Introduction
Non-tuberculous mycobacterial infection is a rarely reported cause of otomastoiditis. Austin and Lockey were the first to describe such a case, in 1976.Reference Austin and Lockey1 Eighty-eight additional cases have since been reported.Reference Avery, Eavey, Della Torre, Ramos and Pasternack2–Reference Wu, Shu and Chen32 A major risk factor for non-tuberculous mycobacterial otomastoiditis in children, as identified in previous reports, is treatment with a ventilation tube. In adults, chronic otitis media of various forms is a predisposing factor. The infection tends to cause long-standing otomastoiditis that can involve the meninges and brain. In addition to prolonged antibiotic treatment, the majority of previously reported cases required operation, and in many cases several mastoid and intracranial procedures were undertaken. No fatal cases have been reported in otherwise healthy and immunologically competent patients.
Humans are surrounded by non-tuberculous mycobacteria, which are present almost everywhere in soil and water. Their most distinguishing characteristic is a complex wax-like cell wall, which protects against a variety of hostile factors such as disinfectants, antibiotics and the immune response, thereby causing the typical granulomatous inflammation seen on histopathology.Reference Falkinham33, Reference Hale34 The cell wall also contributes to the bacteria's slow growth and extreme hydrophobicity, which makes them prone to adhere to household plumbing and other water sources where their relative insensitivity to chlorine give them a comparative advantage over other micro-organisms.Reference Falkinham33 It has been shown by molecular typing that people with non-tuberculous mycobacterial lung infections or sinusitis are infected with the same strain that is isolated from their plumbing system.Reference Falkinham33, Reference Tichenor, Thurlow, McNulty, Brown-Elliott, Wallace and Falkinham35 However, unlike the related Mycobacterium tuberculosis, human-to-human infection has not been reported.
Several species have been described as responsible for non-tuberculous mycobacterial otomastoiditis; the rapidly growing Mycobacterium abscessus, Mycobacterium chelonae and Mycobacterium fortuitum, and the slowly growing Mycobacterium avium complex, are the most frequent.Reference Austin and Lockey1–Reference Wu, Shu and Chen32
The objectives of the current study were: (1) to identify and describe cases of otomastoiditis caused by these bacteria in Sweden, (2) to relate these cases to a comprehensive review of previously reported cases and relevant literature, and (3) to highlight the cases of intracranial engagement (i.e. the most severe cases). The results of this study and review may help clinicians to manage new cases in the absence of data from controlled clinical trials.
Materials and methods
A survey was undertaken among all active ear surgeons in Sweden regarding their personal experience with otomastoiditis caused by non-tuberculous mycobacteria between 2000 and 2012. In addition, searches of laboratory databases and the Swedish database for otomastoiditis were performed.
The survey resulted in the identification of 16 cases of otomastoiditis with a positive culture of non-tuberculous mycobacteria. After approval from each patient or their guardian, complete datasets from hospital charts and bacteriological databases were collected and analysed. One author (LL) performed the data retrieval from hospital charts systematically. Another author (HE) performed the review of the histopathological samples. Accredited microbiological laboratories were responsible for the bacteriological analyses. A different author (KÄ) analysed the laboratory reports, and interpreted bacteriological data and antibiotic treatment systematically.
A literature search was performed of the Medline database using the search terms ‘nontuberculous mycobacteria’, ‘atypical mycobacteria’, ‘otitis media’, ‘otomastoiditis’ and ‘mastoiditis’. Additional articles were retrieved from the reference lists of each article.
Fourteen of the previously reported 89 cases included complete information regarding the following 15 parameters matching the present study: age, sex, occurrence of concomitant or previous ear disease, occurrence of other predisposing disease, time to diagnosis, symptoms at presentation, histopathology, type of bacteria, antibiotic treatment, length of therapy, spread of infection, radiological or intra-operative findings of bone destruction, surgical procedures, need for revision surgery, and sequelae.Reference Flint, Mahadevan, Gunn and Brown6–Reference Hsiao, Liu and Hsueh8, Reference Muller, Kemper, Hartwig, Mooi-Kokenberg, van Altena and Versteegh17, Reference Nylén, Alestig, Fasth, Gustavii, Olofsson and Renvall19, Reference Redaelli de Zinis, Tironi, Nassif and Ghizzardi21–Reference Sugimoto, Ito, Hatano, Nakanishi, Maruyama and Yoshizaki23, Reference TerKonda, Levine, Duvall and Giebink25, Reference Van Aarem, Muytjens, Smits and Cremers28, Reference Wardrop and Pillsbury31 Another 35 cases had 1 or 2 parameters missing.Reference Austin and Lockey1–Reference Dalovisio, Pankey, Wallace and Jones3, Reference Flint, Mahadevan, Gunn and Brown6–Reference Kinsella, Grossman and Black9Reference Liening, Plemmons, Fair, Butler, McAllister and Davis11, Reference Linmans, Stokroos and Linssen12, Reference McAvoy, Carron, Poulik, Altinok and Belenky14–Reference Molloy, Shandilya, Mahesh, McShane and El Nazir16, Reference Neitch, Sydnor and Schleupner18, Reference Nylén, Alestig, Fasth, Gustavii, Olofsson and Renvall19, Reference TerKonda, Levine, Duvall and Giebink25, Reference Ticca, Comparcola, Graziani, Lancella, Marcella and Nicolsi26, Reference Van Ingen, Looiijmans, Piet, Richard, Martin and van Solingen29, Reference Wu, Shu and Chen32 In the remaining 23 reported sporadic cases and in the 17 patients infected from a single rinsing water source, 3 or more of the above parameters were missing.Reference de Juan, Bouthelier Moreno, Fernández Liesa and Lezcano Carrera4, Reference Ferguson and Saulsbury5, Reference Franklin, Starke, Brady, Brown and Wallace7, Reference Hsiao, Liu and Hsueh8, Reference Lee, Tsai, Cheng, Liu, Huang and Liao10, Reference Lowry, Jarvis, Oberle, Bland, Silberman and Bocchini13, Reference Sungkanuparph, Sathapatayavongs and Pracharktam24, Reference Trupiano and Prayson27, Reference Vreede, Kruyt, Nijhuis-Heddes and Sastrowijoto30 In 15 of the sporadic cases, 11 or more of the above parameters were lacking.Reference Franklin, Starke, Brady, Brown and Wallace7 Thus, the statistics presented here for the previously reported cases, compared to the statistics in this study, must be viewed in the light of incomplete data in the historical reports. The numbers of cases from these reports presented below therefore vary depending on the parameter discussed.
