INTRODUCTION
One of the oldest hypotheses in community ecology is that resource competition among closely related species is higher than between distantly related species (Darwin Reference DARWIN1859), leading to competitive exclusion. This phylogenetic limiting similarity hypothesis (Violle et al. Reference VIOLLE, NEMERGUT, PU and JIANG2011) is based on two assumptions: (1) with common ancestry, closely related species are similar in aspects of their morphology, physiology and ecology and, thus, occupy similar ecological niches (phylogenetic niche conservatism: Losos Reference LOSOS2008, Wiens & Graham Reference WIENS and GRAHAM2005) and (2) since there are limits in similarity among stably coexisting species (MacArthur & Levins Reference MACARTHUR and LEVINS1967), few closely related species are expected in local communities (classical niche theory: summarized in Chase & Leibold Reference CHASE and LEIBOLD2003).
Local assemblies of bats regularly contain morphologically similar taxa belonging to the same families and genera. In many cases, these closely related species can only be identified by combined evidence from genetic, karyotypic, morphological and echolocation traits (Barratt et al. Reference BARRATT, DEAVILLE, BURLAND, JONES, RACEY and WAYNE1997, von Helversen et al. Reference VON HELVERSEN, HELLER, MAYER, NEMETH, VOLLETH and GOMBKÖTÖ2001). One group showing such crypticism is the family Vespertilionidae, specifically the subfamily Vespertilioninae (e.g. Koubínová et al. Reference KOUBÍNOVÁ, IRWIN, HULVA, KOUBEK and ZIMA2013, Monadjem et al. Reference MONADJEM, RICHARDS, TAYLOR and STOFFBERG2013). Phylogenetic niche conservatism would predict high similarities in ecological niche utilization between these species, which should lead to competitive exclusion; however, cryptic Vespertilionidae co-occur, which qualifies these communities as excellent systems to test predictions of niche theory, phylogenetic niche conservatism and alternative random processes (Hubbell Reference HUBBELL2001). However, due to their nocturnal life-styles, direct ecological information on taxa making up local communities is difficult to obtain.
Recent advances in methods such as stable isotope analysis (Boecklen et al. Reference BOECKLEN, YARNES, COOK and JAMES2011, West et al. Reference WEST, BOWEN, CERLING and EHLERINGER2006) provide tools to indirectly study community ecology of cryptic species and insights into their feeding ecology (Fleming et al. Reference FLEMING, NUNEZ and STERNBERG1993, Voigt et al. Reference VOIGT, GRASSE, REX, HETZ and SPEAKMAN2008), subtle trophic niche differentiation between morphologically and ecologically similar species (Siemers et al. Reference SIEMERS, GREIF, BORISSOV, VOIGT-HEUCKE and VOIGT2011) and trophic relationships in bat communities (Dammhahn & Goodman Reference DAMMHAHN and GOODMAN2014, Rex et al. Reference REX, CZACZKES, MICHENER, KUNZ and VOIGT2010). Hard tissue, such as hair, provides integrated information about assimilated food over a period of time (DeNiro & Epstein Reference DENIRO and EPSTEIN1978, Reference DENIRO and EPSTEIN1981), when the individual was growing fur (Fraser et al. Reference FRASER, LONGSTAFFE and FENTON2013) and, thus, an indirect indicator of trophic niche utilization. In contrast, stable isotopes from food remains in faecal samples reveal fast dietary changes (Salvarina et al. Reference SALVARINA, YOHANNES, SIEMERS and KOSELJ2013).
Although comparatively species-poor with regards to other tropical communities (e.g. Monadjem et al. Reference MONADJEM, RICHARDS, TAYLOR and STOFFBERG2013), insectivorous Malagasy bats provide a setting to study niche differentiation among coexisting species. Over the past decade considerable advancements have been made concerning aspects of the taxonomy and distribution of Malagasy Vespertilionidae and amongst the Vespertilioninae several cryptic species in the genera Hypsugo, Neoromicia, Pipistrellus and Scotophilus have been described based on concordant molecular genetic and morphological traits (Bates et al. Reference BATES, RATRIMOMANARIVO, HARRISON and GOODMAN2006, Goodman et al. Reference GOODMAN, JENKINS and RATRIMOMANARIVO2005, Reference GOODMAN, RATRIMOMANARIVO and RANDRIANANDRIANINA2006, Reference GOODMAN, TAYLOR, RATRIMOMANARIVO and HOOFER2012, Reference GOODMAN, RAKOTONDRAMANANA, RAMASINDRAZANA, MONADJEM, SCHOEMAN, TAYLOR, NAUGHTON and APPLETONin press). Sympatric vespertilionids in the two Malagasy bat assemblages studied herein do not contain phylogenetic sister species and in several cases involve taxa that are distantly related (Goodman et al. Reference GOODMAN, RAKOTONDRAMANANA, RAMASINDRAZANA, MONADJEM, SCHOEMAN, TAYLOR, NAUGHTON and APPLETONin press), generally supporting the phylogenetic limiting similarity hypothesis. However, coexisting species are morphologically similar, with considerable overlap in size, bioacoustics and habitat use (Goodman et al. Reference GOODMAN, RAKOTONDRAMANANA, RAMASINDRAZANA, MONADJEM, SCHOEMAN, TAYLOR, NAUGHTON and APPLETONin press).
The aim of this study was to study indirectly diet composition of co-occurring Malagasy vespertilionid bats in dry and mesic habitat communities. These data help in understanding the mechanisms stabilizing the local co-existence of different taxa, particularly those similar in external and craniodental morphology. Specifically, we focus on the following hypotheses and predictions: (1) sympatric vespertilionid bats are differentiated into trophic niches, indicated by species-specific stable isotope signatures of time-integrating hair samples. (2) Assuming similar isotopic source spaces in dry and mesic forests, we expect the dry-forest community (six species) to have more trophic diversity (larger isotopic space) than the mesic-forest community (three species) based on hair samples. (3) Seasonal migration probably associated with shifts in food availability (Moussy et al. Reference MOUSSY, HOSKEN, MATHEWS, SMITH, AEGERTER and BEARHOP2013, Voigt et al. Reference VOIGT, HELBIG-BONITZ, KRAMER-SCHADT and KALKO2014), specifically arthropod abundance, might relax local competition and, thus, facilitate co-occurrence. Intensive local surveys at certain sites suggest that species occurring in western Madagascar show seasonal variation in their presence (Rakotondramanana & Goodman Reference RAKOTONDRAMANANA and GOODMAN2011). Since Madagascar's forests vary in stable nitrogen isotopic signatures (Crowley et al. Reference CROWLEY, THORÉN, RASOAZANABARY, VOGEL, BARRETT, ZOHDY, BLANCO, MCGOOGAN, ARRIGO-NELSON, IRWIN, WRIGHT, RADESPIEL, GODFREY, KOCH and DOMINY2011), we compare intra-individual variation in stable nitrogen content of hair and faecal samples, reflecting the local source pool, and anticipate a mismatch if indeed certain individuals or species are migratory.
