INTRODUCTION
Parasitic flatworms continue to inflict immense suffering in humans throughout the world, the most notable example being trematodes of Schistosoma spp. which cause schistosomiasis. Afflicting over 200 million people, the World Health Organization names schistosomiasis among its top 5 disease priorities, and identifies research into the basic biology of these parasites as a primary need (Remme et al. 2002; Chitsulo et al. 2004). S. mansoni muscle contraction is essential for worm survival. There is evidence that voltage-operated Ca2+ channels (VOCCs) play a role in somatic muscle function in schistosomes and other flatworms. Although cDNAs encoding VOCCs have been identified in S. mansoni (Kohn et al. 2001a), their distribution and specific function within the worms are not clear. Depolarization by elevated extracellular K+ induces muscle contraction in whole schistosomes (Fetterer et al. 1980) and Fasciola hepatica worm strip preparations (Graham et al. 1999). However, because these preparations contain both nerve and muscle tissue, they cannot reveal if the depolarization is directly triggering Ca2+ influx at the level of the muscle or if the response is mediated by pre-synaptic transmitter release or some other mechanism. As depolarization induces contraction of individual, dispersed muscle fibres of schistosomes and other platyhelminths as well (Day et al. 1994, 2000; Moneypenny et al. 2001), the most plausible explanation is the opening of VOCC in the plasmalemma of the muscle fibres.
Despite this accumulation of evidence implicating VOCC in somatic muscle function of platyhelminths (and, quite possibly, the action of praziquantel), there has not been a clear elucidation of the pharmacological nature of these channels or of their role mediating contraction. One of our objectives was to characterize the pharmacology of the VOCC presumably underlying the depolarization-induced contractions in flatworm muscle. As functional ryanodine receptors have also been identified in S. mansoni (Silva et al. 1998; Day et al. 2000), Ca2+ mobilization from this intracellular store might also play a role in muscle contraction. The second objective of the present work was to elucidate how intracellular Ca2+ handling modulates the contraction of the muscle fibres of S. mansoni.
MATERIALS AND METHODS
Worms
Worms were recovered from the hepatic and mesenteric vasculature of mice infected with approximately 150 S. mansoni cercariae 45 days previously. Adult, paired worms were used for the studies.
Muscle fibre dissociation
Muscle fibres were dissociated from S. mansoni following the procedure previously described (Day et al. 1996, 1994). In each case, live worms were chopped approximately 100 times with a sterile razor blade, and the resultant worm pieces were incubated in an enzyme-containing dissociation medium (Dulbecco's Modified Eagle's Medium supplemented by 1 mg/ml papain). The pieces were then triturated through a fire-polished Pasteur pipette, resulting in the dissolution of the pieces into a suspension, which included many dissociated muscle fibres. This suspension was plated onto plastic Petri dishes and allowed 1 h for settling and adherence, after which the dissociation medium was exchanged for an incubation medium in which the contraction assays were performed.
Media
Media for the work with the muscle fibres were based on Dulbecco's Modified Eagle's Medium (DMEM without Na2HPO4 or NaHCO3, Sigma). The incubation medium was a modified DMEM, which was DMEM reduced to 67% of normal concentration and to which was added (mM): 2·2 CaCl2, 2·7 MgSO4, 0·04 Na2HPO4, 61·1 glucose, 1·0 dithiothreitol (DTT), 0·01 serotonin, 15 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES) (pH 7·4), and 1% (v/v) antibiotic/antimycotic solution (Gibco). With these modifications the final concentrations of ions were (mM): 4·1 K+, 82·6 Na+, 3·6 Ca2+, 3·3 Mg2+, 93·7 Cl−, 3·3 SO42−, 0·04 PO43−. The dissociation DMEM was this incubation medium supplemented by 1 mM ethyleneglycol-bis-(β-aminoethyl ether) tetraacetic acid (EGTA), 1 mM ethylenediamine tetraacetic acid (EDTA) and 0·1% bovine serum albumin.