Case reports
All cases are summarised in Tables I–V, and four cases are described in detail below.
Case one
A 10-year-old boy with a history of cleft palate surgery and recurrent otitis media, treated several times with ventilation tubes, presented in February 2007 with left-sided otorrhoea and pain behind the ear for the previous month. A mass of polyps was found filling the entire outer ear canal. A month later, a swelling was noted behind the ear.
Histopathological examination revealed a formation of granulomas with and without necrosis, and the presence of lymphocytes, neutrophilic granulocytes, giant cells and sheets of histiocytes (Figure 1).

Fig. 1 Histopathology image from case one, showing confluent granulomas composed of epithelioid histiocytes and multiple multinucleate giant cells. A rim of lymphocytes is seen at the periphery. (H&E; × 100)
Microbial cultures identified M abscessus susceptible to amikacin, clarithromycin, linezolid and tigecycline. Computed tomography (CT) scans showed opacity of the mastoid, and bone defects towards the sigmoid sinus and the bony outer ear canal. Magnetic resonance imaging (MRI) studies showed enhancement of the dura in the middle and posterior fossa. Treatment with amikacin and clarithromycin was commenced.
In April 2007, a left-sided modified radical mastoidectomy was performed. Thick, greyish granulation tissue filled the entire mastoid cavity and middle ear.
In June 2007, a culture from the mastoid cavity again grew M abscessus, now resistant to amikacin; amikacin was terminated and linezolid treatment was started.
Revision surgery was performed in July 2007 and the mastoid cavity was cleaned of granulation tissue. The culture was again positive for M abscessus, now intermediately susceptible to linezolid.
Magnetic resonance imaging studies conducted in September 2007 revealed decreased meningeal enhancement.
Linezolid was terminated in October 2007 because of neurological side effects and was replaced by tigecycline. Abdominal pain, however, led to termination of tigecycline and linezolid was restarted.
In December 2007, linezolid treatment was terminated. Clarithromycin was continued as a monotherapy, but was terminated in May 2008 after a total treatment period of 10 months when MRI studies showed no pathological meningeal enhancement and revealed that the mastoid cavity was dry.
In light of incudostapedial discontinuity and a large conductive hearing loss, ossiculoplasty was performed four years later. Post-operative hearing was satisfactory, with a remaining air–bone gap of 15 dB. At the most recent follow up, in August 2014, the ear was free from disease.
Case two
An otherwise healthy eight-year-old girl with a history of recurrent otitis media, treated at the age of six years with ventilation tubes, developed therapy-resistant purulent secretion from the left ear in September 2006.
In January 2007, she developed insidious mastoiditis, with otorrhoea and polyps in the outer ear canal. Computed tomography scans showed opacity of the middle ear and mastoid bone, destruction of the mastoid process, and a bone defect towards the middle cranial fossa. Mastoidectomy was performed. The mastoid was filled with granulation tissue and pus. Histopathological examination showed an inflammatory response containing lymphocytes, neutrophilic granulocytes and histiocytes. Necrosis was observed, but there was no true formation of granulomas.
A culture was positive for M abscessus. Clarithromycin, imipenem and amikacin treatment was started. Magnetic resonance imaging studies showed widespread inflammation along the dura in the middle and posterior fossa, and an 11-mm rounded structure, interpreted as an inflammation, bulging into the temporal lobe. There was also slight oedema surrounding the lesion in the temporal lobe. The general condition of the patient was good and there were no neurological symptoms. Given the location of the intracranial abscess, it was not possible to surgically remove it. The bacteria were shown to be susceptible to clarithromycin and tigecycline, intermediately susceptible to amikacin, and resistant to imipenem. The treatment protocol was changed to clarithromycin, tigecycline and amikacin. Amikacin treatment was continued for two months.
In February 2007, one month after the mastoidectomy, the ear canal was still swollen and granulation tissue was observed along the incision behind the ear. The mastoid cavity was surgically revised several times during the following months, and the inflammatory changes behind the ear and in the ear canal gradually subsided. During the entire antibiotic treatment period, the patient had severe problems with nausea and vomiting, most likely a result of the tigecycline treatment.
Repeated MRI studies showed a gradual decrease in the intracranial inflammation. Antibiotic treatment was terminated after 11 months, in December 2007. The patient has been in good condition since then, and her hearing has returned to normal. Immunological investigation findings were normal.
Magnetic resonance imaging studies conducted in April 2008 did not show any intracranial inflammatory changes.
At follow up in April 2012, five years after onset of the disease, MRI studies, and eardrum and hearing findings, were all normal.
Case four
A healthy 12-year-old boy with a history of recurrent otitis media and ventilation tube treatment from the age of 6 years was admitted in December 2012 because of persistent purulent otitis media in the right ear during the previous month. He had bathed in a hot tub prior to the development of the ear problems. His general condition was good.
In the right ear, a large polyp coming from the eardrum was observed, as well as chronic perforation. Computed tomography scans showed cloudiness of the mastoid, but no bone destruction. Mastoidectomy was performed. Granulation tissue was filling the medial part of the ear canal and the mastoid.
A culture showed growth of M abscessus susceptible to amikacin, linezolid and tigecycline, and intermediately susceptible to clarithromycin. Amikacin and clarithromycin treatment was started, but amikacin was discontinued after a treatment period of six weeks.
In February 2013, the ear canal was still draining. Debridement and cleaning of the ear canal was performed several times. A culture was positive for M abscessus with an unchanged susceptibility pattern. The audiogram showed a large conductive hearing loss.
One month later, the ear was dry. Magnetic resonance imaging studies showed opacity of the mastoid, but no meningeal or intracranial enhancement. The patient's general condition was good, and he was able to go to school, but he complained of severe tiredness.
On examination in May 2013, the ear was dry with persistent perforation of the eardrum. Clarithromycin was terminated after a total treatment period of five months.
In October 2013, the patient again started to have a sensation of pressure in the ear. In January 2014, after swimming during a holiday in Mexico, the ear began to drain. There was no response to conventional local and systemic antibiotic treatment. In April 2014, insidious mastoiditis was apparent. The ear canal was swollen, with granulation tissue on the eardrum.
