INTRODUCTION
Sponges (phylum Porifera) represent one of the oldest extant multicellular animals (Müller, Reference Müller1998), inhabiting a variety of marine and freshwater ecosystems. They are sessile filter-feeding organisms with a relatively simple body, constituted by an outer epithelial layer (pinacocytes) enclosing the mesohyl, formed by specialized cells, extracellular matrix and a network of canals and chambers. The water is pumped continuously throughout those chambers by flagellated cells (choanocytes) that retain and phagocytize the suspended particles (Simpson, Reference Simpson1984). Despite the absence of conspicuous physical defences, such as shells or spines, sponges are found in environments where the competition by substrate and predation are extremely aggressive (Ruzicka & Gleason, Reference Ruzicka and Gleason2008; Turon et al., Reference Turon, Marti and Uriz2009). This success is probably due to a wide variety of bioactive compounds, which have turned these animals into one of the most prolific sources of natural products (see Blunt et al., Reference Blunt, Copp, Keyzers, Munro and Prinsep2014, and previous reviews of this series). However, many of these substances are not produced by the sponge itself, but by its associated microbiota (Costantino et al., Reference Costantino, Fattorusso, Mangoni, Di Rosa and Ianaro1999; Sipkema et al., Reference Sipkema, Schippers, Maalcke, Yang, Salim and Blanch2011). Recently there has been a trend to isolate, cultivate and identify sponge-associated microorganisms to search for new compounds and to obtain active substances in larger quantities (Taylor et al., Reference Taylor, Radax, Steger and Wagner2007; Schippers et al., Reference Schippers, Sipkema, Osinga, Smidt, Pomponi, Martens and Wijffels2012).
Sponges are known to harbour a species-specific microbiota, which is maintained under strict control in healthy animals (Lee et al., Reference Lee, Lee and Lee2001; Jadulco et al., Reference Jadulco, Brauers, Edrada, Ebel, Wray, Sudarsono and Proksch2002; Webster et al., Reference Webster, Negri, Munro and Battershill2004). In addition, sponges are also exposed to large numbers of microorganisms from the surrounding environment (Pfannkuchen et al., Reference Pfannkuchen, Fritz, Schlesinger, Bayer and Brümmer2009). Sponges filter vast quantities of water, retaining more that 80% of the suspended particles. Therefore, a transient microbiota is always present in its channels, tissues and surfaces (Pile et al., Reference Pile, Patterson and Witman1996). To deal with these environment-derived microorganisms, they present a well-organized innate immune system, which represents an efficient first line of defence and allows immediate responses using molecules that recognize highly conserved microbial structures (Wiens et al., Reference Wiens, Korzhev, Perovic-Ottstadt, Luthringer, Brandt, Klein and Müller2007). Recently, it has been shown that the bases of this system resemble those of higher metazoans (Müller et al., Reference Müller, Koziol, Müller and Wiens1999, Reference Müller, Böhm, Grebenjuk, Skorokhod, Müller and Gamulin2002). The immune reactions in sponges are affected by suppressants such as FK506 and Cyclosporine A (CsA), which are produced by microorganisms and widely used in human organ transplants (Müller et al., Reference Müller, Blumbach, Krasko and Schröder2001; Sabella et al., Reference Sabella, Faszewski, Himic, Colpitts, Kaltenbach, Burger and Fernandez-Busquets2007). This fact suggests that at least part of the symbiotic microbiota may be using a similar strategy to modulate the sponge immune system and avoid elimination.
It is know that several antibiotics, in special macrolides, are able to modulate many components of the immune response (Tamaoki, Reference Tamaoki2004; Altenburg et al., Reference Altenburg, de Graaff, van der Werf and Boersma2011). Consequently, antimicrobial screening can indicate the presence of such compounds. In view of this, and the fact that many bioactive molecules detected in sponges have antibacterial properties, we tested the capacity of these substances isolated from sponge-associated microbionts to inhibit the degranulation of RBL-2H3 cells. This cell line, like mast cells and basophils, respond with degranulation as a consequence of immunological and non-immunological stimulation (Narenjkar et al., Reference Narenjkar, Assem, Wan, Marsh and Ezeamuzie2006; Passante & Frankish, Reference Passante and Frankish2009). Substances that are able to block this process may be potential immunosuppressive molecules.