METHODS
Study species
The focal animals of this study are insectivorous bats (Vespertilioninae). Molecular and karylogical tools, as well as bacular morphology, have been employed to understand the evolutionary history of the group and uncover cryptic species in different areas of the Old World (Koubínová et al. Reference KOUBÍNOVÁ, IRWIN, HULVA, KOUBEK and ZIMA2013, Monadjem et al. Reference MONADJEM, RICHARDS, TAYLOR and STOFFBERG2013), including Madagascar (Goodman et al. Reference GOODMAN, RAKOTONDRAMANANA, RAMASINDRAZANA, MONADJEM, SCHOEMAN, TAYLOR, NAUGHTON and APPLETONin press). Following the higher-level taxonomy of Simmons (Reference SIMMONS, Wilson and Reeder2005), two subfamilies of Vespertilionidae occur on Madagascar: Vespertilioninae containing the genera Scotophilus, Pipistrellus, Hypsugo and Neoromicia; and Myotinae comprising Myotis, with a single Malagasy species, M. goudoti. The following taxa of Vespertilioninae are known from the island (Bates et al. Reference BATES, RATRIMOMANARIVO, HARRISON and GOODMAN2006, Goodman et al. Reference GOODMAN, JENKINS and RATRIMOMANARIVO2005, Reference GOODMAN, RATRIMOMANARIVO and RANDRIANANDRIANINA2006, Reference GOODMAN, TAYLOR, RATRIMOMANARIVO and HOOFER2012, Reference GOODMAN, RAKOTONDRAMANANA, RAMASINDRAZANA, MONADJEM, SCHOEMAN, TAYLOR, NAUGHTON and APPLETONin press): S. marovaza (endemic), S. robustus (endemic), S. tandrefena (endemic), N. matroka (endemic and formerly placed in the genus Eptesicus), N. malagasyensis (endemic), N. robertsi (endemic), Hypsugo sp. nov. (endemic), P. hesperidus for which Madagascar animals are genetically close but distinct from African populations of the same species, and P. raceyi (endemic). On the basis of a recent phylogenetic study of Malagasy taxa, N. malagasyensis and N. robertsi form allopatric sister species; the balance of taxa are not closely related to other congenerics on the island and presumably represent multiple colonization events (Goodman et al. Reference GOODMAN, TAYLOR, RATRIMOMANARIVO and HOOFER2012, Reference GOODMAN, RAKOTONDRAMANANA, RAMASINDRAZANA, MONADJEM, SCHOEMAN, TAYLOR, NAUGHTON and APPLETONin press).
Virtually nothing is known on the natural history of Malagasy Vespertilioninae (Goodman Reference GOODMAN2011), particularly aspects of their reproductive and social biology, habitat utilization and foraging ecology, although some information is available on diet (Rakotoarivelo et al. Reference RAKOTOARIVELO, RANAIVOSON, RAMILIJAONA RAVOAHANGIMALALA, KOFOKY, RACEY and JENKINS2007, Razakarivony et al. Reference RAZAKARIVONY, RAJEMISON and GOODMAN2005). Recent taxonomic studies of these bats using molecular and bacular characters have provided important insights into understanding aspects of community assemblage (Goodman et al. Reference GOODMAN, RAKOTONDRAMANANA, RAMASINDRAZANA, MONADJEM, SCHOEMAN, TAYLOR, NAUGHTON and APPLETONin press). However, in several cases, the different species and even genera living at the same locality cannot be confidently identified in the hand or based on external and craniodental morphology or bioacoustics. For example in the Kirindy Forest, excluding aspects of bacular morphology, the following taxa cannot be confidently separated from one another: Hypsugo sp. nov., Pipistrellus hesperidus and P. raceyi. Taking this aspect into account and to advance on this current study, only genotyped animals or males for which the bacula information is available (Goodman et al. Reference GOODMAN, RAKOTONDRAMANANA, RAMASINDRAZANA, MONADJEM, SCHOEMAN, TAYLOR, NAUGHTON and APPLETONin press) have been used in the stable isotope analyses.
The different species of Vespertilioninae employed in this study are not known to show sexual dimorphism in body size and vary in body mass from about 15 g in S. marovaza to 45 g in S. robustus to the smaller vespers ranging from 3.6 g in Hypsugo sp. nov. to 8.7 g in N. robertsi (Goodman Reference GOODMAN2011, Goodman et al. Reference GOODMAN, RAKOTONDRAMANANA, RAMASINDRAZANA, MONADJEM, SCHOEMAN, TAYLOR, NAUGHTON and APPLETONin press) (Appendix 1).
In general, forest-associated African vespertilionid bats tend to occur at the forest edge ecotone or in the canopy (Monadjem et al. Reference MONADJEM, TAYLOR, COTTERILL and SCHOEMAN2010). The different species studied herein, which occur in sympatry in the Kirindy and Antsahabe Forests, produce low-duty-cycle echolocation calls with broad interspecific overlap in maximum energy frequency from 49.0 to 58.3 kHz (Goodman et al. Reference GOODMAN, RAKOTONDRAMANANA, RAMASINDRAZANA, MONADJEM, SCHOEMAN, TAYLOR, NAUGHTON and APPLETONin press). In general, members of this group show considerable bioacoustic plasticity associated with differences in microhabitat use (Kalko & Schnitzler Reference KALKO and SCHNITZLER1993, Rakotondramanana et al. Reference RAKOTONDRAMANANA, GOODMAN, RAMASINDRAZANA and SCHOEMAN2014).