Treatment with clarithromycin was started. Computed tomography scans showed a cloudy mastoid, but no bone destruction. Mastoidectomy was performed; the mastoid was filled with granulation tissue. The cavity was left open and repeated curettage procedures were carried out.
Cultures showed growth of M abscessus with an unchanged pattern of susceptibility. Amikacin and linezolid were added to the treatment. Linezolid had to be withdrawn after two weeks because of the development of severe anaemia. Amikacin was withdrawn after one month. Clarithromycin single therapy is planned to continue for one more year.
Case 13
This case concerns a girl with a history of recurrent otitis media and several bouts of pneumonia, who had been treated since the age of four years with bilateral ventilation tubes. In March 2010, at the age of 6 years, and after a 10-month period of repeated bouts of infection in the left ear, she developed pain and facial nerve palsy on the same side. Both ear canals were filled with granulation tissue.
Culture from the left ear was positive for M fortuitum susceptible to amikacin, ciprofloxacin and tigecycline, and intermediately susceptible to linezolid. Computed tomography scans showed bilateral opacity of the mastoid air cells and middle ear. Exploration of the right mastoid showed an essentially normal mastoid cavity with some thickened mucosa in the antrum. Exploration of the left mastoid showed a mass of purulent granulation tissue filling the entire mastoid and middle ear, with a defect in the ear canal. Modified radical mastoidectomy was performed.
Histopathological examination showed necrosis and a non-specific inflammatory response containing large amounts of neutrophilic granulocytes, and some lymphocytes, plasma cells and histiocytes. No true granulomas were found. Treatment with amikacin and ciprofloxacin was begun. The facial nerve palsy subsided.
In May 2010, the left ear was revised surgically because of persistent infection, with granulation tissue in the radical cavity and in the wound. The dura of the middle cranial fossa was exposed. A culture from the left ear was again positive for M fortuitum, with unchanged susceptibility. Computed tomography scans and MRI studies revealed bone destruction anterior to the superior semicircular canal, and an abscess in the temporal lobe (Figure 2). Tigecycline was added to the treatment and the amikacin dose was increased. The right ear subsequently cleared. The general condition of the girl was good, except for slight headache and nausea, most likely due to the antimicrobial therapy.
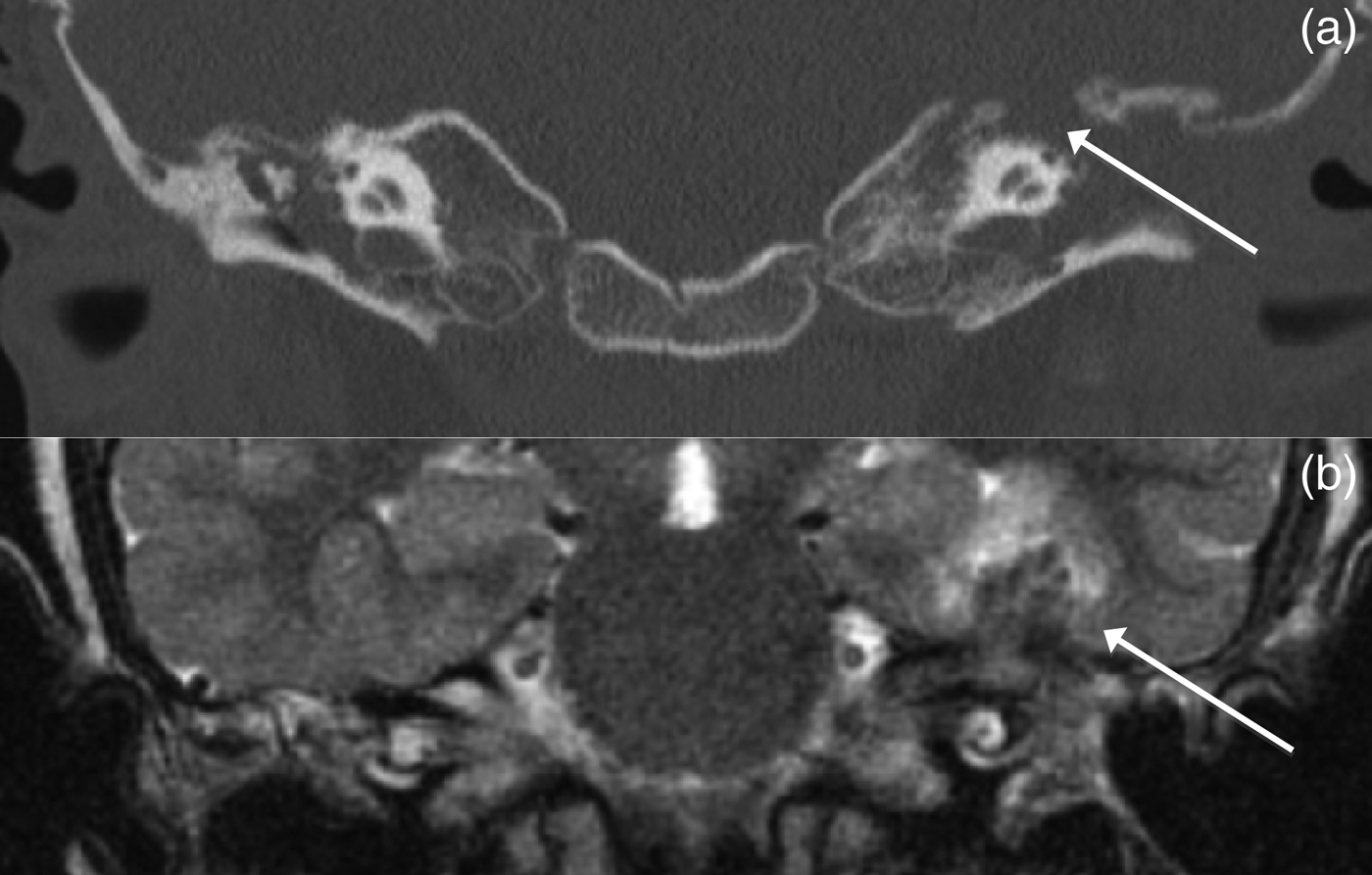
Fig. 2 Coronal computed tomography scan (a) and coronal magnetic resonance imaging scan (b) from case 13, illustrating a defect in the tegmen tympani (arrow in part a) and a corresponding abscess in the temporal lobe (arrow in part b) on the left side.
