Introduction
Our studies on the secondary mineralisation at the small, long-inactive Torrecillas mine, in the northern Atacama Desert of Chile, have thus far yielded a remarkable array of new mineral species, the majority of which are arsenic oxysalts. These include twelve new hydrogen arsenates and four new arsenites (see Kampf et al., Reference Kampf, Nash, Dini and Molina Donoso2019a). The new mineral species mauriziodiniite, described herein, is the fifth new arsenite to be discovered at Torrecillas. Mauriziodiniite, NH4(As2O3)2I, is the ammonium and iodine analogue of lucabindiite, K(As2O3)2Cl. Another potentially new arsenite, the ammonium analogue of lucabindiite, with the ideal formula NH4(As2O3)2Cl, is currently under study.
The name mauriziodiniite honours Maurizio Dini of La Serena, Chile (born 1968). Mr. Dini is an Italian amateur mineralogist who has lived in Chile since 1998. He is a Professor of Sociology at both Universidad Pedro de Valdivia and Universidad Central de Chile. Since 2002, he has collaborated with Chilean geologist Arturo Molina, whom he considers his geological and mineralogical mentor and with whom he has discovered many new minerals. Mr. Dini's principal mineralogical interests are sulfides and sulfosalts of Ag, Sb and As, and secondary minerals, especially arsenites and arsenates. He was involved in the discovery of santarosaite, juangodoyite and sanrománite at the Santa Rosa silver mine (Iquique Province, Chile) and, more recently, in the discovery of the unique secondary arsenic-rich secondary mineral assemblages at Torrecillas. He is a co-author of numerous new mineral descriptions including alcaparrosaite, bariopharmacoalumite, camaronesite, camanchacaite, canutite, chongite, cuatrocapaite-(NH4), cuatrocapaite-(K), currierite, erazoite, gajardoite, joteite, juansilvaite, leverettite, magnesiocanutite, magnesiofluckite, magnesiokoritnigite, mendozavilite-KCa, obradovicite-NaNa, paratacamite-(Mg), picaite, ríosecoite, shilovite, tapiaite, tondiite and torrecillasite (See Pasero, Reference Pasero2020 and references therein). Maurizio Dini and one of the authors (AAMD) collected mauriziodiniite; Mr. Dini recognised it as a potentially new mineral and provided the holotype specimen. He has given permission for the mineral to be named in his honour. Note that the compound name mauriziodiniite is proposed because of the similarity of ‘diniite’ to the existing mineral named dinite. Also, note that mauriziodiniite is an analogue of another mineral with a compound name – lucabindiite. Furthermore, quite serendipitously, the compound name includes the letter sequence ‘iodin’.
The new mineral and the name have been approved by the International Mineralogical Association (IMA2019-036, Kampf et al., Reference Kampf, Nash and Molina Donoso2019c). The description is based upon one holotype specimen deposited in the collections of the Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, CA 90007, USA, catalogue number 67365.
Occurrence
The new mineral was found on one specimen at the Torrecillas mine, Salar Grande, Iquique Province, Tarapacá Region, Chile (~20°58'13′S, 70°8′17″W). Torrecillas Hill, on which the Torrecillas mine is located, is composed of four different rock units. The Coastal Range Batholith (mainly gabbros) extends from the seashore to the Pan-American Road along the base of Torrecillas Hill. At the foot of Torrecillas Hill is a small area of contact metamorphic rocks in which garnet crystals occur in metamorphosed shales. Higher on the hill, the rocks are predominantly porphyritic andesitic lavas of the Jurassic La Negra Formation (García, Reference García1967; Buchelt and Tellez, Reference Buchelt, Tellez, Bahlburg, Breitkreuz and Giese1988). The Torrecillas deposit, in which the new minerals were found, consists of two main veins rich in secondary arsenic and copper minerals that intersect metamorphosed marine shales and lavas. These mineralised veins are genetically related to the aforementioned porphyritic andesitic lavas. More information on the geology and mineralogy of the area is provided by Gutiérrez (Reference Gutiérrez1975).
The rare secondary chlorides, arsenates and arsenites (and associated sulfates) have been found at three main sites on the hill: an upper pit measuring ~8 m long and 3 m deep, a lower pit ~100 m from the upper pit and measuring ~5 m long and 3 m deep, and a mine shaft adjacent to the lower pit and lower on the hill. Mauriziodiniite was found in a recent excavation a few metres above the shaft.
Mauriziodiniite is a secondary alteration phase occurring on matrix consisting of native arsenic, arsenolite and pyrite in association with calcite, cuatrocapaite-(NH4) (Kampf et al., Reference Kampf, Chukanov, Möhn, Dini, Molina Donoso and Friis2019b), lavendulan, magnesiokoritnigite (Kampf et al., Reference Kampf, Nash, Dini and Molina Donoso2013) and torrecillasite (Kampf et al., Reference Kampf, Nash, Dini and Molina Donoso2014). The secondary assemblages at the Torrecillas deposit are interpreted as principally having formed from the oxidation of native arsenic and other As-bearing primary phases, followed by later alteration by saline fluids derived from evaporating meteoric water under hyperarid conditions (cf. Cameron et al., Reference Cameron, Leybourne and Palacios2007); however, considering the proximity of the Torrecillas deposit to the Pacific Ocean, it seems possible that the frequent dense coastal camanchaca fogs have also played a role in the alteration of the veins and the formation of the secondary minerals, particularly in the recent past, since the exhumation of the deposit well above sea level on Torrecillas Hill (see Kampf et al., Reference Kampf, Nash, Dini and Molina Donoso2019a).
Physical and optical properties
Mauriziodiniite occurs as hexagonal tablets with bevelled edges, exhibiting the forms {100}, {101} and {001} (Fig. 1). Tablets are up to 0.3 mm in diameter and grow in irregular clusters (Fig. 2). No twinning was observed. Crystals are transparent, with pearly to adamantine lustre and white streak. The mineral does not fluoresce in longwave or shortwave ultraviolet light. The Mohs hardness is ~1 based on scratch tests. The tenacity is sectile and tablets are flexible, but not elastic. Cleavage is perfect on {001}. The sectile tenacity and perfect cleavage contribute to a fracture with curved, irregular and stepped characteristics. The density was not measured because of the paucity of material and the difficulty of observing the small crystals in Clerici solution. The calculated density is 3.916 g/cm3 for the empirical formula and 3.977 g/cm3 for the ideal formula. The mineral is insoluble at room temperature in concentrated HCl or concentrated H2SO4.

