Introduction
Melcherite is the second natural hexaniobate. The first described was peterandresenite (Friis et al., Reference Friis, Larsen, Kampf, Evans, Selbekk, Sánchez and Kihle2014) and hansesmarkite was recently discovered (Friis et al., Reference Friis, Weller and Kampf2017). Polyoxometalates of niobium are dominated by the Linqdvist hexaniobate ion, (Nb6O19)8–, and its synthesis and stability requires alkaline conditions. The crystal structure of these compounds was first described by Lindqvist (Reference Lindqvist1953). Hexaniobates are negatively charged clusters of six mutually edge-sharing NbO6 octahedra forming a super-octahedron (Nyman, Reference Nyman2011). Possible polyoxoniobate applications include their use as reagents in the break-down of nerve agents and in the development of filter media protection against chemical warfare agents (Kinnan et al., Reference Kinnan, Creasy, Fullmer, Schreuder-Gibson and Nyman2014). Polyoxometalates have also been investigated in coordination chemistry, leading to the development of hybrid organometallic hexametalate complexes (Abramov et al., Reference Abramov, Vicent, Kompankov, Gushchin and Sokolov2016), and the synthesis of new polyoxoniobates coordinated to copper complexes (Wang et al., Reference Wang, Niu and Niu2008).
The mineral is named in honour of Geraldo Conrado Melcher (1924–2011). He was professor at the Department of Mining Engineering at the Polytechnic School, University of São Paulo and was also a pioneer in Jacupiranga carbonatite studies (Melcher, Reference Melcher, Tuttle and Gittins1966).
Both the description and name were approved by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA2015-018). Type material is deposited in the Museu de Geociências, Instituto de Geociências, Universidade de São Paulo, Rua do Lago, 562, 05508-080 – São Paulo, SP, Brazil. Specimen number: DR982. Part of the cotype sample has been deposited at the University of Arizona Mineral Museum, RRUFF Project (deposition no. R130752).
Occurrence
The mineral occurs in the carbonatite of the Jacupiranga mine (24°43′47″S, 48°06′37″W), Cajati County, São Paulo, Brazil (Menezes Filho and Martins, Reference Menezes Filho and Martins1984). For general information about this carbonatite see Menezes Filho et al. (Reference Menezes Filho, Atencio, Andrade, Downs, Chaves, Romano, Scholz and Persiano2015). This is also the type locality for zirkelite (Hussak and Prior, Reference Hussak and Prior1895), quintinite (Chao and Gault, Reference Chao and Gault1997), menezesite (Atencio et al., Reference Atencio, Coutinho, Doriguetto, Mascarenhas, Ellena and Ferrari2008) and pauloabibite (Menezes Filho et al., Reference Menezes Filho, Atencio, Andrade, Downs, Chaves, Romano, Scholz and Persiano2015). Although the joint occurrence of menezesite, pauloabibite and melcherite has not been observed, these minerals may be related genetically. Pauloabibite is trigonal NaNbO3, isostructural with ilmenite (Menezes Filho et al., Reference Menezes Filho, Atencio, Andrade, Downs, Chaves, Romano, Scholz and Persiano2015). The synthetic analogue of pauloabibite was reported by Kinomura et al. (Reference Kinomura, Kumata and Muto1984) and Kumata et al. (Reference Kumata, Kinomura and Muto1990) from a two-step synthesis method, involving the preparation of Na8Nb6O19·13H2O (a hexaniobate) followed by hydrothermal reaction with NaOH in a silver-lined vessel at 250°C. Menezesite is a heteropolyoxoniobate, cubic (□,Ba,K)12(□,Mg)3Zr4(BaNb12O42)·12H2O (Atencio et al., Reference Atencio, Coutinho, Doriguetto, Mascarenhas, Ellena and Ferrari2008). According to Nyman et al. (Reference Nyman, Bonhomme, Alam, Rodriguez, Cherry, Krumhansl, Nenoff and Sattler2002), the heteropolyanions of W, Mo and V are formed simply by acidification of solutions of their oxoanions. Under similar conditions, these oxoanion precursors are not available for Nb, and Nb-oxo chemistry is dominated by formation of the Lindquist ion [Nb6O19]8– (present in melcherite). However, heteropolyniobate (present in menezesite) formation is favoured in hydrothermal reactions of aqueous, alkaline precursor mixtures. A competing phase to the formation of polyoxoniobates in hydrothermal aqueous reactions involving Nb and an alkali hydroxide is NaNbO3, avoided by using short reaction times (i.e. 24 hours or less) (Nyman et al., Reference Nyman, Bonhomme, Alam, Rodriguez, Cherry, Krumhansl, Nenoff and Sattler2002). So melcherite could have originally formed under acid conditions, and afterwards, under basic conditions, menezesite and pauloabibite could have formed.
Quintinite, menezesite, pauloabibite and melcherite occur in the so-called ‘intermediate zone’, characterized by a high dolomite and slightly anomalous ‘pyrochlore’ content. Associated minerals are dolomite, calcite, magnetite, pyrrhotite, tochilinite, ‘pyrochlore’, pyrite and fluorapatite. Melcherite formed as a carbonatite vug mineral.
Habit and physical properties
Melcherite forms irregular, tabular crystals up to 200 µm in maximum dimension (Fig. 1). The mineral is transparent and displays a vitreous lustre; it is beige and the streak is white. It is non-fluorescent under both short (254 nm) and long wavelength (366 nm) ultraviolet radiation. The mineral displays perfect cleavage on {001}. Fracture was not determined. Twinning and parting were not observed. The Mohs hardness and density were not measured due to the paucity of material but the calculated density is 3.733 g/cm3 [based on the empirical formula (Ba0.99K1.00)Σ1.99(Na1.02Ca0.96)Σ1.98(Mg0.95 Mn0.05)Σ1.00Nb6.02O19.00·6H2O]. Refractive indices were not measured due to paucity of material. The mean refractive index is estimated as 1.924 using the Gladstone-Dale relationship (Mandarino, 1981).