Study sites
Two ecologically different zones of Madagascar have been included in this study: an area of dry forest with little disturbance of natural habitat at 60 m asl in the central west (Kirindy/Centre National de Formation, d’Etudes et de Recherches en Environnement et Foresterie (CNFEREF), Morondava region, hereafter referred to as Kirindy Forest) and an area with a patchwork of montane forest and anthropogenic grassland-agricultural habitat at 1300 m asl in the humid east (Antsahabe, Anjozorobe region).
Rainfall in the Kirindy Forest is approximately 800 mm per year, with a pronounced 7–9-mo dry season, which commences in April (Sorg & Rohner Reference SORG, ROHNER, Ganzhorn and Sorg1996). Average daily temperatures range from a minimum of 19°C to a maximum of 30°C (Sorg & Rohner Reference SORG, ROHNER, Ganzhorn and Sorg1996). The soils at this site are unconsolidated and derived from eroded sedimentary Pliocene sandstone (Bourgeat Reference BOURGEAT, Ganzhorn and Sorg1996). No permanent flowing watercourses exist in this forest block and the ephemeral Kirindy River forms the major drainage during the rainy season. The native vegetation is western dry deciduous forest (Moat & Smith Reference MOAT and SMITH2007).
In contrast, the climate of the Antsahabe area, situated in the Anjozorobe-Angavo forest corridor (Goodman et al. Reference GOODMAN, RASELIMANANA and WILME2007) is notably cooler and more mesic, with annual average rainfall of approximately 1240 mm (Raselimanana & Goodman Reference RASELIMANANA and GOODMAN2007). There is a distinct reduction in rainfall between April and September. Average daily temperatures range from a minimum of 9°C to a maximum of 27°C. This zone rests on base formations of granitic migmates with gneiss and mica schists (Battistini Reference BATTISTINI, Battistini and Richard-Vindard1972). The decomposition of these formations results in ferralitic yellow-red soils. Within and around the forest corridor, a complex of permanently flowing streams and rivers drain the zone. The native vegetation at the site is evergreen humid forest (Moat & Smith Reference MOAT and SMITH2007).
Sampling and specimens
The different Vespertilionidae handled in the Kirindy Forest were captured in vegetationally homogeneous sites along the banks of the Kirindy River surrounded by intact dry deciduous forest (for details see Rakotondramanana & Goodman Reference RAKOTONDRAMANANA and GOODMAN2011). Trees at this site were generally without leaves during the period from September to early December. During the latter month is the start of the leafing season, which coincides with the commencement of the rainy season. In the case of the Antsahabe site, bats were captured within montane forest, at the ecotone between forest and anthropogenic open areas, and in valley bottoms often associated with zones cleared for agriculture, specifically rice paddy.
Bats were captured with mist nets, 6 m or 12 m in length with mesh size from 28 mm to 36 mm. In the Kirindy Forest nets were generally placed in positions traversing the Kirindy River channel and in the Antsahabe area in positions crossing streams and small rivers, bordering rice fields, or trails in the forest. Nets were in active use for 3–4 h each evening, starting from sunset, and frequently checked for captured bats.
The animals used in this study were collected and the voucher specimens are held in the Field Museum of Natural History, Chicago, and the Département de Biologie Animale, Université d’Antananarivo, Antananarivo. These specimens used herein were also employed in a study on the systematics of Malagasy Vespertilioninae (Goodman et al. Reference GOODMAN, RAKOTONDRAMANANA, RAMASINDRAZANA, MONADJEM, SCHOEMAN, TAYLOR, NAUGHTON and APPLETONin press). These collections were made in strict accordance with the terms of research permits issued by Malagasy authorities (Direction du Système des Aires Protégées, Direction Générale de l’Environnement et des Forêts, and Madagascar National Parks), following national laws. Animals were captured, manipulated and dispatched using guidelines accepted by these different national authorities and the scientific community for the handling of wild animals (Sikes et al. Reference SIKES and GANNON2011).
Hair samples were clipped from the lower back of collected individuals, placed in individual vials and left open until the samples were air-dried; they were either stored without any preservative (in a dry state) or in 70% ethanol. For 76 individuals we analysed samples with and without preservative and found high correlations for both stable isotopes (δ13C: r = 0.81, df = 74, P < 0.0001; δ15N: r = 0.97, df = 74, P < 0.0001), therefore we subsequently pooled samples of different preservation types. We collected hair samples from specimens of eight species of Vespertilionidae, all endemic to Madagascar, with the exception of Pipistrellus hesperidus (Appendix 1). Our samples included all of the vespertilionid taxa known to occur in the Kirindy and Antsahabe areas. We obtained between one and 49 (median 11) hair samples per species (Appendix 1).
As mentioned earlier, there is a marked dry season in western Madagascar, between May and November, presumably resulting in notable differences in density and diversity of arthropods (Dammhahn & Kappeler Reference DAMMHAHN and KAPPELER2008, Rakotoarivelo et al. Reference RAKOTOARIVELO, RANAIVOSON, RAMILIJAONA RAVOAHANGIMALALA, KOFOKY, RACEY and JENKINS2007). In an attempt to control for seasonality, we analyzed samples collected between September and March, i.e. during the end of the dry season and the rainy season, with the majority of samples being collected between November and March. No information is available on when the bat species concerned or any tropical Old World bat species (Fraser et al. Reference FRASER, LONGSTAFFE and FENTON2013) replace their hair; at Kirindy sympatric non-volant mammals moult once per year at the end of the dry season (November) (Dammhahn & Kappeler Reference DAMMHAHN and KAPPELER2010, Reference DAMMHAHN and GOODMAN2014, M. Dammhahn, pers. obs.). Since this site shows considerable seasonal differences in temperature, precipitation and food availability and bats generally follow the mammalian pattern of fur change between seasons (Fraser et al. Reference FRASER, LONGSTAFFE and FENTON2013), we assume that our study animals moult also at the end of the dry season. To assess potential migratory patterns of bat species, we collected both faecal and hair samples from the same individuals, which included 26 bats (17 Pipistrellus raceyi, nine Hypsugo sp. nov.) at Kirindy and 31 individuals (26 Neoromicia matroka, five N. robertsi) at Antsahabe. As noted above, due to difficulties in species determination in the field based on external morphological characters, only hair samples from P. hesperidus are available.
To establish a habitat baseline for stable isotopes, we collected three soil samples at each of the two study sites. Further, we collected arthropod samples from Malaise traps (SLAM Trap Standard, 110 × 110 × 110 cm, MegaView Science, Taiwan) placed in close proximity to the netting sites and active during the same nights bats were captured; separate traps were installed both on the ground and at least 3 m off the ground.