Whole-cell recording
All recordings were made using the whole-cell configuration of the patch-clamp technique (Hamill et al. 1981). Patch electrodes were double pulled and heat polished to give resistance of 1–5 MΩ. A high resistance seal between electrode tip and the muscle fibre membrane was most easily formed on fibres that were significantly contracted. After formation of the whole cell configuration, membrane capacitance and series resistance were compensated to permit optimal control of membrane potential in voltage clamp experiments. Current signals were amplified (List Electronic EPC-7), digitized (CED1401; Cambridge Electronic Design, Cambridge, UK), and transferred to a personal computer. Current signals were filtered (5 kHz; Kemo Instruments) prior to digitization. Voltage steps were generated and digitized signals were analysed using software written by Dr John Demptser (Department of Physiology, University of Strathclyde, Glasgow, Scotland).
To record mixed cationic current under voltage clamp, the electrode solution contained (mM): 135 KCl, 10 HEPES, 10 glucose, 2 EGTA (adjusted to pH 7·3 with KOH). In experiments to examine the existence and nature of voltage-operated calcium channel currents, the electrode solution contained (mM): 140 CsCl, 10 HEPES, 10 glucose, 2 EGTA (pH adjusted to pH 7·3 with NaOH).
Contraction assay
The muscle contraction data are based on visual observation of contractions of individual muscle fibres in response to microperfusion with test medium via a micropipette. Each fibre selected for testing was identified as quiescent and fit our previous morphological characterization as a frayed fibre (Fig. 1) (Day et al. 1994). Recent studies of F. hepatica musculature suggest that these frayed fibres are in fact bundles of incompletely separated fibres (Kumar et al. 2003). This may indeed also be the case for the schistosome fibres, but the frayed morphology still provides a plentiful, readily identifiable fibre type to control the studies.

Fig. 1. (A) Adult (paired) male and female Schistosoma mansoni. The posterior half of the female worm can be seen emerging from the gynecophoral canal formed by the folding of the male body. (B and C) Individual contractile muscle fibres dispersed from adult male worms.
After selection of a fibre, the micropipette was brought to within 50 μm of the cell and test medium was pressure ejected directly onto the fibre. In each 35 mm Petri dish, 20–25 muscle fibres were tested, and each was individually scored as either contracting or not contracting in response to the microperfusion.
A muscle fibre was deemed to have contracted when it shortened to less than 50% of its original length, but this criterion was rarely necessary; since the fibres have no imposed load, the contractions were almost always all or none. Putative blockers or toxins were added to the incubation medium 15 min before the assessment of contractility.
Data are presented as the percentage of fibres that contracted in response to the test medium. Each datum presented is the mean±S.E.M. for 4–8 dishes prepared on at least 3 different days (20–25 fibres tested in each dish). The concentration-response data were analysed by nonlinear regression using Prism 3.0 (Graph Pad Software, Inc. San Diego, CA). The mean effective concentration value (IC50 or EC50) was described as molar concentrations, and the 95% confidence interval of the goodness of fit was indicated between parentheses. The difference between groups was evaluated through Student's t-test.
Drugs
Ca2+ channel blockers were obtained from Sigma (St Louis, USA). Stock solutions were made in water (diltiazem) or dimethyl sulfoxide (DMSO) (nicardipine, nifedipine, nimodipine, nitrendipine). Alomone Laboratories (Jerusalem, Israel) was the source of the cyclopiazonic acid (CPA) and thapsigargin, which were dissolved in DMSO. Ryanodine, caffeine and ω-conotoxins were obtained from Sigma and dissolved in 16% ethanol or water (caffeine and ω-conotoxins). In each case, the final DMSO and ethanol concentrations were never higher than 0·1% and 0·01%, respectively, and these vehicles had no measurable contractile effects on the preparations at these concentrations.
RESULTS
The predominant voltage-gated currents in muscle fibres from S. mansoni (Fig. 1) examined thus far are outward K+ currents (Day et al. 1993, 1995; Blair and Anderson, 1994; Cobbett and Day, 2003). However, abrogating these currents by replacing K+ in the intracellular solution with Cs+ allows the visualization of small voltage-gated inward currents in individual schistosome muscle fibres (Fig. 2). The size of the inward current in any particular fibre was amplified when 5 mM BaCl2 was added to the extracellular medium during the recording. The inward currents did not show any evidence of inactivation within the period of the 250 ms depolarizing pulse. From a holding potential of −70 mV, the peak inward current was observed at pulses to +20 mV. The currents were comparatively small, with a maximum of 90·5±5·7 pA (n=12). Like the inward currents measured in other platyhelminth muscle fibres, these currents were very labile, running down to amplitudes difficult to measure within a few minutes of the initiation of whole cell recording.