Fig. 1. Melcherite from the Cajati mine, São Paulo, Brazil.
Mineral chemistry
Melcherite crystals were embedded in epoxy resin and polished. In the back-scattered electron images, we can see that the crystals are zoned (Fig. 2). The chemical analyses (Table 1) were done by means of a Cameca SX100 electron microprobe (wavelength dispersive spectroscopy mode, 15 kV, 10 nA and 20 µm beam diameter). H2O was inferred from the crystal structure determination. H2O was initially assumed by difference prior to the matrix correction (PAP) and then calculated by stoichiometry post matrix correction due to software limitations. Analyses from the brighter areas of the melcherite crystal, (Fig. 2 back-scattered electron image) have the following composition: (Ba1.75K0.19)Σ1.94(Na1.80Ca0.19)Σ1.99(Mg0.96Mn0.02Al0.02)Σ1.00Nb6.02O19.00·6H2O (mean of four analytical points). Those from the darker areas correspond to (Ba0.99 K1.00)Σ1.99(Na1.02Ca0.96)Σ1.98(Mg0.95Mn0.05)Σ1.00Nb6.02O19.00·6H2O (mean of eight analytical points). The enrichment in Ba is coupled to the enrichment in Na and depletion of K and Ca. The analyses were obtained in points of several shades of grey observed in back-scattered electron images distributed in different crystals. These analyses were ordered by ascending Ba atoms per formula unit, numbered from 1 to 25, and served as the basis for the construction of the graph in Fig. 3.

Fig. 2. Back-scattered electron image of melcherite.

Fig. 3. Chemical variability in melcherite.
Table 1. Chemical composition of melcherite from the Cajati mine (in wt.%).