In August 2010, MRI studies showed regression of the abscess in the temporal lobe. Nausea became an increasing problem, leading to withdrawal of tigecycline. Ciprofloxacin was changed to moxifloxacin.
In October 2010, MRI studies revealed that the size of the abscess was unchanged, but the surrounding oedema in the temporal lobe had increased. The granulation tissue in the mastoid cavity had also increased. Hearing tests revealed bilateral sensorineural hearing loss in the high frequency region, most likely due to the ototoxicity of amikacin. Amikacin was therefore withdrawn after six months and tigecycline was restarted.
In November 2010, the left ear was surgically revised, with exploration of the mastoid and middle fossa, and removal of infected tissue from the middle ear, mastoid and temporal lobe. Histopathological examination of tissue from the brain and middle ear showed a weak inflammatory response containing the formation of small granulomas, some with central necrosis, the presence of a few giant cells and some lymphocytes, and fibrosis. The mastoid and middle ear were obliterated with fat, and the ear canal was closed. No neurological symptoms developed. A culture for mycobacteria was negative. Treatment with tigecycline and moxifloxacin was continued.
In January 2011, tigecycline treatment was discontinued. Monotherapy with moxifloxacin was continued for another 10 months and thereafter discontinued given the increasing problems with nausea.
Since 2011, the patient has suffered from juvenile idiopathic arthritis. Histopathological examination of biopsies from the femur and tibia showed fragmented trabecular bone and bone marrow, with an inflammation response containing lymphocytes and neutrophilic granulocytes. Fibrosis and necrosis were observed, but there was no formation of granulomas. Cultures from this region were negative.
At examination in May 2013, almost three years after diagnosis, the patient's general condition was good. The right eardrum was thickened. The audiogram showed severe bilateral high frequency sensorineural hearing loss at 4 kHz and above, with 60 dB conductive hearing loss in the left ear and 20 dB low frequency conductive hearing loss in the right ear. The hearing situation has been acceptable with a bone-anchored hearing aid. Repeated MRI studies showed a slight regression of a 4 × 5 × 6 mm large enhancement in the base of the left temporal lobe.
In the beginning of 2014, repeated MRI studies revealed that the abscess was increasing in size in the temporal lobe. There were no neurological symptoms. In April 2014, the abscess was removed surgically, and treatment with moxifloxacin and tigecycline was commenced. There was also an abscess in the left mastoid laterally, which was removed three weeks later.
Histopathological examination showed necrosis and granulomas consistent with mycobacterial infection. Cultures from these sites were, however, negative, despite the lack of antibiotic treatment prior to surgery. Extensive immunological investigation has not revealed any immunological impairment. Despite histopathological suspicion of active infection, antibiotic treatment has been discontinued and the patient will be followed up with repeated MRI studies.
At the time of writing, the patient was doing fine apart spells of dizziness and nausea. The latest MRI studies and CT scans, from April 2015, did not show any remaining inflammatory enhancement in the temporal lobe. However, there was a focal herniation from the temporal lobe through the bony defect anterior to the superior semicircular canal, and small bony defects of the lateral and superior semicircular canals themselves. Tentative diagnoses of the dizziness and nausea spells include temporal lobe epilepsy or vestibular dysfunction; however, the definite diagnosis is currently unclear.
Results and discussion
Case identification and literature review
This presentation and analysis is the largest sample of case reports of non-tuberculous mycobacteria otomastoiditis, with the most comprehensive data extraction published to date. The survey resulted in the identification of 16 sporadic cases. Only one case was identified through the laboratory database alone. Because these cases are easy to remember given the severity of the disease, we believe that the majority of cases occurring during the period have been identified. However, half of the cases were identified after 2009, suggesting that some cases from the years 2000 to 2009 may have been missed. Additionally, because a positive mycobacterial culture was part of the inclusion criteria, cases (probably only a few) for which mycobacterial culture was not performed, or false negatives, are missed by definition.
Since 1976, 72 sporadic cases have been reported in 31 publications.Reference Austin and Lockey1–Reference Linmans, Stokroos and Linssen12, Reference McAvoy, Carron, Poulik, Altinok and Belenky14–Reference Wu, Shu and Chen32 In addition, 17 patients were infected from the same source of ear rinsing water in an ENT practice in 1987,Reference Lowry, Jarvis, Oberle, Bland, Silberman and Bocchini13 making a total of 89 previously published cases.
Patient and symptom characteristics
Fifteen children and one adult (female) were identified in this study (Table I). Seventy-seven per cent of the patients in previous reports were children (aged less than 16 years). In this study, the median age of the children at the time of diagnosis was 6 years (range, 1–14 years). That is somewhat higher compared to previous reports (3 years and 6 months).Reference Avery, Eavey, Della Torre, Ramos and Pasternack2–Reference Kinsella, Grossman and Black9, Reference Liening, Plemmons, Fair, Butler, McAllister and Davis11–Reference Muller, Kemper, Hartwig, Mooi-Kokenberg, van Altena and Versteegh17, Reference Nylén, Alestig, Fasth, Gustavii, Olofsson and Renvall19–Reference Plemmons, McAllister, Liening and Garces20, Reference Stewart, Troendle-Atkins, Starke and Coker22, Reference TerKonda, Levine, Duvall and Giebink25–Reference Wardrop and Pillsbury31 Males predominated in this study, which is in line with the previous reports (56 per cent boys). Children of all ages appear to be affected, with the exception of M avium complex infections, which tend to affect the youngest children.