Stable isotope analysis
Prior to analyses, all samples were oven-dried at 60°C until weight was constant to remove tissue water. For determination of carbon and nitrogen isotope ratios, 1 mg of either homogenized soil, homogenized faeces parts of arthropods (abdomen, legs) or whole specimens of small arthropods, or whole hairs of bat specimens were enclosed into tin capsules. Mass spectrometer analyses were carried out at the Centre for Stable Isotope Research & Analysis (KOSI) in Göttingen, Germany, using an isotope ratio mass spectrometer (Delta Plus, Finnigan MAT, Bremen, Germany) in an online system after passage through an element analyzer (NA 1110, Carlo Erba, Milan, Italy). Since the ratio between the heavy and the light isotopes is small and subject to natural fluctuations, the isotope data are compared with a standard and presented in δ notation calculated as follows:
Where δX is δ15N or δ13C, and R is the respective 15N/14N or 13C/12C ratio. The international standards are atmospheric air for nitrogen and PDB (Pee Dee Belemnite marine carbonate) for carbon. Analytical error was calculated based on the within-run standard deviations of the working standard, acetanilide (6–26 per run), and ranged 0.05‰ to 0.30‰ for δ15N and 0.02% to 0.11‰ for δ13C.
Data analyses
We analysed δ13C–δ15N bi-plots based on mean values of species hair samples to characterize and compare the two vespertilionid communities. Following Layman et al. (Reference LAYMAN, ARRINGTON, MONTANA and POST2007), we calculated four different measures of community trophic diversity: (1) total δ15N range signifying the number of trophic levels; (2) total δ13C range indicating variation in basal resources; (3) the total area covered by the community calculated as a minimum convex polygon indicating the isotope niche space covered by the community; and (4) the mean distance of each species to the community centroid (mean δ15N and mean δ13C over all species) representing average trophic diversity in the community.
We used three approaches to test for within-community differences in stable isotope signatures of time-integrating hair samples among species for Kirindy and Antsahabe. First, we explored isotopic niche overlap based on relative overlap of standard ellipses for each pair of species using the function overlap of the R package siar (Parnell et al. Reference PARNELL, INGER, BEARHOP and JACKSON2010). Standard ellipses were calculated based on δ13C and δ15N signatures of each individual per species. Second, we compared differences in centroid location across species in the δ13C–δ15N bi-plot (Turner et al. Reference TURNER, COLLYER and KRABBENHOFT2010). To assess whether species occupy different portions of the δ13C–δ15N bi-plot, we compared differences in Euclidean distances between species centroids (i.e. arithmetic mean of δ13C and δ15N per species) for each pairwise combination of species. Using a residual permutation procedure (RPP), these differences were compared to a null distribution. This procedure shuffles residual vectors of individual observations (δ13C–δ15N pair of one species) to the community centroid and generates null model distributions based on 9999 random permutations of residual vectors. The RPP allows for statistical testing of species differences from zero, i.e. the null hypothesis of no difference between the pairs of species and is less sensitive to unbalanced data sets than traditional multivariate analyses. For differences between centroid locations, we calculate the parametric Hotelling's T2 test statistics, which is a multivariate analogue of the t-test. All calculations of centroid location test statistics were based on Turner et al. (Reference TURNER, COLLYER and KRABBENHOFT2010). Third, we applied multivariate analysis, which incorporate within-species variation. Based on MANOVA, we tested whether species differ in stable isotope signatures of time-integrating hair samples. Using univariate ANOVAs and subsequent post hoc Tukey HSD tests, we further examined whether these differences are due to interspecific variation in δ13C or δ15N and whether species pairs differ from each other.
As explained above, since determination in the hand of certain Malagasy vespertilionid bats to species level is difficult, we further explored whether species-specific stable isotopic signatures allow species discrimination within the more species-rich Kirindy Forest community. We calculated linear discriminant function analyses with leave-one-out cross-validation for δ13C and δ15N signatures of hair samples of all unambiguously determined specimens of all species combined and species as a grouping factor. Scotophilus spp. are excluded from all multivariate analyses and dispersion statistics due to small sample sizes.
Further, we tested for between-species differences in stable carbon and nitrogen signatures of faecal samples – reflecting diet the night an individual was captured – using Mann-Whitney U-tests due to small and unequal sample sizes. Finally, we calculated the difference between stable isotope ratios in hair and faecal samples (Δhair-faeces15N, Δhair-faeces13C) collected the same day from the same individual and tested for between species differences using Mann-Whitney U-tests. All statistics were performed in the statistical package R 2.15 (www.r-project.org) and tests were two-tailed with accepted significance levels of P ≤ 0.05.
RESULTS
Soil and arthropod samples
Soil and insect samples, collected in proximity to bat netting sites, were analysed to provide a habitat baseline of isotopic variation. The stable isotope signatures of soil samples collected at each site were similar in δ15N but differed in δ13C. The median δ13C was −26.9‰ (range: −27.0‰ to −26.5‰) at Kirindy and −19.0‰ (range: −19.0‰ to −18.6‰) at Antsahabe. The median δ15N was 6.0‰ (range: 5.7‰ to 6.2‰) at Kirindy and 6.4‰ (range: 6.3‰ to 6.6‰) at Antsahabe.
We classified insects collected in Malaise traps into orders. Insects from Kirindy ranged widely in δ15N from very low values in Lepidoptera (range: 2.1‰ to 5.6‰, n = 3) to high values in Diptera (7.5‰ to 12.9‰, n = 7) and intermediate values in Coleoptera (4.5‰ to 11.0‰, n = 11) and Hymenoptera (4.3‰ to 12.1‰, n = 3). Stable carbon was lowest in Coleoptera (range: −27.2‰ to −23.3‰) and Diptera (−27.5‰ to −22.3‰) and highest in Hymenoptera (−24.7‰ to −23.1‰) and in Lepidoptera (−24.4‰ to −22.6‰). At Antsahabe, the median δ15N for insects (n = 7) was 7.6‰ (range: 1.8‰ for Lepidoptera to 7.8‰ for Coleoptera), the median δ13C was −22.6‰ (range: −28.8‰ for Lepidoptera to −21.9‰ for Coleoptera). Overall, insects showed high variation in both stable isotopes (Figure 1), supporting the assumption that isotopic variation between bat species reflect different prey types in their diet.