1. (Ba1.75K0.19)Σ1.94(Na1.80Ca0.19)Σ1.99(Mg0.96Mn0.02Al0.02)Σ1.00Nb6.02O19.00·6H2O (n = 4)
2. (Ba0.99K1.00)Σ1.99(Na1.02Ca0.96)Σ1.98(Mg0.95Mn0.05)Σ1.00Nb6.02O19.00·6H2O (n = 8)
b.d.l. = below detection limits.
Chemical composition varies from Ba2Na2Mg[Nb6O19]·6H2O to (BaK)(NaCa)Mg[Nb6O19]·6H2O. Coupled heterovalent substitutions at two sites are verified. As discussed by Hatert and Burke (Reference Hatert and Burke2008), where a heterovalent substitution occurs at a given crystallographic site, the charge balance can also be maintained by coupling this substitution to another heterovalent substitution at a different site. At the Ba site, the atom Ba2+ is replaced progressively by K+, and to maintain charge balance, the atom Na+ is replaced progressively by Ca2+ at the Na site. The substitution mechanism is Ba2+ + K+ ↔ Na+ + Ca2+. The boundary site occupancies between the two members of the series is (BaK)(NaCa)Mg[Nb6O19]·6H2O. We could imagine a solid-solution series from Ba2Na2Mg[Nb6O19]·6H2O to K2Ca2Mg[Nb6O19]·6H2O, with two mineral species, but the composition varies only from the first end-member to the intermediate member. As no analyses correspond to predominant K and Ca, only one mineral species is defined.
The formula BaCa2Mg[Nb6O19]·6H2O (Andrade et al., Reference Andrade, Atencio and Menezes Filho2015) is incorrect because Na was not identified. The change in formula was previously approved executively by CNMNC IMA Newsletter No. 29 (Hålenius et al., Reference Hålenius, Hatert, Pasero and Mills2016): “Soon after the approval of the new mineral melcherite (IMA No. 2015-018; see CNMNC Newsletter 25), the authors of the proposal have communicated results of subsequent analytical work on this mineral, which verifies essential contents of sodium. The new data were examined carefully by the CNMNC officers and were found reliable. The revised simplified formula, Ba2Na2Mg[Nb6O19]·6H2O, has been approved executively.” A fragment of the darker part was extracted from the polished section for crystal structure determination.
Crystal structure determination
Powder X-ray diffraction data (XRD) were obtained using a Siemens D5000 diffractometer equipped with a Göbel mirror and a position-sensitive detector using CuKα radiation and 40 kV and 40 mA at the Instituto de Geociências of the Universidade de São Paulo (Table 2). Unit-cell parameters refined from the powder data are as follows: trigonal, space group: R ![]() $\bar 3$, a = 9.022(2) Å, c = 23.410(6) Å, V = 1650.2(8) Å3 and Z = 3.
$\bar 3$, a = 9.022(2) Å, c = 23.410(6) Å, V = 1650.2(8) Å3 and Z = 3.
Table 2. Powder X-ray diffraction data for melcherite.

The strongest reflections are given in bold.
A single-crystal X-ray study was carried-out using a Bruker APEX II CCD diffractometer with graphite-monochromated MoKα (λ = 0.71073 Å) radiation and gave the following data: trigonal, space group: R ![]() $\bar 3$, a = 9.0117(6) Å, c = 23.3986(16) Å, V = 1645.64(19) Å3 and Z = 3. The X-ray absorption correction was applied to intensity data using the program SADABS from Bruker.
$\bar 3$, a = 9.0117(6) Å, c = 23.3986(16) Å, V = 1645.64(19) Å3 and Z = 3. The X-ray absorption correction was applied to intensity data using the program SADABS from Bruker.
The SHELXL-97 package (Sheldrick, Reference Sheldrick2008) was used for the direct methods structure solution and its subsequent refinement. The Ba and Na sites were refined assuming full but joint occupation by Ba/K and Na/Ca respectively, which yielded occupancy values close to those indicated by the empirical formula based on the electron microprobe analysis. A final difference-Fourier synthesis allowed the H atom positions of the water molecule to be located, which were then refined with soft restraints of 0.86 Å on the O–H distances and 1.40 Å on the H–H distance, and with U iso values fixed at ~1.5 times that of the O atom. Refinement of this final model converged to an R 1 of 0.017 and the crystal chemical formula obtained is (Ba1.06K0.94)(Na1.09Ca0.91)Nb6Mg[O18.98(OH)0.02]Σ19.00·6H2O, where a small fraction of the oxygen atoms in the hexaniobate polyanion is assumed to be replaced by OH groups in order to balance the slight positive charge deficiency associated with the Ba/K and Na/Ca sites. Details of the data collection and structure refinement are given in Tables 3 and 4. Selected bond distances and associated bond-valence sum calculations, using the parameters of Brese and O'Keefe (Reference Brese and O'Keeffe1991), are given in Table 5.
Table 3. Structure refinement results for melcherite.