MATERIALS AND METHODS
Sponges
Samples of Metania reticulata (Bowerbank, 1863) (Haplosclerida: Metaniidae) were collected in the Negro River, in the Brazilian Amazon Central Basin region (Manaus, Amazon state, Brazil) (Figure 1), during the dry season (January). The sponges, including gemmules, were attached to trees positioned 10 m from the river margins and 1 m from the ground. The samples were collected with forceps, placed in sterile tubes and maintained at 8°C until processed.

Fig. 1. The freshwater sponge Metania reticulata attached to vertical tree branches in the Negro river during the dry season.
Microbial isolation and culture
The external portions of the sponges were carefully removed with sterile scalpels and pieces (1 cm3) were collected from the interior of each specimen. These samples were homogenized, diluted in 50 mL of DYP medium (10 g of dextrose, 2.5 g of yeast extract and 5 g of peptone per litre of MilliQ water), and distributed in tubes. After 3 days of incubation at 25°C, each culture was serially diluted and 100 µL of each dilution was spread in DYP agar plates (DYP medium plus agar 16 g L−1). The isolated microorganisms were tested for antibacterial activity as described below, and the strains that showed positive results were incubated in 350 mL of DYP medium. After growth, the medium was collected and filtered with a 0.22 µm membrane and the retained microorganisms were used for DNA extraction and molecular fingerprinting by amplification of target sequences. Amplification of 18S and 16S rRNA genes was performed using the primers ITS1 and ITS4 (White et al., Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990), 27FB and 1492RAB (Turque et al., Reference Turque, Cardoso, Silveira, Vieira, Freitas, Albano, Gonzalez, Paranhos, Muricy and Martins2008), and the products sequenced by capillary electrophoresis in a Megabace 1000 platform (GE Healthcare). Consensus sequences were then manually edited and analysed by BLAST searches at NCBI (National Center for Biotechnology Information). All sequences were deposited in GenBank.
Extraction of exudates from microorganism cultures
The filtered culture medium (350 mL) of each strain was passed through Sep–Pak C18 cartridges (Waters) and the analytes were eluted consecutively with ethanol 50% (ET050), ethanol 100% (ET100), hexane, dichloromethane and acetone. Each sample was evaporated in reduced pressure at 60°C, and the ethanol 50 and 100% fractions were lyophilized after the ethanol evaporation and stored at −20°C. The substances not retained by the Sep–Pak columns were passed through 10 and 1 kDa membranes. Smaller compounds were recovered from the filtrate by anion exchange chromatography with Amberlite IR-400 resin (Sigma). Each fraction was dried, weighed and stored at −20°C.
Antibacterial activity assay
The microorganisms used were Staphylococcus aureus (AS), Salmonella enteritidis (SE), Pseudomonas aeruginosa (PA), Proteus vulgaris (PV), Klebsiella pneumoniae (KP) and Micrococcus luteus (ML), which were incubated at 37°C for 24 h in Brain-Heart infusion (BHI) prior to the assays. The fractions obtained from the liquid culture were dissolved to 10 mg mL−1 in 50% BHI for testing and the active extracts were dissolved to 2 mg mL−1 for re-testing. The hydrophobic extracts were diluted in DMSO (dimethyl sulphoxide, 0.2 mg µL−1) to permit the dilution in aqueous medium. In the assays, 50 µL of each culture (1 × 108 CFU) were distributed in tubes with 400 µL of 50% BHI and 50 µL of the sample. For each treatment, controls without sample and a blank, without sample and microorganisms, were maintained. Aliquots (200 µL) of each test tube were read in 96-well plates, immediately prior to the test (T 0) as well as after 24 h of incubation (T 24). The optical density of the cultures was registered at 600 nm in a SpectraMax microplate reader (Molecular Devices), and the effect in the bacterial concentration was determined by comparing the optical density in the T 0 to the value registered in the T 24, for each treatment. The results (N = 9) were plotted as percentage using the readings from the controls as baseline (zero) and the effects on the growth as positive (promoter) or negative (inhibitory) bars.
HPLC purification
The HPLC was performed in a LC-10 system connected to UV detector SPD-10 (Shimadzu) and a Capcell C18 column (Shiheido). The extract showing highest antimicrobial activity (MERETb.762 ET100: 100 µg) was diluted in 50 µL of 50% acetonitrile and eluted with a gradient of acetonitrile (B) in 0.1% trifluoroacetic acid (A). B gradient was 0% (0–1 min), 0–100% (1–5 min), 100% (5–40 min), 100–0% (40–45 min) and 0% (45–60 min). The substances were detected at 214 nm wavelength and the flow rate was 1 mL min−1. The fractions were manually collected, evaporated and stored at −20°C until the degranulation assay.