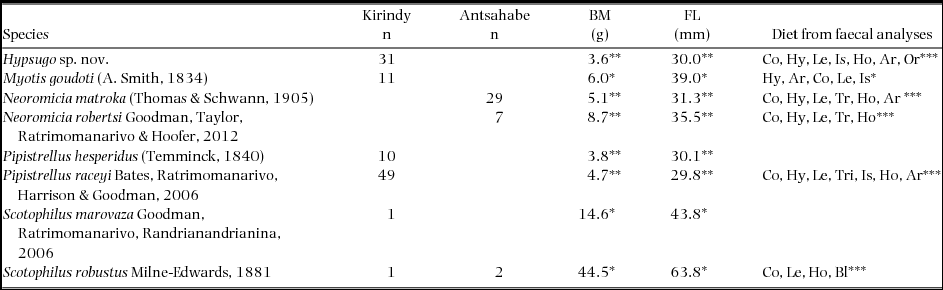
Figure 1. Mean (± 1 SD) δ13C and δ15N of hair samples (filled circles) and of faecal samples (filled squares) of all Vespertilionidae bats documented in the dry deciduous Kirindy Forest (a) and in the mesic zone at Antsahabe (b) of Madagascar. The grey area indicates mean (± 1 SD) isotope values for insects. Key to species – Hspn: Hypsugo sp. nov.; Mgou: Myotis goudoti; Nmat: Neoromicia matroka; Nrob: N. robertsi; Phes: Pipistrellus hesperidus; Prac: P. raceyi; Smar: Scotophilus marovaza; Srob: S. robustus.
Overview and comparison of the vespertilionid bat community structures
We calculated several measures to describe and compare the trophic structure of the vespertilionid communities of Kirindy and Antsahabe based on δ13C-δ15N bi-plots of species mean values. In the Kirindy Forest, six species co-occurred and ranged in δ15N over 6.0‰ from a minimum of 7.3‰ in Scotophilus marovaza to a maximum of 13.3‰ in Myotis goudoti with a community mean of 11.3‰ (Figure 1a). At Antsahabe, three species co-occurred and ranged in δ15N over 2.4‰ from a minimum of 6.8‰ in S. robustus to a maximum of 9.2‰ in Neoromicia matroka with a community mean of 8.0‰ (Figure 1b). Hence, the Kirindy Forest community spanned over two trophic levels, whereas the Antsahabe community foraged only on one trophic level. For δ13C, the Kirindy community had a mean of −21.0‰ and a total range of 4.2‰, with a minimum of −22.6‰ for M. goudoti and a maximum of −18.4‰ in S. robustus (Figure 1a). The Antsahabe community had a mean δ13C of −21.7‰ and ranged over 1.0‰ with a minimum of −22.1‰ in N. robertsi and a maximum of −21.2‰ in N. matroka (Figure 1b). The mean distance to the community centroid was 2.2‰ in Kirindy (range: 0.4‰ to 4.0‰) and 1.0‰ (range: 0.5‰ to 1.3‰) in Antsahabe. The total stable isotope area covered for the two communities was 10.5‰2 for Kirindy and 1.0‰2 for Antsahabe.
Trophic niche differentiation within the Kirindy Forest community
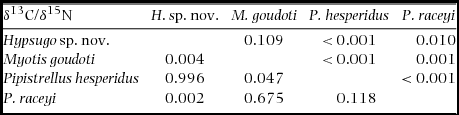
For the Kirindy Forest community, multivariate analysis of stable isotope ratios of hair samples revealed an effect of species (MANOVA, Pillai's trace = 0.69, F(3,194) = 17.0, P < 0.0001). Preliminary analyses showed that neither sampling year (2010–2013) nor sex explained significant parts of the variance (all P > 0.2). Overall, species differed in δ15N (F(3, 97) = 7.16, P = 0.0002, R2 = 0.18) and in δ13C (F(3,97) = 33.2, P < 0.0001, R2 = 0.51) (Figure 1a). Post hoc pairwise comparisons showed differences between all species pairs in at least one stable isotope (Table 1). The stable carbon and nitrogen isotope signatures of individual samples from Scotophilus robustus and S. marovaza fall outside the inter-quartile range of all other species (Figure 1a).
Table 1. Post hoc pairwise differences in stable isotopes of hair samples of vespertilionid species in the Kirindy Forest bat community. Shown are P values for δ13C above diagonal and δ15N below diagonal. Note Scotophilus spp. are excluded due to small sample sizes.
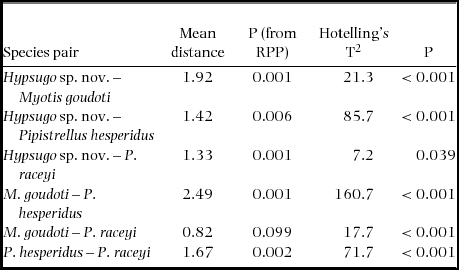
The results of the multivariate analyses were confirmed by dispersion statistics, an approach, which is less sensitive to inhomogeneous sample sizes. The Euclidean distances between centroids of species, i.e. the species mean value of δ13C and δ15N based on hair samples, differed from zero for all pairwise comparisons of groups (P < 0.04 for Hotelling's T2-test) (Table 2). Relative pairwise overlap of estimated standard ellipses between most species pairs was low (< 0.14 for all species pairs) and moderate (0.38 and 0.51) for Hypsugo sp. nov.–Pipistrellus raceyi.
Table 2. Mean Euclidean distances based on δ13C–δ15N bi-plots between centroids of species in the Kirindy Forest community. Statistical difference from zero was assessed based on residual permutation procedure (RPP) with 9999 random permutations and using the multivariate parametric Hotelling's T2 test statistics. Note Scotophilus spp. are excluded from this analysis due to small sample sizes.
Linear discriminant function analysis with leave-one-out cross-validation for δ13C and δ15N signatures of hair samples correctly classified species in 63.4% of cases in the Kirindy Forest community; classification was better for P. raceyi (77.6%) and P. hesperidus (80.0%) than for Hypsugo sp. nov. (45.2%) and Myotis goudoti (36.4%).