Table I Patient and symptom characteristics

*Deficiency in the interleukin-12/interferon-gamma axis was later diagnosed. Y = years; VT = ventilation tube; M = male; SOM = serous otitis media; F = female; ROM = recurrent otitis media; TM = tympanic membrane; IgA = immunoglobulin A; FN = facial nerve
Fifteen patients were regarded as having no other significant non-ear predisposing disease at the time of diagnosis. One patient had immunoglobulin A (IgA) deficiency. This condition may lead to an increased frequency of infections involving the mucosal membranes (e.g. respiratory infections).Reference Hammarström, Vorechovsky and Webster36 Although it is unknown whether the risk of mycobacterial infections is elevated in such cases, it has been shown that mice with IgA deficiency are more susceptible to infections with Mycobacterium bovis bacillus Calmette-Guérin.Reference Rodríguez, Tjärnlund, Ivanji, Singh, Garcia and Williams37 One of the patients (case 16) was diagnosed with a deficiency in the interleukin-12/interferon-gamma axis, which may lead to a higher susceptibility to mycobacterial infections.Reference Newport, Huxley, Huston, Hawrylowicz, Oostra and Williamson38 Only one of the children in the previous reports suffered from an immunodeficiency condition (common variable immunodeficiency), and the infection in this child led to a fatal disseminated infection.Reference Trupiano and Prayson27 In the previous reports, other significant non-ear diseases were rarely reported.
In this study, all children except one had ventilation tubes or perforations of the eardrum at the time of diagnosis or shortly before. The adult female had a previously diagnosed perforation with infection. She developed otomastoiditis shortly after tympanoplasty surgery. Only one patient had no previously known ear disease (case 16). None of the patients had cholesteatoma. Thus, it seems unlikely that a non-tuberculous mycobacteria infection in the middle ear starts without a defect of the eardrum. The results from this study are in accordance with previous reports, in which 82 per cent (54 out of 66) of the children had a history of ventilation tubes,Reference Avery, Eavey, Della Torre, Ramos and Pasternack2, Reference Dalovisio, Pankey, Wallace and Jones3, Reference Ferguson and Saulsbury5–Reference Hsiao, Liu and Hsueh8, Reference Liening, Plemmons, Fair, Butler, McAllister and Davis11–Reference Lowry, Jarvis, Oberle, Bland, Silberman and Bocchini13, Reference Moerman, Dierick, Mestdagh, Boedts and Van Cauwenberge15, Reference Muller, Kemper, Hartwig, Mooi-Kokenberg, van Altena and Versteegh17, Reference Nylén, Alestig, Fasth, Gustavii, Olofsson and Renvall19, Reference Plemmons, McAllister, Liening and Garces20, Reference TerKonda, Levine, Duvall and Giebink25, Reference Van Aarem, Muytjens, Smits and Cremers28–Reference Vreede, Kruyt, Nijhuis-Heddes and Sastrowijoto30 and perforation of the eardrum was present in another 8 per cent (5 out of 66 children).Reference Lowry, Jarvis, Oberle, Bland, Silberman and Bocchini13–Reference Moerman, Dierick, Mestdagh, Boedts and Van Cauwenberge15 In adults, previous reports indicate that ear disease, such as perforation of the eardrum, chronic otitis media or previous irradiation, can predispose an individual to non-tuberculous mycobacteria infection (11 out of 21 patients).Reference Austin and Lockey1, Reference Franklin, Starke, Brady, Brown and Wallace7, Reference Hsiao, Liu and Hsueh8, Reference Lee, Tsai, Cheng, Liu, Huang and Liao10, Reference Lowry, Jarvis, Oberle, Bland, Silberman and Bocchini13, Reference Neitch, Sydnor and Schleupner18, Reference Redaelli de Zinis, Tironi, Nassif and Ghizzardi21, Reference Sugimoto, Ito, Hatano, Nakanishi, Maruyama and Yoshizaki23, Reference Sungkanuparph, Sathapatayavongs and Pracharktam24, Reference Wu, Shu and Chen32
The median time to diagnosis was two months (range, one to five months). Compared to previous reports, where the median time to diagnosis was four months, the results from this study might suggest that the time to diagnosis has decreased. However, it takes several months in many cases for the diagnosis to be apparent. This could partly be because these bacteria grow more slowly than other bacteria, but the main reason is probably that non-tuberculous mycobacteria infections are rare and consequently usually not suspected. Additionally, most cases do not have alarming symptoms such as high fever, severe pain or an altered general condition.
The most common presentation in this study was a therapy-resistant secretion from the ear canal (94 per cent), followed by polyps (81 per cent) and insidious mastoiditis (44 per cent). In previous reports, mastoiditis was present initially in only 14 per cent of cases (8 out of 57).Reference Kinsella, Grossman and Black9, Reference Moerman, Dierick, Mestdagh, Boedts and Van Cauwenberge15, Reference Muller, Kemper, Hartwig, Mooi-Kokenberg, van Altena and Versteegh17–Reference Nylén, Alestig, Fasth, Gustavii, Olofsson and Renvall19, Reference Stewart, Troendle-Atkins, Starke and Coker22, Reference Van Aarem, Muytjens, Smits and Cremers28, Reference Wardrop and Pillsbury31 Whether this difference is a true difference is unclear. It may instead reflect disparity in the definition of mastoiditis and inconsistent reporting. The cases of mastoiditis in this study and in previously reported studies were generally insidious, with a lack of toxic symptoms, unlike mastoiditis caused by other bacteria. One patient in this study had transient facial nerve palsy at presentation (case 13). Facial nerve weakness was present in 12 per cent of patients (7 out of 58) in previous reports.Reference Redaelli de Zinis, Tironi, Nassif and Ghizzardi21, Reference Trupiano and Prayson27, Reference Van Ingen, Looiijmans, Piet, Richard, Martin and van Solingen29, Reference Wu, Shu and Chen32 In only one of these cases did the palsy persist at follow up (House–Brackmann facial nerve grade II).Reference Redaelli de Zinis, Tironi, Nassif and Ghizzardi21 Thus, facial nerve palsy in these infections has good prognosis.