Fig. 1. Mauriziodiniite tablets on cuatrocapaite-(NH4); field of view = 0.84 mm across, holotype specimen #67365.

Fig. 2. Crystal drawing of mauriziodiniite; clinographic projection.
Optically, mauriziodiniite is uniaxial (–) with ɛ = 1.770(5) (measured in white light). ω is significantly greater than 2.00 based upon measurement in 2.00 index liquid. Because liquids of higher index were not available, direct measurement of ω was not possible. Measurement of the ω – ɛ birefringence using a Berek compensator provided an approximate value of 0.30, which allows the calculation of ω as 2.07. The mineral is nonpleochroic.
Raman spectroscopy
Raman spectroscopy was done on a Horiba XploRa+ micro-Raman spectrometer using an incident wavelength of 532 nm at 50% power, laser slit of 50 μm, 1800 gr/mm diffraction grating and a 100x (0.9 NA) objective. In addition to mauriziodiniite, spectra were recorded from 4000 to 60 cm–1 for gajardoite, KCa0.5(As3+2O3)2Cl2⋅5H2O, cuatrocapaite-(NH4), (NH4,K)3(NaMg□)(As3+2O3)6Cl6⋅16H2O, and a potentially new phase that corresponds to the NH4 analogue of lucabindiite (with some enrichment in I), (NH4,K)(As2O3)2(Cl,I) [henceforth referred to as ‘lucabindiite-(NH4)’]. In all cases, the laser was oriented perpendicular to the plates. The aforementioned phases all occur in the same general assemblage at Torrecillas, all occur as colourless hexagonal plates, and all have structures containing identical planar neutral As2O3 (arsenite) sheets that are included in the same layer sequences: (Cl,I)–As2O3–(K,NH4)–As2O3–(Cl,I). The structures of gajardoite and cuatrocapaite differ from that of mauriziodiniite and ‘lucabindiite-(NH4)’ in that they each contain an additional layer consisting of cations and H2O.
The four spectra exhibit many similarities (Fig. 3). Two of the most prominent bands in all four spectra are at 680–667 cm–1 and 545–530 cm–1, which can be assigned to As3+O3 stretching and bending, respectively. It should be noted that the Raman modes associated with arsenite groups vary considerably depending upon their As–O–As linkages (cf. Szymanski et al., Reference Szymanski, Marabella, Hoke and Harter1968; Bahfenne and Frost, Reference Bahfenne and Frost2010). We were unable to find any past Raman studies on compounds containing hexagonal As2O3 sheets similar to those occurring in the structures of these four minerals; however, the aforementioned band assignments seem generally consistent with earlier studies.