Both δ13C and δ15N measured in faecal samples of 28 individuals (17 P. raceyi, 11 Hypsugo sp. nov.) showed high within-species variation (median, inter-quartile range, δ13C: P. raceyi −25.0‰, −25.7‰ to −24.6‰, Hypsugo sp. nov. −25.1‰, −25.7‰ to −24.2‰; δ15N: P. raceyi 6.1‰, 3.1‰ to 7.4‰, Hypsugo sp. nov. 4.5‰, 1.5‰ to 5.9‰). Overall species did not differ in either isotope (MWU-tests, δ13C: W = 86, P = 0.942; δ15N: W = 55, P = 0.110). For 26 individuals (17 P. raceyi, nine Hypsugo sp. nov.), we calculated the difference between stable isotope ratios in hair and faecal samples (Δ15N, Δ13C). Hair samples were enriched in stable nitrogen and carbon as compared to faecal samples (median, inter-quartile range; Δ15N: 7.9‰, 5.1‰ to 10.4‰; Δ13C: 2.9‰, 2.1‰ to 3.7‰). Neither Δ15N nor Δ13C differed between species (MWU-test, Δ13C: W = 58, P = 0.339; Δ15N: W = 86, P = 0.634).
Trophic niche differentiation within the Antsahabe community
Multivariate analysis revealed an effect of species (MANOVA, Pillai's trace = 0.31, F(1,33) = 8.0, P = 0.0020). Overall, species differed in δ15N (F(1,34) = 9.62, P = 0.0039, R2 = 0.22) and in δ13C (F(1,34) = 12.6, P < 0.0012, R2 = 0.27). Post hoc pairwise comparison showed differences between sympatric species pairs in both stable isotopes (δ15N: P = 0.0039, δ13C: P = 0.0012); Neoromicia robertsi had lower δ15N and lower δ13C than N. matroka. The stable carbon and nitrogen isotope signatures of the single individual of Scotophilus robustus fell outside the inter-quartile range of the other two taxa known from the site (Figure 1b). Dispersion statistics confirmed the results of the multivariate analyses. The Euclidean distances between the centroids of the two Neoromicia spp. differed from zero (MD = 1.48, PRPP = 0.001, Hotelling's T2 = 15.5, P = 0.0019). Relative overlap between estimated standard ellipses between N. matroka and N. robertsi was low (0.10 and 0.13).
Both δ13C and δ15N measured in faecal samples of 31 individuals (26 N. matroka, 5 N. robertsi) showed low within-species variation (median, inter-quartile range, δ13C: N. matroka −26.0‰, −26.3‰ to −25.8‰; N. robertsi −25.9‰, −25.9‰ to −25.7‰; δ15N: N. matroka 3.7‰, 2.5‰ to 4.2‰, N. robertsi 4.6‰, 4.1‰ to 5.1‰). Species differed in δ15N (MWU-test, W = 26, P = 0.036) but not in δ13C (W = 51, P = 0.452). Hair samples were enriched in stable nitrogen and carbon compared to faecal samples (median, inter-quartile range; Δ15N: 5.5‰, 4.2‰-6.4‰; Δ13C: 4.5‰, 3.9‰-5.2‰). Both Δ15N and Δ13C differed between species (MWU-test, Δ13C: W = 107, P = 0.011; Δ15N: W = 112, P = 0.004).
DISCUSSION
Using stable carbon and nitrogen isotope measurements in time-integrating hair samples, we found distinct trophic niches among coexisting Vespertilioninae in two different Malagasy communities.
The dry-forest vespertilionid community of Kirindy Forest
Assuming an average enrichment of 3–5‰ in δ15N per trophic level (Vanderklift & Ponsard Reference VANDERKLIFT and PONSARD2003), we found that the Kirindy Forest vespertilionids ranged over two trophic levels. These levels are not discrete but species fall along a continuous gradient in δ15N, similar to that found in assemblages of bats in the Neotropics (Rex et al. Reference REX, CZACZKES, MICHENER, KUNZ and VOIGT2010) and in northern Madagascar (Dammhahn & Goodman Reference DAMMHAHN and GOODMAN2014). Arthropods sampled in Kirindy ranged widely in δ15N (Dammhahn & Kappeler Reference DAMMHAHN and KAPPELER2010). Low δ15N signatures of Scotophilus spp. suggest higher proportions of Lepidoptera (depleted in δ15N) in their diet as compared to the smaller vespertilionids. However, small sample sizes for both Scotophilus spp. limit more-detailed analyses.
Stable carbon varied by c. 4‰ in the dry-forest vespertilionids, suggesting vertical microhabitat stratification of bats, as δ13C increases in trees as a function of distance from the ground (canopy effect: Medina & Minchin Reference MEDINA and MINCHIN1980). Accordingly, Scotophilus spp. with the highest δ13C potentially forage in the upper canopy, Pipistrellus hesperidus in the middle layer, and the three other species in the lower canopy. Similar patterns of δ13C and vertical stratification in feeding height have been found in Neotropical (Voigt Reference VOIGT2010) and Malagasy bat assemblages (Dammhahn & Goodman Reference DAMMHAHN and GOODMAN2014).
Based on hair stable isotopes, members of the Kirindy vespertilionid community are separated into distinct isotopic niches with species differing in arthropod prey (δ15N) or foraging height (δ13C). This is particularly notable for the morphologically cryptic species, P. raceyi, P. hesperidus and Hypsugo sp. nov., and suggests that fine-grained niche differentiation stabilizes the coexistence of these morphologically similar species. Theoretically, species differences in moulting patterns (Fraser et al. Reference FRASER, LONGSTAFFE and FENTON2013), i.e. the timing of hair growth and isotopic assimilation, could contribute to the low isotopic niche overlap. The period of fur change is unknown for the study species. Pooling samples collected around and after the known moulting period of other syntopic non-volant mammals in Kirindy (Dammhahn & Kappeler Reference DAMMHAHN and KAPPELER2010, Reference DAMMHAHN and GOODMAN2014; M. Dammhahn, pers. obs.) should, with regards to our results, minimize the potential effect of species differences in moulting patterns.