Fig. 2. Whole-cell currents of Schistosoma mansoni muscle fibres. (A) Voltage-gated, cationic inward currents recorded in the presence of 5 mM extracellular BaCl2. The evoked inward currents are shown above a graphical representation of the applied voltage protocol, with the largest current evoked by the 250 ms step to +20 mV. (B) Current-voltage relationship obtained from one of those fibres. The average peak current occurred at +20 mV. The solid circles ([bull ]) represent the peak current and the hollow circles (○) the sustained current (the last 10 ms of the 250 ms step).
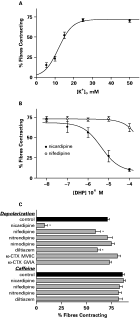
Individual muscle fibres were microperfused with a medium identical to the incubation medium, except that the [K+] was elevated, and the [Cl−] concentration was diminished by substitution with gluconate in order to keep constant the osmolarity and the [K+]×[Cl−] product. The depolarization with elevated extracellular K+ contracted the dispersed muscle fibres (Fig. 3A) in agreement with previous data (Day et al. 1994). The percentage of fibres contracting increased in a concentration-dependent manner reaching a maximum of about 75% of the tested fibres contracting, and a half-maximal percentage of contraction (EC50) was obtained with 12 mM [K+]o.

Fig. 3. (A) Effect of elevated extracellular K+ on the percentage of individual muscle fibres contracting. Individual muscle fibres were microperfused with medium identical to the incubation medium, except that the [K+] was elevated as indicated and the [Cl−] concentration was diminished by substitution with gluconate to keep a constant [K+]×[Cl−] product. The extracellular [K+] required to elicit half-maximal contractions was 12 mM. (B) Concentration-response curves for the inhibition of the contractions by the dihydropyridines nifedipine and nicardipine. Nicardipine produced half of its effect on the contractions at 4·1 μM. (C) The effect of a panel of other calcium channel blockers (at 100 μM) and toxins (at 0·1 μM) on the depolarization- and the caffeine-induced contractions. All of the compounds were added to the incubation medium 15 min before testing by the application of extracellular medium with [K+]o elevated to 25 mM or with 5 mM caffeine. Data are expressed as mean and S.E.M. of at least 6 replicates. * P<0·05 in relation to control (Student's t-test).
The voltage-gated inward divalent cation currents reported here, along with the depolarization-induced contractions, suggest the presence of VOCCs in the muscle fibres. Therefore, we tested the ability of various voltage-gated Ca2+ channel blockers to inhibit these contractions. The fibres were tested by depolarization with elevated extracellular [K+] close to the plateau of the maximum response (25 mM).
The calcium channel blockers by themselves produced no observable changes in the fibres during the 15 min pre-incubation period. The depolarization-induced contractions were significantly blocked by nicardipine, with an IC50 of 4·1 μM [95% C.I.: 1·1–16 μM], but the closely related DHP nifedipine was much less effective (Fig. 3B). There was no significant inhibition produced by the DHPs nimodipine and nitrendipine, even at 100 μM. At the same concentration, the benzothiazepine diltiazem inhibited the contractions, but only to about the same extent as nifedipine (Fig. 3C).
As the sensitivity of these contractions to classical blockers of L-type VOCCs proved equivocal, we tested conotoxins (CTX) known to block DHP-insensitive channels in mammals (Oliveira et al. 1994). Neither ω-CTX GVIA, more selective for mammalian N-type channels, nor ω-CTX MVIIC, more selective for mammalian P- and Q-type channels, had any effect at concentrations up to 100 nM (Fig. 3C).