Degranulation assay
The amount of β-hexosaminidase released by the cells was measured as described previously (Yan et al., Reference Yan, Nanamori, Sun, Zhou, Cheng, Li, Zheng, Xiao, Xie, Ye and Wang2006). The rat basophilic leukaemia cells (RBL–2H3) were cultivated in Eagle medium with 15% foetal bovine serum. The cells were differentiated toward neutrophil-like upon incubation with dibutyryl–cAMP (0.2 mm) for 48 h and cultured overnight in 24-well tissue culture plates (2 × 105 per well). Then, washed twice with PBS and incubated 5 min with or without the HPLC fractions FII and FIV (10 µg mL−1) and CsA (10 µm). Control cultures were incubated without the presence of inhibitors. After incubation for 15 min with formyl-methionyl-leucyl phenylalanine (fMLF, 100 nm) the degranulation was terminated by placing the plates on ice. The amount of β-hexosaminidase released into the medium was determined by incubating 20 µL of supernatant or cell lysate with 10 µL of 1 mm p-nitrophenyl-N-acetyl-D-glucosamide (pNAG) in sodium citrate buffer (0.1 m; pH 4.5) at 37 °C for 1 h. At the end of the incubation, 200 µL of 0.1 m Na2CO3 and 0.1 m NaHCO3 (pH 10) were added and the absorbance was determined in a microplate reader at 405 nm. Total β-hexosaminidase was determined by lysing the RBL–2H3 cells in 0.1% Triton X-100. Data were collected from three independent experiments and presented as the percentage of the β-hexosaminidase released in relation to the total.
Mass spectrometry
Mass spectra were acquired on a LCMS-IT-TOF mass spectrometer (Shimadzu), equipped with a standard electrospray probe. The electrospray probe was adjusted to ~200 µL min−1. The CDL and the heat block temperatures were maintained at 200°C and the needle voltage at 3.6 kV, applying a drying gas flow (nitrogen) of 3 L min−1 and a nebulizer gas flow (nitrogen) of 1.5 L min−1. The mass spectrometer was calibrated with sodium trifluoracetate and its typical cone-voltage induced fragments. About 50 pmol of each sample was injected into electrospray transport solvent. The ESI mass spectra were obtained in the continuous acquisition mode, scanning from m z−1 50 to 1000 with a scan time of 7 s.
RESULTS
Fourteen bacterial and six fungal strains were isolated from the freshwater sponge samples. Two bacterial strains (MERETb.761, GenBank accession no KF305316; and MERETb.762, no KF305317) and one fungus (MERETf.010, no KF305318) showed natural antimicrobial activity. Both bacterial strains inhibited the fungus Aspergillus sp., that was grown in the same culture plate, producing an inhibition zone where the bacteria grew over the hypha (Figure 2). The fungus MERETf.010 was the only microorganism isolated from the number 10 sponge sample. An average of six to eight colonies of both bacteria and fungi were observed in each one of the other plates in these initial cultures, indicating that MERETf.010 strain inhibited all other microorganisms present in this sample. These three strains were then selected for further testing and cultured until the liquid medium acquired a dark brownish colour, which took about 45 days. In this stage, the cultures were then extracted and assayed for antibacterial activity as described in the experimental section.

Fig. 2. (A) Inhibitory effect of the bacterial strains MERETb.761 and 762 against the fungus Aspergillum sp., both isolated from the same sponge sample (no. 7). (B) Detail of the bacteria MERETb.762 (opaque mass in the central area) growing over the fungal hyphae.