The two children in this study with infections caused by M avium complex were one and six years old. The younger child had not been treated with ventilation tubes prior to the infection. Previous reports indicate that children with M avium complex infections are younger than children infected with other species of non-tuberculous mycobacteria (median of 2 years vs 5.5 years).Reference de Juan, Bouthelier Moreno, Fernández Liesa and Lezcano Carrera4, Reference Flint, Mahadevan, Gunn and Brown6, Reference Kinsella, Grossman and Black9, Reference Muller, Kemper, Hartwig, Mooi-Kokenberg, van Altena and Versteegh17, Reference Stewart, Troendle-Atkins, Starke and Coker22, Reference TerKonda, Levine, Duvall and Giebink25–Reference Trupiano and Prayson27, Reference Wardrop and Pillsbury31 Only 44 per cent (4 out of 9) of the children infected with M avium complex reported previously had been treated with ventilation tubes,Reference Flint, Mahadevan, Gunn and Brown6, Reference Kinsella, Grossman and Black9, Reference Muller, Kemper, Hartwig, Mooi-Kokenberg, van Altena and Versteegh17, Reference Stewart, Troendle-Atkins, Starke and Coker22, Reference TerKonda, Levine, Duvall and Giebink25–Reference Trupiano and Prayson27, Reference Wardrop and Pillsbury31 compared to 96 per cent (55 out of 57) of the children infected with other strains of non-tuberculous mycobacteria (ventilation tubes or perforation).Reference Avery, Eavey, Della Torre, Ramos and Pasternack2, Reference Ferguson and Saulsbury5–Reference Franklin, Starke, Brady, Brown and Wallace7, Reference Linmans, Stokroos and Linssen12, Reference Lowry, Jarvis, Oberle, Bland, Silberman and Bocchini13, Reference Van Aarem, Muytjens, Smits and Cremers28–Reference Vreede, Kruyt, Nijhuis-Heddes and Sastrowijoto30M avium complex infections in young children typically affect neck lymph nodes. This was also the case in one of the patients in this study. In 30 per cent (3 out of 10) of previously reported M avium complex cases, engagement of the neck was evident.Reference de Juan, Bouthelier Moreno, Fernández Liesa and Lezcano Carrera4, Reference Flint, Mahadevan, Gunn and Brown6, Reference Kinsella, Grossman and Black9, Reference Muller, Kemper, Hartwig, Mooi-Kokenberg, van Altena and Versteegh17, Reference Stewart, Troendle-Atkins, Starke and Coker22, Reference TerKonda, Levine, Duvall and Giebink25–Reference Trupiano and Prayson27, Reference Wardrop and Pillsbury31 It is reasonable to suggest that M avium complex middle ear infections in children are in some cases caused by ingesting infected soil, as opposed to other strains of non-tuberculous mycobacteria that almost invariably infect the middle ear via a defect in the eardrum.
In this study, there was also one case of neck lymph node enlargement in a patient with M abscessus infection. This has not been described previously in patients with non-tuberculous mycobacteria ear infections other than M avium complex. Because mycobacteria could not be isolated from the lymph nodes of this case, and because the histopathological analysis suggested reactivity rather than granulomatous inflammation, it might have reflected an immunological reaction rather than a lymph node infection.
Histopathology
Histopathological material was available in 10 cases in this study (Table II). In each case, the initial histopathology assessment did not differ from the review by the relevant author (HE). A histopathological picture of granulomas with or without necrosis, and with a surrounding inflammatory response, was seen in eight cases. This was regarded as consistent with, but not diagnostic of, mycobacterial infection. The two patients without granulomas showed a non-specific inflammatory response rich in neutrophilic granulocytes and sheets of histiocytes. In the correct clinical setting, this could be suggestive of an infectious aetiology. None of the cases in this study showed a positive special stain reaction (Ziehl–Neelsen stain (n = 4), auramine-rhodamine stain (n = 1) or immunohistochemical stain (n = 1)). In 67 per cent (20 out of 30) of the cases in previous reports, granulomas with necrosis suggestive of mycobacterial infections were observed, and in 33 per cent (10 out of 30), a non-specific inflammatory response was reported.Reference Austin and Lockey1, Reference Flint, Mahadevan, Gunn and Brown6–Reference Hsiao, Liu and Hsueh8, Reference Lee, Tsai, Cheng, Liu, Huang and Liao10, Reference McAvoy, Carron, Poulik, Altinok and Belenky14, Reference Moerman, Dierick, Mestdagh, Boedts and Van Cauwenberge15, Reference Muller, Kemper, Hartwig, Mooi-Kokenberg, van Altena and Versteegh17, Reference Nylén, Alestig, Fasth, Gustavii, Olofsson and Renvall19–Reference Van Aarem, Muytjens, Smits and Cremers28, Reference Wardrop and Pillsbury31
Table II Histopathology

N/A = not available; Neg = negative
The classic histopathological picture of a mycobacterial infection is typically observed in patients with tuberculosis. The classic granulomatous inflammation includes grouped collections of epithelioid histiocytes; that is, granulomas often with central necrosis, lymphocytes surrounding the granulomas, sheets of histiocytes, and the presence of multinucleate giant cells (Figure 1). Non-tuberculous mycobacteria, especially slow-growing species, can have a histological picture indistinguishable from tuberculosis. Fast-growing species may also give a more suppurative inflammatory response (i.e. rich in neutrophilic granulocytes). A similar granulomatous pattern can sometimes be seen in non-mycobacterial infections. Sarcoidosis also generates a granulomatous response, but often with less inflammation and less necrosis.Reference Hale34, Reference Rosai39
Non-specific inflammatory patterns, such as necrosis outside granulomas, granulation tissue, chronic inflammation, and sheets of histiocytes, are often present in both tuberculosis and in non-tuberculous mycobacteria infections. As could be expected, the histopathological picture cannot reliably differentiate between different types of mycobacteria.Reference Hale34, Reference Rosai39
Microbiology and medical treatment
Cultures were positive for M abscessus in 12 cases, positive for M fortuitum in 2 cases and positive for M avium complex in 2 cases (Table III). M abscessus was by far the most common species in both this study and in previous reports (75 per cent and 58 per cent, respectively).
Table III Microbiology and medical treatment

*See case report. †Additional six weeks’ clarithromycin treatment because of mastoiditis (culture negative for non-tuberculous mycobacteria), which developed six months after treatment was discontinued. ‡Amikacin and ethambutol started after five months. Mth = months; wk = weeks; cont = continued; N/A = not available
M abscessus and M fortuitum belong to the group of rapidly growing mycobacteria. They are phylogenetically different and have a different antibiotic susceptibility pattern compared to the slow-growing mycobacteria (e.g. M avium complex). In general, M abscessus and M fortuitum infections in otherwise healthy persons are associated with wound infections, but they also cause lung infections in the predisposed.Reference Petrini40 The American Thoracic Society and the Infectious Disease Society of America have issued recommendations on the diagnosis and treatment of these infections.Reference Griffith, Aksamit, Brown-Elliott, Catanzaro, Daley and Gordin41 Although drug susceptibility testing for these bacteria is generally regarded as difficult to standardise and interpret, these societies recommend that the treatment of rapidly growing non-tuberculous mycobacteria infections be based on drug susceptibility testing results. Rapidly growing mycobacteria are uniformly resistant to standard anti-tuberculosis drugs such as rifamycin, ethambutol and isoniazid. In this study, two patients (cases 5 and 11) were treated with rifamycin and ethambutol at some point, which must be regarded as erroneous.