The ecology of most species involved in this study is not well known. For Myotis goudoti, stomach content analysis of individuals collected in western Madagascar suggests that it mainly forages on Coleoptera, Isoptera and Araneae (Razakarivony et al. Reference RAZAKARIVONY, RAJEMISON and GOODMAN2005). Faecal samples of P. raceyi, collected during the wet season at Kirindy, mainly contained remains of Coleoptera, Isoptera, Lepidoptera and Hymenoptera, and for Hypsugo sp. nov. Isoptera, Lepidoptera, Coleoptera and Hymenoptera (C. F. Rakotondramanana, unpubl. data). Flying termites (Isoptera) are an ephemeral phenomenon in Kirindy (M. Dammhahn, unpubl. data), and, thus, likely of lower importance at a seasonal level in the diet of these species as indicated by faecal analysis. Concerning the relative proportions of Coleoptera (enriched in δ15N) and Lepidoptera (depleted in δ15N) in the diet of P. raceyi and Hypsugo sp. nov., the results of the faecal analysis and stable isotope signatures of hair samples are concordant.
In contrast to hair samples, within-species variation in δ13C and δ15N of faecal samples was high and there was broad overlap in both stable isotopes for P. raceyi and Hypsugo sp. nov. Thus, such short-term information would indicate a broad dietary spectrum in both species. For a subset of individuals, we compared stable isotope signatures in hair (seasonal turnover) and faeces (fast turnover) to assess whether they both originate from the same local source pool (see Voigt et al. Reference VOIGT, HELBIG-BONITZ, KRAMER-SCHADT and KALKO2014 for a similar approach). As revealed by a previously conducted controlled feeding experiment, faecal stable isotopes generally reflect dietary isotope signatures (mean ± SD, Δ13C: −0.1‰ ± 0.8‰) but are slightly lower in δ15N (Δ15N: 1.5‰ ± 1.5‰) (Salvarina et al. Reference SALVARINA, YOHANNES, SIEMERS and KOSELJ2013). Assuming these same relationships between diet and faecal stable isotopes and the general pattern of trophic enrichment (δ15N: 3‰ to 5‰, δ13C: 1‰ to 3‰; Vanderklift & Ponsard Reference VANDERKLIFT and PONSARD2003), hair samples should be enriched by 3‰ to 8‰ in δ15N and 0 to 4‰ in δ13C as compared with faecal samples. This relationship was generally the case in our paired samples and suggests the same resource pool for hair and faecal stable isotopes. However, some individuals of Hypsugo sp. nov. and P. raceyi have relatively high Δ15N and Δ13C. Several non-mutually exclusive explanations for this pattern can be presented. (1) Assimilated parts of the food (as reflected by hair) might have higher ratios of stable isotopes than non-assimilated parts (as reflected by the faeces) because proteins and fat are removed and keratin is enriched during the digestion process. On the basis of a mealworm diet, Salvarina et al. (Reference SALVARINA, YOHANNES, SIEMERS and KOSELJ2013) found no difference in the C/N mass ratio between food and faeces, rendering this explanation unlikely. Moreover, this mechanism should affect all sampled individuals in the same manner. (2) Isotopes in hair also reflect other physiological mechanisms, which involve the mobilization of body substances to meet metabolic requirements of homeostasis and generally augment δ15N and δ13C ratios in tissues; examples relevant to the tropics include nutritional and water stress (Ambrose & DeNiro Reference AMBROSE and DENIRO1986, Mirón et al. Reference MIRÓN, HERRERA, RAMIREZ and HOBSON2006, Voigt & Matt Reference VOIGT and MATT2004). Individual differences in these physiology-related additional enrichments should result in high variation in hair isotopes; however, we found high variation in faecal sample isotopes. (3) Since hair samples integrate dietary information over relatively longer periods, including up to 4 mo in P. kuhlii (Alagaili et al. Reference ALAGAILI, JAMES and MOHAMMED2011), some individuals might have fed on food with lower δ15N (and δ13C) during the period of sampling as compared with the time period when the hair was growing. This would imply short-term within-species specialization but a long-term generalist feeding ecology, which is also suggested by the high within-species variation in faecal stable isotopes. (4) Bats may have foraged in forest areas with a lower stable isotope baseline directly preceding faeces sampling. The stable isotope ecology of Kirindy Forest is typical for a dry deciduous forest in Madagascar (Crowley et al. Reference CROWLEY, THORÉN, RASOAZANABARY, VOGEL, BARRETT, ZOHDY, BLANCO, MCGOOGAN, ARRIGO-NELSON, IRWIN, WRIGHT, RADESPIEL, GODFREY, KOCH and DOMINY2011) but characterized by high small-scale spatial heterogeneity in δ15N (Dammhahn & Kappeler Reference DAMMHAHN and KAPPELER2014). Within our sample, bats were captured within a limited area along the Kirindy River, which is isotopically homogeneous and depleted in δ15N as compared with other parts of the forest (Dammhahn & Kappeler Reference DAMMHAHN and KAPPELER2014, M. Dammhahn, unpubl. data). Although specific foraging areas are unknown for the study species they are presumed to be in close proximity to the capture sites. If indeed foraging took place outside of the sampling area, this would have led to increased stable nitrogen in faeces and hair samples. However, only faecal stable nitrogen was augmented in the studied bats.
Since there are no differences in Δ15N and Δ13C between species, intraspecific variation should not affect our general conclusions based on the hair samples. Since individual variation in Δ15N and Δ13C is principally driven by variation in faecal stable isotopes, we did not find signs of migration from other areas with different isotopic signatures or indication of between-species variation in the timing of moult. If hair and faecal samples originated from different source pools, we would expect lower δ15N in hair as compared with faeces (Δ15N < 3‰), specifically in cases of movements between more mesic (lower δ15N) and dry habitats (higher δ15N) (see figure 2 in Crowley et al. Reference CROWLEY, THORÉN, RASOAZANABARY, VOGEL, BARRETT, ZOHDY, BLANCO, MCGOOGAN, ARRIGO-NELSON, IRWIN, WRIGHT, RADESPIEL, GODFREY, KOCH and DOMINY2011). However, it cannot be excluded that some local movements occur along the western lowland areas, as some forests to the north of Kirindy also have high δ15N (Crowley et al. Reference CROWLEY, THORÉN, RASOAZANABARY, VOGEL, BARRETT, ZOHDY, BLANCO, MCGOOGAN, ARRIGO-NELSON, IRWIN, WRIGHT, RADESPIEL, GODFREY, KOCH and DOMINY2011).