Fig. 3. Raman spectra of gajardoite, cuatrocapaite-(NH4), I-rich ‘lucabindiite-(NH4)’ and mauriziodiniite.
In the 3700–3000 cm-1 region (expanded insets in Fig. 3), both gajardoite and cuatrocapaite-(NH4) show prominent broad bands corresponding to O–H stretching, which are absent in the spectra of mauriziodiniite and ‘lucabindiite-(NH4)’; however, all four spectra exhibit a relatively sharp band in the range 3185–3169 cm–1. This band can be assigned to N–H stretching associated with the NH4+ units (cf. Mairesse et al., Reference Mairesse, Barbier, Wignacourt, Rubbens and Wallart1978). The band is only weakly displayed in the gajardoite spectrum; however, this is an indication that a small amount of NH4 is present in this mineral, presumably accommodated in the partially occupied K site in the structure, even though it was not reported in the original chemical analyses (Kampf et al., Reference Kampf, Nash, Dini and Molina Donoso2016).
Composition
Analyses (3 points) were performed at the University of Utah on a Cameca SX-50 electron microprobe with four wavelength dispersive spectrometers and using Probe for EPMA software. Analytical conditions were 15 keV accelerating voltage, 20 nA beam current and a beam diameter of 15 μm. Raw X-ray intensities were corrected for matrix effects with a ϕρ(z) algorithm (Pouchou and Pichoir, Reference Pouchou, Pichoir, Heinrich and Newbury1991). No other elements were detected by EDS or by WDS wavescans. There was minor damage from the electron beam. The analytical results are provided in Table 1. Loss of volatiles (NH4, Cl and I), perhaps associated with the minor beam damage, could account for the low analytical total.
Table 1. Compositional data (in wt.%) for mauriziodiniite.

S.D. – standard deviation.
The empirical formula based on 4 As atoms per formula unit is (NH4)0.94K0.03(As2O3)2I0.92Cl0.03. The simplified structural formula is (NH4,K)(As2O3)2(I,Cl) and the idealised formula is NH4(As2O3)2I, which requires (NH4)2O 4.82, As2O3 73.19, I 23.47, O = I –1.48, total 100.00 wt.%. The Gladstone-Dale compatibility 1 – (K P/K C) for the empirical formula is –0.013 and for the ideal formula is 0.004, both in the range of superior compatibility (Mandarino, Reference Mandarino2007).
X-ray crystallography and structure refinement
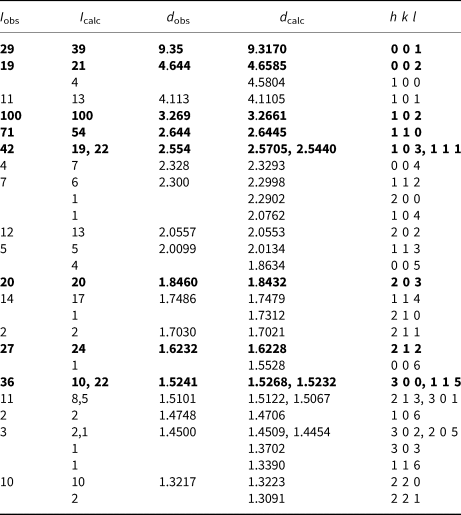
Both powder and single-crystal X-ray studies were carried out using a Rigaku R-Axis Rapid II curved imaging plate microdiffractometer, with monochromatic MoKα radiation. For the powder-diffraction study, a Gandolfi-like motion on the φ and ω axes was used to randomise the sample, and observed d values and intensities were derived by profile fitting using JADE 2010 software (Materials Data, Inc.). The powder data presented in Table 2 show good agreement with the pattern calculated from the structure determination. Unit-cell parameters refined from the powder data using JADE 2010 with whole pattern fitting are a = 5.2832(10), c = 9.3094(19) Å and V = 225.03(10) Å3.
Table 2. Powder X-ray data (d in Å) for mauriziodiniite.

The strongest lines are given in bold.
The Rigaku CrystalClear software package was used for processing the structure data, including the application of an empirical absorption correction using the multi-scan method with ABSCOR (Higashi, Reference Higashi2001). The structure was solved by the charge-flipping method using SHELXT (Sheldrick, Reference Sheldrick2015a). The atom coordinates were then transformed to correspond to those of synthetic NH4(As2O3)2I (Pertlik, Reference Pertlik1988), with which mauriziodiniite is isostructural. Refinement proceeded by full-matrix least-squares on F 2 using SHELXL-2016 (Sheldrick, Reference Sheldrick2015b). The NH4 site was refined with joint occupancy by N and K and the I site was refined with joint occupancy by I and Cl providing the formula [(NH4)0.65K0.35)](As2O3)2(I0.94Cl0.06); however, the resulting U eq for the NH4 site of 0.088(11) suggested a lower K and higher N content. Consequently, the site was instead assigned an occupancy of N0.97K0.03, more consistent with the EPMA. This resulted in a more reasonable U eq for the NH4 site of 0.039(7). The difference Fourier revealed one possible H site at (0, 0, 0.424), 0.71 Å from the N site along the 6-fold axis; however, no other possible H sites were found and no H sites were included in the final refinement. The data collection and refinement details are given in Table 3, atom coordinates and displacement parameters in Table 4, selected bond distances in Table 5 and bond-valence sums in Table 6. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 3. Data collection and structure refinement details for mauriziodiniite.