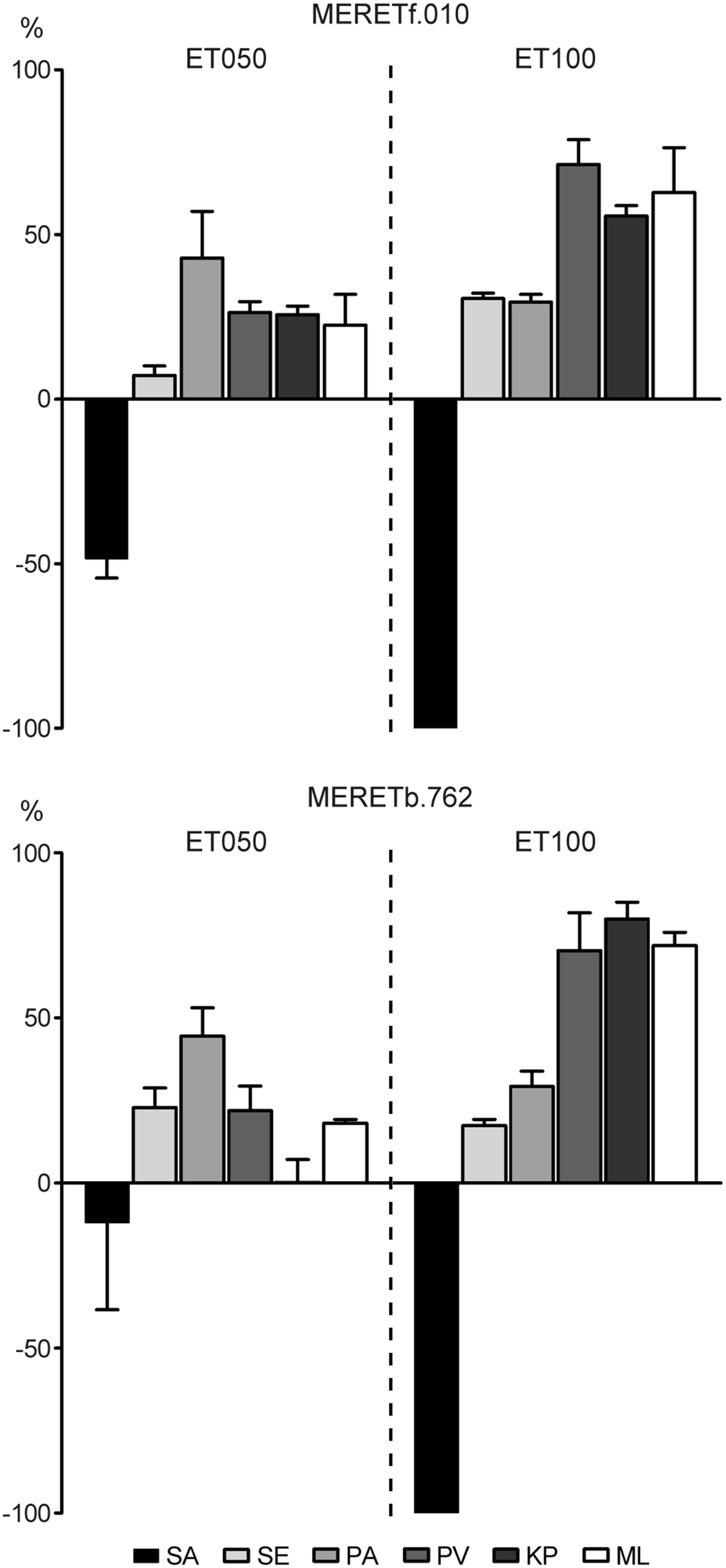
The results showed that the ET050 and ET100 fractions from the fungus MERETf.010 and the bacteria MERETb.762 inhibited specifically the growth of S. aureus (10 mg mL−1, Figure 3). Both ET100 fractions completely abolished bacterial growth. However, when the same fractions were tested in lower concentrations (2 mg mL−1), only the bacterial ET100 maintained the full inhibitory effect, while that from the fungi was slightly reduced (97.2 ± 2.8%).

Fig. 3. Effects of the ethanolic extracts ET050 and ET100 from the fungi MERETf.010 and the bacteria MERETb.762 on liquid cultures of S. aureus (SA), S. enteritidis (SE), P. aeruginosa (PA), P. vulgaris (PV), K. pneumoniae (KP) and M. luteus (ML). The results were plotted using the readings from the controls as baseline (zero) and the effects on the growth as positive (promoter) or negative (inhibitory) bars.