The mesic vespertilionid community at Antsahabe
Vespertilionidae bats at Antsahabe represent one trophic level and show low variation in both stable isotopes. As predicted by ecological niche theory (Chase & Leibold Reference CHASE and LEIBOLD2003), vespertilionids coexisting at Antsahabe are clearly separated into isotopic niches. Neoromicia robertsi and N. matroka show considerable morphological similarity, with the former being larger (Goodman et al. Reference GOODMAN, TAYLOR, RATRIMOMANARIVO and HOOFER2012), and both are differentiated based on stable isotopes. Lepidoptera and Coleoptera showed high variation in both stable isotopes, supporting the assumption that isotopic differences between bat species reflect varying prey sources in their diet. Low sample size and diversity of insects collected at Antsahabe impair detailed interpretations of the potential food sources consumed by these bats. The ecology of Neoromicia spp. is not well known; analyses of arthropod remains in faecal samples revealed that Coleoptera are the main dietary component for both of the locally occurring Neoromicia, followed by Hymenoptera and Lepidoptera (C. F. Rakotondramanana, unpubl. data).
At Antsahabe, within-species variation in faecal stable isotopes is low and Δ15N and Δ13C indicate that hair and faecal samples resemble the same stable isotope source pool. Hence, we do not find any indication of seasonal migration in these two Neoromicia spp. At this site, the majority of bats were captured outside of the forest in bottomlands with rice paddy or wet habitat, which are characterized by elevated δ13C due to the high prevalence of C4-plants (Marshall et al. Reference MARSHALL, BROOKS, LAJTHA, Michener and Lajtha2007). Stable carbon signatures of bats were lower than soil samples collected in close proximity to the netting sites, indicating that bats predominantly foraged in the forest areas, i.e. C3-plant dominated areas.
Comparison of the two vespertilionid assemblages
The dry-forest community at Kirindy contains more vespertilionid species than the mesic-forest community at Antsahabe and consequently occupies larger isotopic space. In general, the representation of Vespertilionidae bats in communities across Madagascar is variable and Kirindy has the highest known species richness (Goodman et al. Reference GOODMAN, RAKOTONDRAMANANA, RAMASINDRAZANA, MONADJEM, SCHOEMAN, TAYLOR, NAUGHTON and APPLETONin press). The trophic space occupied by the six insectivorous vespertilionid species at this site is similar to that covered by the complete community of 16 species from four families, including frugivorous/nectarivorous Pteropodidae, at Ankarana in northern Madagascar (Dammhahn & Goodman Reference DAMMHAHN and GOODMAN2014) and twice as large as the isotopic space covered by a community of eight lemur species, including folivores, frugivores and omnivores, at Kirindy (Dammhahn & Kappeler Reference DAMMHAHN and KAPPELER2014). This considerable trophic diversity, also reflected in high distances to the community centroid, suggests that competitive interactions are a structuring force of the Kirindy vespertilionid community. In contrast, the trophic space covered by three species at Antsahabe is small and species have similar distances to the community centroid as the bats in the Ankarana community (Dammhahn & Goodman Reference DAMMHAHN and GOODMAN2014).
Conclusions: coexistence of closely related species – the case of Malagasy vespertilionids
Based on niche theory (summarized in Chase & Leibold Reference CHASE and LEIBOLD2003) closely related species, which typically possess high similarities in morphology, behaviour, resource and habitat use, are not expected to coexist. In contrast to predictions of this phylogenetic limiting similarity hypothesis (Violle et al. Reference VIOLLE, NEMERGUT, PU and JIANG2011), bat communities worldwide typically harbour several – often cryptic – congeneric/confamiliar species (Bloch et al. Reference BLOCH, STEVENS and WILLIG2011, Fahr & Kalko Reference FAHR and KALKO2011, Rex et al. Reference REX, KELM, WIESNER, KUNZ and VOIGT2008). Comparative analyses of New and Old World bat assemblages (Schoeman & Jacobs Reference SCHOEMAN and JACOBS2011, Stevens & Willig Reference STEVENS and WILLIG2000) as well as community-wide stable isotope analyses (Dammhahn & Goodman Reference DAMMHAHN and GOODMAN2014) indicate that competitive interactions appear to be relaxed and not a prevailing structuring force at the community level.
Here, we show for two Malagasy bat assemblages, the presence of fine-grained trophic niche differentiation among syntopic cryptic Vespertilionidae. Similarly, subtle niche differences between closely related syntopic European Myotis spp. have been described based on stable isotope analyses (Lam et al. Reference LAM, MARTIN-CREUZBURG, ROTHHAUPT, SAFI, YOHANNES and SALVARINA2013, Siemers et al. Reference SIEMERS, GREIF, BORISSOV, VOIGT-HEUCKE and VOIGT2011) and DNA barcoding of arthropod remains in faecal samples (Krüger et al. Reference KRÜGER, CLARE, GREIF, SIEMERS, SYMONDSON and SOMMER2014). In the New World, congeneric coexisting short-tailed fruit bats (Phyllostomidae: Carollia) are distinguished isotopically (York & Billings Reference YORK and BILLINGS2009). These different results, including those presented herein, suggest differences in feeding behaviour – not measurable based on classically used morphological characters – presumably contribute to the coexistence of closely related species.
ACKNOWLEDGEMENTS
We thank the Ministère des Forêts et de l’Environnement for providing research authorizations for the capture and collection of animals. For facilitating our work, we are also grateful to colleagues at Fanamby at Antsahabe and the director of Kirindy/CNFEREF. The fieldwork was supported by grants from the Irene Pritzker Foundation, John D. and Catherine T. MacArthur Foundation, and Volkswagen Foundation. We are grateful to Amyot Kofoky, Ara Monadjem, Beza Ramasindrazana, Fanja Ratrimomanarivo, Peter Taylor and Corrie Schoeman for help with different aspects of the fieldwork. We thank Peter M. Kappeler for support and Reinhard Langel (KOSI) for technical help in the lab.
Appendix 1. Sample sizes (n) of hair samples obtained from all specimens of eight vespertilionid bat species of the Kirindy and Antsahabe assemblages. All species are endemic to Madagascar, except Pipistrellus hesperidus, which forms a genetically distinct population from the African mainland. Information on diet, body mass (BM) and forearm length (FL) of bat species are largely from Goodman (Reference GOODMAN2011)*, Goodman et al. (in press)** and unpubl. data***. Diet from faecal analyses is based on unpublished sources (C. F. Rakotondramanana, unpubl. data), order reflects prevalence in faecal samples: Ar – Araneae, Bl – Blattaria, Co – Coleoptera, Ho – Homoptera, Hy – Hymenoptera, Is – Isoptera, Le – Lepidoptera and Tr – Trichoptera.