* The structure refinement did not include H sites.
R int = Σ|F o2–F o2(mean)|/Σ[F o2]. GoF = S = {Σ[w(F o2–F c2)2]/(n–p)}½. R 1 = Σ||F o|–|F c||/Σ|F o|. wR 2 = {Σ[w(F o2–F c2)2]/Σ[w(F o2)2]}½; w = 1/[σ2(F o2) + (aP)2 + bP] where a is 0.073, b is 2.3342 and P is [2F c2 + Max(F o2,0)]/3.
Table 4. Atom coordinates and displacement parameters (Å2) for mauriziodiniite.

Table 5. Selected bond distances (Å) for mauriziodiniite.

Table 6. Bond-valence analysis for mauriziodiniite. Values are expressed in valence units (vu).

Multiplicities indicated by ×↓→; bond strengths based upon site occupancies; K+–O and As3+–O bond-valence parameters are from Gagné and Hawthorne (Reference Gagné and Hawthorne2015), NH4+–O are from Garcia-Rodriguez et al. (Reference García-Rodríguez, Rute-Pérez, Piñero and González-Silgo2000), As3+–Cl are from Brese and O'Keeffe (Reference Brese and O'Keeffe1991) and As3+–I are from I.D. Brown (private communication).
Description of the structure
Mauriziodiniite is isostructural with lucabindiite, (K,NH4)(As2O3)2(Cl,Br) (Garavelli et al., Reference Garavelli, Mitolo, Pinto and Vurro2013), and with a series of (K,NH4)(As2O3)2(Cl,Br,I) synthetics reported by Pertlik (Reference Pertlik1988). The structure (Fig. 4) contains three types of layers: (1) a planar neutral As2O3 (arsenite) sheet with hexagonal symmetry (Fig. 4); (2) an NH4+ layer that links adjacent arsenite sheets via bonds to their O atoms; and (3) an I– layer that links adjacent arsenite sheets via bonds to their As atoms. The layer sequence is I–As2O3–NH4–As2O3–I. The bond-valence sum for the I site (0.74 valence units) is somewhat low; however, it is much higher than those observed for Cl in the lucabindiite (0.31 vu) and gajardoite (0.24 vu) structures. This phenomenon is probably caused by the repulsive effect of the lone electron pair of the As3+.

Fig. 4. Arsenite sheet viewed along c in the structures of mauriziodiniite, lucabindiite, gajardoite and cuatrocapaite-(NH4). The As atoms are red and the O atoms are white.
Gajardoite [(K,NH4)Ca0.5(As2O3)2Cl2⋅5H2O; Kampf et al., Reference Kampf, Nash, Dini and Molina Donoso2016] and the series cuatrocapaite-(NH4)–cuatrocapaite-(K) [(NH4,K)3(NaMg□)(As2O3)6Cl6⋅16H2O; Kampf et al., Reference Kampf, Chukanov, Möhn, Dini, Molina Donoso and Friis2019b] have structures with Cl–As2O3–(K,NH4)–As2O3–Cl layer sequences that are topologically equivalent to those in lucabindiite and mauriziodiniite; however, the gajardoite and cuatrocapaite structures both incorporate an additional layer. Gajardoite incorporates a disordered Ca–H2O layer and the cuatrocapaite series incorporates a disordered Na–Mg–H2O layer. The structures of mauriziodiniite, gajardoite and cuatrocapaite-(NH4) are compared in Fig. 5. Torrecillasite [Na(As2O3)2Cl; Kampf et al., Reference Kampf, Nash, Dini and Molina Donoso2014] also has a layer structure, with the sequence Cl–As2O3–Na–As2O3–Cl; however, the As2O3 layer in torrecillasite is geometrically and topologically different from that in the other minerals.

Fig. 5. The structures of mauriziodiniite, gajardoite and cuatrocapaite-(NH4).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2019.75.
Acknowledgements
Reviewers Peter Leverett and Igor Pekov are thanked for their constructive comments on the manuscript. A portion of this study was funded by the John Jago Trelawney Endowment to the Mineral Sciences Department of the Natural History Museum of Los Angeles County.