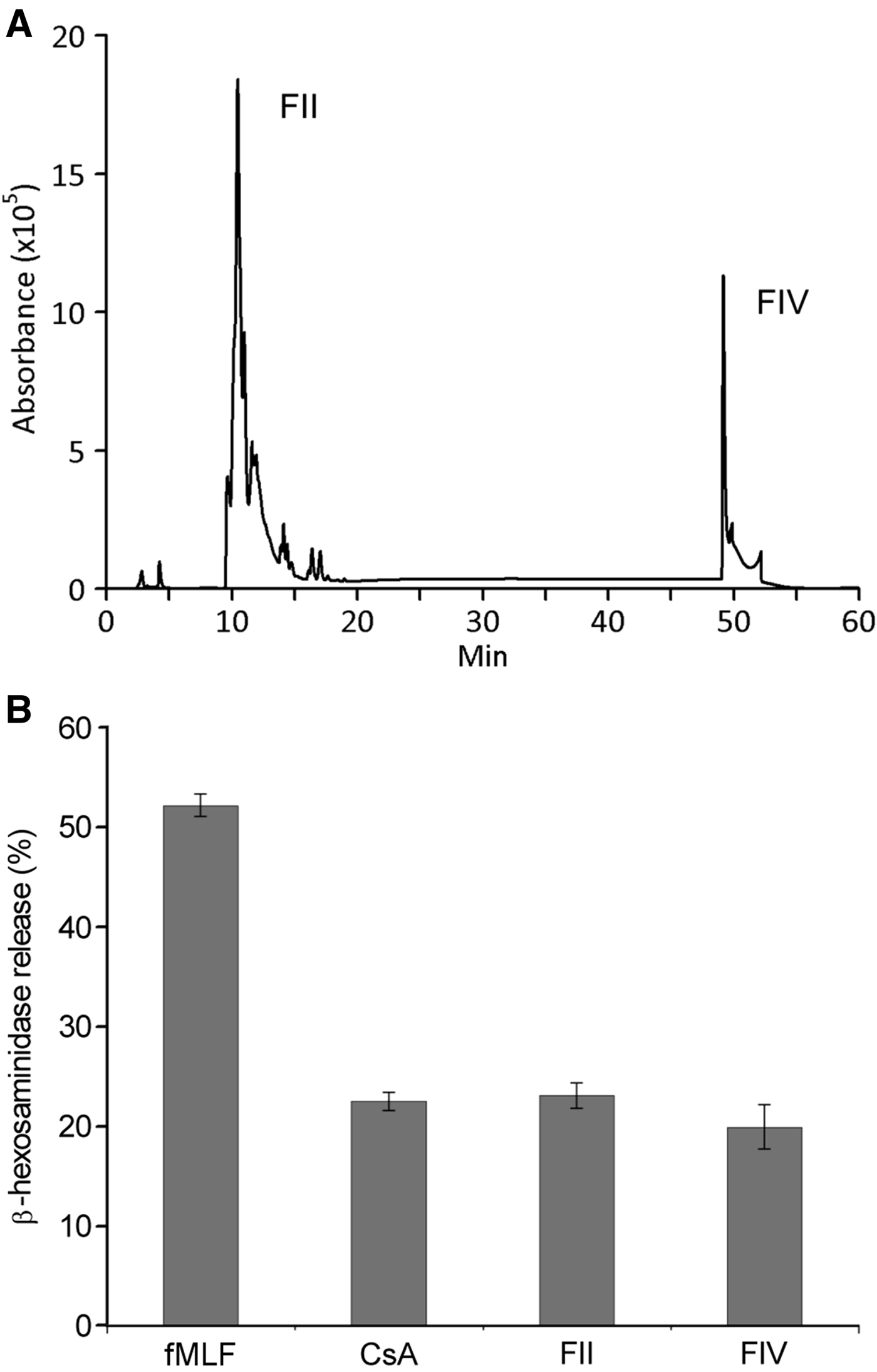
Considering the specificity and the stronger antibiosis of ET100 from MERETb.762 against S. aureus, this fraction was then selected and analysed by HPLC. The HPLC profile of ET100 showed two main peaks, FII and FIV (Figure 4A), which were collected and tested in the RBL–2H3 degranulation assay. The results show that the fraction FII inhibited the β-hexosaminidase release down to 23.1 ± 1.3% and the FIV to 19.9 ± 2.2%, similar to the CsA effect, with 22.5 ± 0.9% (Figure 4B). When these fractions were analysed by mass spectrometry, peaks of 168.0170 m z−1 for FII and 215.5131 m z−1 for FIV were observed, predicting the formulas C12H6N4O8 and C25H4N2O6, respectively, both corresponding to nitroaromatic compounds. The predicted formula for fraction FII corresponds to tetranitrobiphenyl, which is described as mutagenic in bacteria and carcinogenic in animal cells (Hirayama et al., Reference Hirayama, Iguchi and Watanabe1990; Purohit & Basu, Reference Purohit and Basu2000). There is no reference in the scientific literature for fraction FIV predicted formula. The FII and FIV were submitted to nuclear magnetic resonance analysis in the Analytical Center of Chemistry Institute (São Paulo University). However, the spectra were inconclusive due to the purity of compounds.

