy is switched to pyrimethamine and sulfadiazine. Pyrimethamine and sulfadiazine may be used initially in the late second and third trimesters when acute infection is detected.
French T. gondii programme is based on serological screening of mothers to give prophylactic measures to seronegative women to avoid maternal infection. When seroconversion occurs during pregnancy, in complement to prophylactic treatment, a prenatal diagnosis is recommended. This prenatal diagnosis is based on monthly ultrasonography examination and molecular testing for T. gondii DNA on amniotic fluid. Amniocentesis must be performed after 18 amenorrhea weeks and 4 weeks after maternal infection. In case of detection of DNA (PCR positive) in amniotic fluid, diagnosis of congenital toxoplasmosis is made. However, if the PCR is negative, this does not mean that the fetus is free of congenital infection. Children must be followed at birth to detect congenital infection. In France, since surveillance of congenital toxoplasmosis by the National Reference Centre for Toxoplasmosis (beginning in 2007), approximately 10% of false-negative diagnoses are identified annually (data from NRC for toxoplasmosis). One explanation for these observations is that T. gondii is arrested in the placenta and crosses the barrier a few days to weeks later.
Each country needs to evaluate the cost of screening pregnant women, treatment of congenitally infected children and human suffering based on resources and the prevalence of T. gondii in general population (Scallan et al., Reference Scallan, Hoekstra, Angulo, Tauxe, Widdowson, Roy, Jones and Griffin2011; Jones et al., Reference Jones, Kruszon-Moran, Elder, Rivera, Press, Montoya and McQuillan2018; Suijkerbuijk et al., Reference Suijkerbuijk, van Gils, Bonačić Marinović, Feenstra, Kortbeek, Mangen, Opsteegh, de Wit and van der Giessen2018; Binquet et al., Reference Binquet, Lejeune, Seror, Peyron, Bertaux, Scemama, Quantin, Béjean, Stillwaggon and Wallon2019; Bobić et al., Reference Bobić, Villena and Stillwaggon2019). Stillwaggon et al. (Reference Stillwaggon, Carrier, Sautter and McLeod2011) provided an extensive guideline for estimating the costs of preventive maternal screening for and the social costs resulting from toxoplasmosis based on studies in Europe and the USA. While estimating these costs, the value of all resources used or lost should be considered, including the cost of medical and non-medical services, wages lost, cost of in-home care, indirect costs of psychological impacts borne by the family for lifetime care of a substantially cognitively impaired child; cost of fetal death was estimated to be 5 million dollars (Stillwaggon et al., Reference Stillwaggon, Carrier, Sautter and McLeod2011). A study on the cost-effectiveness of screening from Austria estimated a lifetime cost of 103 Euros per birth under prenatal screening compared with 323 Euros without screening (Prusa et al., Reference Prusa, Kasper, Sawers, Walter, Hayde and Stillwaggon2017). Although it is unethical to value human life in terms of dollars, each nation must balance public funding for all the needs of its people, including the prevention of crippling ailments.
Where it has been carried out with thoroughness, persistence and determination, as in France, education appears to have contributed to a reduction in the incidence of T. gondii infection during pregnancy. It should be included in the instructions given in antenatal clinics and by obstetricians and midwives dealing with individual patients. Individual instruction given in person is likely to be most effective but should be supplemented by booklets printed in the various languages of the patients and by videos in the waiting rooms of antenatal clinics.
Acknowledgements
This research was supported in part by an appointment of Camila K. Cerqueira-Cézar and Fernando H. A. Murata to the Agricultural Research Service (ARS) Research Participation administered by the Oak Ridge Institute for Science and Education (ORISE) through an inter-agency agreement between the US Department of Energy (DOE) and the US Department of Agriculture (USDA). ORISE was managed by ORAU under DOE contract number DE-SC 0014664. All opinions expressed in this paper were the authors' and did not necessarily reflect the policies and views of USDA, ARS, DOE or ORAU/ORISE.
Author contributions
J.P.D. and I.V. wrote the review; F.H.A.M., C.K.C. and O.C.H.K. helped with literature and evaluation of data.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
Not applicable.