M abscessus is mostly susceptible or intermediately susceptible to the macrolides clarithromycin and azithromycin, and to the aminoglycoside amikacin. Some strains may also be susceptible to linezolid and imipenem. Other reviews suggest that M abscessus isolates are susceptible to tigecycline.Reference Petrini40M fortuitum is regarded as more susceptible to antibiotics, and clinical isolates are usually susceptible to macrolides (clarithromycin and azithromycin), fluoroquinolones, sulphonamide, doxycycline, amikacin and imipenem.Reference Griffith, Aksamit, Brown-Elliott, Catanzaro, Daley and Gordin41 However, M fortuitum isolates possess an inducible resistance mechanism for macrolides; therefore, these drugs should be used with caution, and monotherapy with macrolides should be avoided.Reference Griffith, Aksamit, Brown-Elliott, Catanzaro, Daley and Gordin41
The American Thoracic Society and the Infectious Disease Society of America recommend the use of clarithromycin (or azithromycin) in combination with an injectable agent such as amikacin or imipenem (or cefoxitin, which is no longer available in Sweden) for serious skin, soft tissue and bone infections.Reference Griffith, Aksamit, Brown-Elliott, Catanzaro, Daley and Gordin41 The suggested minimum treatment time is four months for skin and tissue infections, and six months for bone infections, to provide a high likelihood of cure. Surgery is usually indicated for extensive disease or abscesses. Among the 14 patients in this study with M abscessus or M fortuitum infections, only 11 were treated for 6 months or more, and only 8 patients received the recommended drug combination of macrolides and amikacin, in some cases this was administered together with other drugs. Twelve patients were nonetheless cured, confirming that surgery and the patients’ own defences have important roles to play. However, case four had recurrence of the M abscessus infection after a period of apparent cure, suggesting that the treatment time was too short initially. Furthermore, case 13, with intracranial extension of the disease, is not definitely cured. Neurosurgical intervention was recently performed in order to diminish the infection load in the temporal lobe. A culture from this lesion was nevertheless negative. This patient was treated for 20 months with different drug combinations, implying a hampered immunological response to the infection.
M avium complex was the cause of otomastoiditis in two patients (cases 15 and 16). The recommended therapy for M avium complex disease, apart from surgery, includes clarithromycin (or azithromycin), together with ethambutol and rifampicin (or rifabutin), and in severe cases, amikacin as well. Drug susceptibility testing is generally not recommended for the latter three drugs because of a low correlation between in vitro test results and clinical responses. For macrolides, drug susceptibility testing may be conducted, but is mostly indicated in cases of therapy failure because strains that have not been exposed to antibiotics are generally considered to be susceptible. Remarkably, one patient (case 16) was reported to have a macrolide-resistant strain, but this may be attributable to a laboratory error. One of the two cases of M avium complex infection (case 16) was treated according to the recommendations for 13 months, whereas the other one was treated for 9 months with macrolides and rifabutin. Nonetheless, both were cured.
Disease spread and surgical treatment
In this study, all cases except two were examined with CT scans, MRI studies or both. In addition to opacity of the temporal bone, bone destruction was almost compulsory (80 per cent) (Table IV). Spread outside the mastoid and evidence of bone destruction were more common in this study compared to previous reports. The presence or absence of bone destruction of the mastoid in previous reports was, however, difficult to judge because intra-operative findings and imaging findings were not always described in detail. Nevertheless, bone destruction was distinctly described in almost half of the cases (17 out of 40).Reference Austin and Lockey1–Reference Dalovisio, Pankey, Wallace and Jones3, Reference Flint, Mahadevan, Gunn and Brown6–Reference Kinsella, Grossman and Black9, Reference Linmans, Stokroos and Linssen12, Reference McAvoy, Carron, Poulik, Altinok and Belenky14–Reference Muller, Kemper, Hartwig, Mooi-Kokenberg, van Altena and Versteegh17, Reference Nylén, Alestig, Fasth, Gustavii, Olofsson and Renvall19, Reference Redaelli de Zinis, Tironi, Nassif and Ghizzardi21–Reference Ticca, Comparcola, Graziani, Lancella, Marcella and Nicolsi26, Reference Van Aarem, Muytjens, Smits and Cremers28, Reference Van Ingen, Looiijmans, Piet, Richard, Martin and van Solingen29, Reference Wardrop and Pillsbury31, Reference Wu, Shu and Chen32
Table IV Disease spread and surgical treatment

CT = computed tomography; MRI = magnetic resonance imaging
Intracranial spread was apparent in three cases in this study (19 per cent). In one of these (case one), only the dura was engaged. In two cases (cases 2 and 13), the dura and the temporal lobe were engaged. None of the three patients with intracranial spread had any neurological symptoms. In previous reports, 8 per cent of cases (4 out of 52) had evidence of intracranial spread.Reference Austin and Lockey1–Reference de Juan, Bouthelier Moreno, Fernández Liesa and Lezcano Carrera4, Reference Flint, Mahadevan, Gunn and Brown6–Reference Kinsella, Grossman and Black9, Reference Linmans, Stokroos and Linssen12, Reference McAvoy, Carron, Poulik, Altinok and Belenky14–Reference Trupiano and Prayson27, Reference Van Ingen, Looiijmans, Piet, Richard, Martin and van Solingen29–Reference Wu, Shu and Chen32 The lymphatic glands were enlarged in two cases in this study. In 6 per cent (3 out of 52) of the cases in previous reports, the infection had spread into the soft tissues. In 81 per cent (42 out of 52), the infection was limited to the temporal bone as indicated by operative and/or imaging findings. In 6 per cent (3 out of 52), the disease was limited to the middle ear.Reference Austin and Lockey1–Reference de Juan, Bouthelier Moreno, Fernández Liesa and Lezcano Carrera4, Reference Flint, Mahadevan, Gunn and Brown6–Reference Kinsella, Grossman and Black9, Reference Linmans, Stokroos and Linssen12, Reference McAvoy, Carron, Poulik, Altinok and Belenky14–Reference Trupiano and Prayson27, Reference Van Ingen, Looiijmans, Piet, Richard, Martin and van Solingen29–Reference Wu, Shu and Chen32