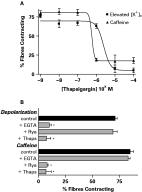
We have previously reported that dispersed schistosome muscle fibres are contracted by caffeine (0·5–5 mM) which, among other effects, is known to stimulate the release of sarcoplasmic reticulum Ca2+ via ryanodine receptors (Day et al. 2000). However, Graham et al. (1999) found that 5 mM caffeine was also myoexcitatory on muscle strips from the parasitic platyhelminth F. hepatica, and that this excitation was inhibited by 100 μM nifedipine, hence concluding that the caffeine inhibition was via blockade of VOCCs. Therefore, we examined the effect of VOCC blockers on the caffeine-induced contractions in the schistosome dispersed muscle fibre preparation. The contractions induced by 5 mM caffeine were not inhibited by any of the VOCC blockers tested (Fig. 3C). On the other hand, the 5 mM caffeine-induced contractions of schistosome muscle fibres were blocked by pre-incubation with 0·01–10 μM thapsigargin, an inhibitor of the sarcoplasmic reticulum Ca2+-ATPase (SERCA), with an IC50 of 0·46 μM [95% C.I.: 0·19–1·1 μM] (Fig. 4A). The alternate SERCA blocker cyclopiazonic acid (CPA) was not effective against caffeine at concentrations as high as 10 μM (data not shown).

Fig. 4. (A) Concentration–response curves for thapsigargin blockade of the contractions elicited by 25 mM [K+]o and 5 mM caffeine. Thapsigargin was added to the bathing medium 15 min before each assay. (B) Effect of disrupting sarcoplasmic reticulum calcium mobilization on the contractions elicited by depolarization or caffeine. EGTA=the omission of Ca2+ from the extracellular bathing medium along with the inclusion of 0·5 mM EGTA; Rya=the inclusion of 10 μM ryanodine for 15 min prior to assay; Thaps=the inclusion of 10 μM thapsigargin 15 min prior to assay. Data are expressed as mean and S.E.M. of 6–8 replicates. * P<0·05 in relation to control.
Blockade of the caffeine-induced contractions suggests that thapsigargin inhibits the intracellular stores of Ca2+, which is the function with which it is most readily ascribed in other cell types. However, in these schistosome muscle fibres, thapsigargin also inhibited the depolarization-induced contractions with an IC50 of 3·03 μM [95% C.I.: 1·77–5·2 μM], about 10 times less potently than it inhibits caffeine-induced contractions. This difference in potency could be because (1) the depolarization-induced contractions utilize the thapsigargin-sensitive stores, but they are less dependent on that calcium than are caffeine-induced contractions, or (2) the thapsigargin is having an additional pharmacological effect at the high concentrations used. The next series of experiments examined these possibilities.
As expected, the depolarization-induced contractions required extracellular Ca2+. Removal of the external Ca2+ by EGTA blocked the contractions elicited by elevated extracellular K+, but this did not block contractions elicited by caffeine (Fig. 4B). Even though the depolarization-induced contractions require the extracellular Ca2+, it is possible that the influx is triggering a Ca2+-dependent release of an intracellular store. If indeed the depolarization-induced contractions are utilizing the intracellular stores, it would be expected that these contractions would also be inhibited by ryanodine, a classical tool used for depleting intracellular calcium pools. However, the depolarization-induced contractions are not blocked by 10 μM ryanodine (Fig. 4B), a concentration that blocks caffeine-induced contractions (Day et al. 2000). This suggests that the depolarization-induced contractions do not utilize the intracellular stores; therefore thapsigargin's inhibition of those contractions cannot be explained by a depletion of the intracellular stores.
DISCUSSION
Blair and Anderson (1994) demonstrated the presence of voltage-gated inward currents carried by divalent cations in muscle fibres isolated from Bdelloura candida, but the currents were so short-lived that a pharmacological characterization was not possible. Voltage-gated divalent currents were also recorded from Girardia tigrina muscle fibres, but they were also quite labile (Cobbett and Day, 2003). Here, we report currents of the same nature in muscle fibres from S. mansoni. As in the other platyhelminth muscle fibres, these currents were lost soon after obtaining whole-cell access. Although this type of current rundown is not rare for Ca2+ currents, it is not clear if the channels mediating these currents in platyhelminths are indeed more labile than similar currents in other organisms, or if the relatively small size of the fibres leads to the rapid loss of some factors necessary for normal function. In either case, neither we nor others have been successful in pharmacologically characterizing these currents in flatworm muscle using whole-cell patch-clamp techniques.