Weighting scheme: w = 1/[σ2(F o2) + (0.0146P)2 + 5.6144P], where P = [max(0,F o)2 + (2F c)2]/3.
Table 4. Final fractional coordinates and displacement parameters of atoms in melcherite.

Table 5. Selected bond lengths and bond valences of the refined melcherite structure.

*Bond valence in valence units.
Melcherite is a hexaniobate that has structural layers parallel to the xy plane that stack along the c axis with simultaneous 1/3 [1 ![]() $\bar 1$ 0] displacement so as to produce an R lattice. The melcherite structure (Figs 4 and 5) is built by layers of [(Ba,K)(O,H2O)9] polyhedra and the [Nb6O19]8− super-octahedron (Lindqvist anion) interconnected by [(Na,Ca)O6] polyhedra. There is a significant distortion present in the Nb–O octahedron forming the hexaniobate polyanion, as measured by the octahedral angle variance (OAV), = 113.650°2, and quadratic elongation (OQE), = 1.040 indices (Robinson et al., Reference Robinson, Gibbs and Ribbe1971). The results are comparable to the NbO6 octahedra present in the crystal structure of peterandresenite and hansesmarkite (Table 6). Ba/K is coordinated by six oxygens and three water molecules. Na/Ca is coordinated by six oxygen atoms in a distorted octahedron and the OAV and OQE values are 354.100°2 and 1.113, respectively. Mg2+ cations are bonded to six water molecules each and are not associated with Lindqvist oxygen ions. The comparison with MnO6 in peterandresenite and hansesmarkite shows that the octahedral coordination of the Mg cation is relatively undistorted, as indicated by the values of OAV = 12.285°2 and OQE = 1.003 (Table 6).
$\bar 1$ 0] displacement so as to produce an R lattice. The melcherite structure (Figs 4 and 5) is built by layers of [(Ba,K)(O,H2O)9] polyhedra and the [Nb6O19]8− super-octahedron (Lindqvist anion) interconnected by [(Na,Ca)O6] polyhedra. There is a significant distortion present in the Nb–O octahedron forming the hexaniobate polyanion, as measured by the octahedral angle variance (OAV), = 113.650°2, and quadratic elongation (OQE), = 1.040 indices (Robinson et al., Reference Robinson, Gibbs and Ribbe1971). The results are comparable to the NbO6 octahedra present in the crystal structure of peterandresenite and hansesmarkite (Table 6). Ba/K is coordinated by six oxygens and three water molecules. Na/Ca is coordinated by six oxygen atoms in a distorted octahedron and the OAV and OQE values are 354.100°2 and 1.113, respectively. Mg2+ cations are bonded to six water molecules each and are not associated with Lindqvist oxygen ions. The comparison with MnO6 in peterandresenite and hansesmarkite shows that the octahedral coordination of the Mg cation is relatively undistorted, as indicated by the values of OAV = 12.285°2 and OQE = 1.003 (Table 6).

Fig. 4. Crystal structure of melcherite. (Ba,K) = yellow; (Na,Ca) = pink; Mg = green; Nb = blue; O = red and OW = grey.