Fig. 4. (A) HPLC profile of the ethanolic extract of the bacteria MERETb.762 culture medium, with the peaks FII and FIV. (B) Release of β-hexosaminidase by RBL–2H3 cells stimulated by fMLF and inhibitory activity of Cyclosporine A (CsA) and fractions FII and FIV on fMLF-stimulated cells. The results are presented as percentage of the total β-hexosaminidase in the cultures.
DISCUSSION
Brazil has a quite representative fauna of freshwater Porifera, with 52 of the 200 species described worldwide (Custódio & Hajdu, Reference Custódio and Hajdu2011). In several areas, these animals are an important fraction of the invertebrate biomass, known as cauxi by the local populations, and recognized as the cause of dermatitis and other health problems (Volkmer-Ribeiro et al., Reference Volkmer-Ribeiro, Lenzi, Oréfice, Pelajo-Machado, Alencar, Fonseca, Batista, Manso, Coelho and Machado2006; Cruz et al., Reference Cruz, Alencar, Medina, Volkmer-Ribeiro, Gattás and Luna2013). Nevertheless, the available literature is mostly limited to systematic or ecological studies, with a single article dealing with biochemical aspects (Barros et al., Reference Barros, Volkmer-Ribeiro and Veiga Junior2013). This is the first report on bioactive substances obtained from freshwater sponges of the Amazon region, and this result can raise the awareness for these organisms in the region.
The inhibition induced by the substances isolated from the bacteria MERETb.762 can be acting through different pathways to exert the immunosuppressive effect. Cyclosporine A works by binding to cytosolic proteins, cyclophilins, and avoiding signal transduction from membrane receptors (Narenjkar et al., Reference Narenjkar, Assem, Wan, Marsh and Ezeamuzie2006). However, CsA not only interfere with intracellular processes but also with the extracellular release of lytic components through degranulation induced by bacterial molecules (Passante & Frankish, Reference Passante and Frankish2009). The fMLF bacterial peptide activates the G protein and the downstream signalling molecules, and stimulates the RBL–2H3 degranulation (Yan et al., Reference Yan, Nanamori, Sun, Zhou, Cheng, Li, Zheng, Xiao, Xie, Ye and Wang2006). This can be inhibited by the deactivation of the Ca+/calmodulin mediated by CsA. In addition, CsA also can suppress c-jun NH2-terminal kinase (JNK) and p38 mitogen-activated protein kinases (Funaba et al., Reference Funaba, Ikeda and Abe2003).
A phylogenetic analysis based on the 16S rRNA gene of MERETb.762 (1404 nts) revealed similarities scores of 99% with several Bacillus strains, a common genus found in different marine sponges (Taylor et al., Reference Taylor, Radax, Steger and Wagner2007). The inhibition of degranulation evoked by fractions FII and FIV represent the first report of this activity for a sponge-associated bacterium. When the physiology of symbiotic organisms is analysed, the production of immunosuppressants is commonly mentioned as a possible strategy to avoid expulsion from their hosts (Corsaro et al., Reference Corsaro, Venditti, Padula and Valassina1999). The production of such compounds by MERETb.762 suggests a possible mechanism by which at least some sponge-associated microorganisms can maintain this relation.
The effects of several antimicrobial agents on the immune system, both at cell mediated and humoral levels, was recognized a long time ago (Finch, Reference Finch1980; Reato et al., Reference Reato, Cuffini, Tullio, Mandras, Roana, Banche, Foa and Carlone2004). However, this characteristic is not tested in screenings that usually focus only on antibiosis. Since sponges and their associated microbiota are known to produce numerous substances with such activity, immunosuppressive assays on newly isolated compounds could reveal new and interesting molecules.
ACKNOWLEDGEMENTS
The authors thank Dr S.P. Zanotto, Amazonas State University, for supplying the sponge samples used in this work; Dr R. Curi, who supplied the RBL–2H3 cells and Ms A. Brito, who kindly supplied the bacteria used in the antimicrobial test, both from University of São Paulo.
FINANCIAL SUPPORT
This work was supported by CNPq and FAPEAM (National Institute of Science and Technology: Energy, Environment and Biodiversity – INCT–CEAB).