Based on the hypothesis that these currents are carried by VOCCs and provide a Ca2+ influx during depolarization-induced contractions, we used the contraction assay to characterize their pharmacology. The sensitivity to the voltage-operated Ca2+ channel blockers belonging to the class of dihydropyridine (DHP) was atypically variable. Nicardipine blocked the depolarization-induced contractions, but at relatively high concentrations; other closely-related DHPs did not. Although this kind of selective DHP sensitivity is not unprecedented, it is unusual. Importantly, this differential sensitivity to DHPs is consistent with reports from whole worm studies in schistosomes. Specifically, Semeyn (1987) showed that resting muscle tension and spontaneous contractile activity in whole worms were sensitive to L-type VOCCs blockers, with the same type of varied sensitivity reported here. Specifically, nicardipine was quite effective at 10 μM, while nifedipine and diltiazem had very little effect even at 100 μM.
It is worth noting that even where DHP sensitivity is observed, concentrations required are relatively high. For example nicardipine's IC50 for inhibiting schistosome muscle fibre contraction is 4 μM. In contrast, the reported IC50 for nifedipine blockade of depolarization-induced contractions in isolated rat aorta (Tunctan et al. 2000) or other arteries (van der Lee et al. 1998) is between 1 and 10 nM, and in non-vascular smooth muscles, blockade is also in the nanomolar range (Godfraind et al. 1986).
Also unusual but not unique is the apparent sensitivity of the schistosome muscle VOCCs to high concentrations of thapsigargin. Although thapsigargin does deplete the intracellular stores, as attested by its effect on caffeine-induced contractions, at higher concentrations it also blocks the depolarization-induced contractions. Since the depolarization-induced contractions are not blocked by ryanodine at concentrations known to block the release of intracellular stores, the effect cannot be attributed to thapsigargin's effect on intracellular stores. As thapsigargin has been found to interact directly with VOCC at high concentrations (Buryi et al. 1995), this is the most plausible explanation of the blockade in schistosome muscle.
Graham et al. (1999) also reported some unusual pharmacology for the Ca2+ influxes in muscle strips of the platyhelminth F. hepatica. Specifically, the caffeine-induced contractions are inhibited by 100 μM nicardipine and not by ryanodine. In this preparation, the caffeine's myoexcitation is not mediated by release of intracellular Ca2+, but rather by some effect on the sarcolemmal Ca2+ channels. However, in S. mansoni, caffeine's myoexcitation depends on the release of intracellular Ca2+ since its effect is fully blocked by ryanodine and thapsigargin, but not by any L-type VOCC blocker.
Previous data from whole animal studies had provided compelling physiological evidence for the presence of VOCC somewhere in schistosomes. The data reported here fix at least one site of voltage-gated Ca2+ fluxes at the muscle itself, and they confirm an atypical or inconsistent L-type pharmacology for the channels mediating those currents.
Schistosomes have cDNAs encoding the alpha subunits of high-voltage activated VOCC, including 1 L-type channel and 2 non-L-type channels (Kohn et al. 2001b). The L-type α1 subunit, SmCav1, has about 50% of homology with vertebrate homologues, but features only 6 of the 13 amino acid residues thought to be involved in DHP interaction (Hockerman et al. 1997; Mitterdorfer et al. 1998; Striessnig et al. 1998; Yamaguchi et al. 2000). Since SmCav1 has not yet been localized to the muscle, drawing correlations between the lack of structural conservation and the variable DHP pharmacology reported here in the schistosome muscle is not warranted. However, if the structure of SmCav1 proves indicative of the L-type channels in these muscle fibres, the lack of structural conservation and the inconsistent DHP sensitivity would substantiate the attribution of DHP sensitivity to these conserved residues. Alternatively these atypical L-type channels could be related to the recently described nifedipine-insensitive voltage-dependent Ca2+ channels in mammalian mesenteric vessels, which among other features, are high-voltage activated channels resistant to nifedipine and peptide blockers of N and P/Q Ca2+ channels (Itonaga et al. 2002).