Fig. 5. Lindquist polyanions [Nb6O19]8– stacking sequence in the crystal structure of melcherite.
Table 6. Selected interatomic bond lengths (Å) and octahedral distortion indices for melcherite (Ba2Na2MgNb6O19·6H2O), peterandresenite (Mn4Nb6O19·14H2O) and hansesmarkite (Ca2Mn2Nb6O19·20H2O).
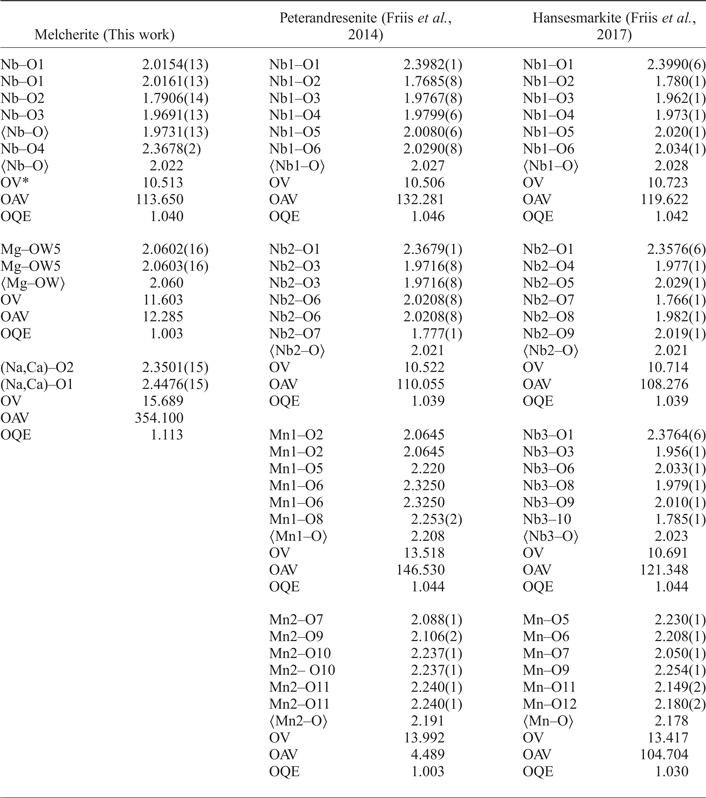
*OV = octahedral volume (Å3), OAV = octahedral angle variance (°2), and OQE = octahedral quadratic elongation (Robinson et al. Reference Robinson, Gibbs and Ribbe1971).
The mineral is similar structurally to the synthetic compounds Cs6Na2(Nb6O19)·18H2O and Rb6(H2Nb6O19).19H2O, studied by Nyman et al. (Reference Nyman, Alam, Bonhomme, Rodriguez, Frazer and Welk2006) (Table 7). They have the same space group as melcherite, R ![]() $\bar 3$. The unit-cell dimensions and arrangement of the Lindqvist ion [Nb6O19]8– are very similar. The crystallographic parameters of melcherite are compared with those of the other hexaniobate minerals in Table 8.
$\bar 3$. The unit-cell dimensions and arrangement of the Lindqvist ion [Nb6O19]8– are very similar. The crystallographic parameters of melcherite are compared with those of the other hexaniobate minerals in Table 8.
Table 7. Comparative data for melcherite and synthetic compounds (all trigonal, R ![]() $\bar 3$).
$\bar 3$).

*This work.
†Nyman et al. (Reference Nyman, Alam, Bonhomme, Rodriguez, Frazer and Welk2006).
Table 8. Comparison of melcherite with other naturally-occurring hexaniobates.

1: Friis et al. (Reference Friis, Larsen, Kampf, Evans, Selbekk, Sánchez and Kihle2014); 2: Friis et al. (Reference Friis, Weller and Kampf2017)
Acknowledgements
We acknowledge the Sao Paulo Research Foundation for financial support (Grants: 2011/22407-0 and 2013/03487-8); the Principal Editor: Peter Williams, reviewers Robert T. Downs, Hexiong Yang (University of Arizona) and Peter Leverett, and the members of the Commission on New Minerals and Mineral Names of the International Mineralogical Association (CNMNC-IMA) for their helpful suggestions and comments.
