A previous report suggested that SR in S. mansoni muscle is rather sparse and, therefore, might not contain sufficient Ca2+ stores to support contraction (Silk and Spence, 1969). Recent studies with these muscle fibres demonstrated otherwise; caffeine elicits contraction of the fibres that is blocked by ryanodine (Day et al. 2000) and thapsigargin, two classic tools that deplete SR of Ca2+. In addition, all the physiological data support an intracellular store of Ca2+ substantial enough to support muscle contraction. Indeed, molecular studies (de Mendonça et al. 1995; Talla et al. 1998) have corroborated previous biochemical and binding studies by showing the presence of a sarco-endoplasmic reticulum ATPases (Cunha et al. 1996; Cunha and Noël, 1998) and ryanodine receptors (Silva et al. 1998) in schistosomes. The caffeine-induced contractions of dispersed cells were blocked by the SERCA inhibitor thapsigargin, but not by CPA, even at a concentration (10 μM) 10 times higher than its IC50 for the inhibition of SERCA activity in the microsomal fraction of S. mansoni (Cunha et al. 1996). This lack of CPA inhibition was unexpected, considering that caffeine is supposed to mobilize Ca2+ from the same intracellular Ca2+ store where SERCA are found. The high concentrations of thapsigargin necessary to inhibit the contraction induced by caffeine (IC50 of 0·46 μM) could be partially explained by the 140 to 250-fold lower sensitivity of Schistosoma SERCA as compared to mammalian enzyme (Noël et al. 2001).
In summary, the present data suggest that VOCC with atypical pharmacology are present in the sarcolemma and mediate the Ca2+ influx elicited by depolarization. So, both influx and mobilization of Ca2+ from the sarcoplasmic reticulum contribute to the cytosolic Ca2+ increase and control of S. mansoni muscle fibre contraction (Fig. 5).

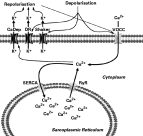
Fig. 5. The model for Ca2+ flows in Schistosoma mansoni muscle. The data suggest that depolarization produces an influx of Ca2+ through a pharmacologically atypical L-type voltage-operated Ca2+ channel (VOCC). Data presented here suggest that this Ca2+ influx is sufficient to support contraction. The elevated intracellular Ca2+ activates Ca2+-dependent K+ channels (CaDep) and the depolarization also activates at least 2 kinds of voltage-gated K+ channels, a delayed rectifier (DR) and a shaker-type (Shaker), all of which mediate a K+ efflux, membrane repolarization and concomitant closure of the VOCCs. Ca2+ is cleared from the cytosol into the sarcoplasmic reticulum by sarco-endoplasmic reticular ATPases (SERCA). Ca2+ can also be released from the sarcoplasmic reticulum via ryanodine receptor channels (RyR). Each of the molecules represented here has been demonstrated to be present on the muscle fibres: Ca2+-dependent K+ channel activity has been measured in patches isolated from S. mansoni fibres (Blair et al. 1991). Voltage-dependent K+ currents, both delayed rectifier and “A”-type currents have been measured in whole-cell recordings from both S. mansoni, and a shaker channel (Skv1.1) has been cloned from S. mansoni and localized to the somatic muscle (Day et al. 1993, 1996; Cobbett and Day; 2003). There is both physiological and pharmacological evidence for ryanodine receptors in muscle fibres (Silva et al. 1998; Day et al. 2000). At least 2 distinct SERCA isoforms are present in schistosomes (de Mendonça et al. 1995; Talla et al. 1998). Three voltage-gated Ca2+ channel α1 subunits have been cloned from S. mansoni, but they have not been definitively localized (Kohn et al. 2001).
The authors thank Dr Fred Lewis at the Biomedical Research Institute (Rockville, MD, USA) and Dr Lygia dos Reis Correia (FIOCRUZ, Rio de Janeiro, Brazil) for providing schistosome-infected mice for these studies. Financial support: this work was funded by NIH grant R01-AI49162 to T.A.D. and A.G.M. and by aFAPERJ grant to F.N.F.N. is a fellow of CNPq/Brazil and D.L.M.-S. was recipient of a CAPES Fellowship (Brazil).