Thirteen patients in this study were operated upon with different middle-ear and mastoid procedures. Five patients had two or more surgical revisions. There was a high frequency of surgery both in this study and in previous reports. Seventy-four per cent (55 out of 74) of the patients in previous reports underwent surgery.Reference Austin and Lockey1–Reference Wu, Shu and Chen32 In many cases, surgery preceded the diagnosis, which was also noted in this study. In 54 per cent (40 out of 74) of cases, various types of mastoidectomy were performed, with preservation of the ear canal combined with exploration of the middle ear. In 11 per cent (8 out of 74), the initial surgery resulted in a radical cavity.Reference Flint, Mahadevan, Gunn and Brown6–Reference Hsiao, Liu and Hsueh8, Reference McAvoy, Carron, Poulik, Altinok and Belenky14, Reference Nylén, Alestig, Fasth, Gustavii, Olofsson and Renvall19, Reference Redaelli de Zinis, Tironi, Nassif and Ghizzardi21, Reference Sungkanuparph, Sathapatayavongs and Pracharktam24 In 4 per cent (3 out of 74), only the middle ear was explored.Reference Lowry, Jarvis, Oberle, Bland, Silberman and Bocchini13 In 3 per cent (2 out of 74), mastoidectomy was combined with neck surgery, and in one patient, mastoidectomy was combined with exploration of the posterior cranial fossa.Reference Dalovisio, Pankey, Wallace and Jones3, Reference de Juan, Bouthelier Moreno, Fernández Liesa and Lezcano Carrera4, Reference Flint, Mahadevan, Gunn and Brown6 In this study, revision surgery was performed at a lower frequency (5 out of 15) compared to previous reports (22 out of 55).Reference Austin and Lockey1–Reference Wu, Shu and Chen32 In many of the cases in the previous reports, the wound ruptured and/or granulation tissue filled the ear canal after surgery. Repeated debridement of the radical cavity was performed in some cases, as well as conversion of the mastoidectomy into a radical cavity, which was also noted in this study.
The three patients in this study not operated upon were diagnosed after the culture of polyps in the ear canal (cases 6, 9 and 12). These patients had M abscessus infections, with no signs of bone destruction on imaging (cases 6 and 9), and their general medical condition was good. The ears improved readily whilst on systemic antibiotic treatment or local broad-spectrum antibiotic treatment only, and healed without sequelae. In the case treated with local antibiotics only (case 12), histopathological examinations were not performed. It may be argued that the infection was not invasive in this case. Seventeen of the 19 patients in previous reports that were not operated upon had M abscessus infections. Information regarding initial presentation, presence of bone destruction and follow-up data are unfortunately lacking in most of these cases.Reference Ferguson and Saulsbury5, Reference Franklin, Starke, Brady, Brown and Wallace7, Reference Lee, Tsai, Cheng, Liu, Huang and Liao10, Reference Lowry, Jarvis, Oberle, Bland, Silberman and Bocchini13, Reference Molloy, Shandilya, Mahesh, McShane and El Nazir16, Reference TerKonda, Levine, Duvall and Giebink25, Reference Van Ingen, Looiijmans, Piet, Richard, Martin and van Solingen29
Two patients in this study had an abscess in the temporal lobe. It was not possible to remove the abscess in one patient (case two) because of its location. This patient received antibiotic treatment for almost one year and has subsequently been followed up for five years with repeated MRI studies, with complete radiological disappearance of the abscess. This patient has been regarded as cured, suggesting that prolonged antibiotic treatment may be sufficient for intracranial disease. The second patient (case 13) is still not cured, despite repeated neurosurgical interventions, bringing forward a tremendously difficult decision in this particular patient. Knowledge regarding intracranial infections with non-tuberculous mycobacteria in immune competent individuals is sparse. There are case reports of meningitis and intracranial abscesses due to M avium complex infections, and of infections with M fortuitum associated with shunts and epidural catheters.Reference Madaras-Kelly, DeMasters and Stevens42, Reference Arkun, Gordon, Lincoln, Levi, Bello and Keller43 A case of pituitary gland infection with Mycobacterium malmoense, preceded by a mastoidectomy two months earlier, and treated successfully with surgery combined with appropriate antimicrobial drugs, has also been reported.Reference Florakis, Kontogeorgos, Anapliotou, Mazarakis, Richter and Brück44
Follow-up time and sequelae
The median follow-up time after diagnosis in this study was 3 years and 5 months (range, 10 months to 7 years and 9 months) (Table V). In 11 patients, the ear canal and the eardrum were normal at follow up, and hearing was normal in 8 of these patients. Three patients were left with radical cavities, and in all of these patients there was persistent hearing loss. One patient suffered a persistent eardrum perforation and conductive hearing loss. One patient was left with severe high frequency bilateral sensorineural hearing loss and a major conductive hearing loss in the affected ear. These figures are in accordance with previous reports, in which 63 per cent (42 out of 67) of patients recovered with normal hearing and a normal ear canal and eardrum.Reference Austin and Lockey1–Reference Franklin, Starke, Brady, Brown and Wallace7, Reference Kinsella, Grossman and Black9–Reference Wu, Shu and Chen32 Sequelae in this study and in previous reports were related to the severity of the disease and extensiveness of the surgery.
Table V Follow-up time and sequelae

Y = years; mth = months; CHL = conductive hearing loss; TM = tympanic membrane; cont = continued; BAHA = bone-anchored hearing aid; SNHL = sensorineural hearing loss
Conclusion
Non-tuberculous mycobacteria infection is a rare but important and potentially serious cause of otomastoiditis. Analysis of the patients in this study and in previously published reports gives a consistent picture of the clinical and microbiological characteristics of this disease. In the absence of clinical trials, our results may be used to support clinicians in the management of these patients.
Otomastoiditis due to rapidly growing non-tuberculous mycobacteria species share a similar clinical picture, disease extent, treatment strategy and outcome. However, otomastoiditis due to slow-growing mycobacteria (i.e. M avium complex) tend to affect the youngest children, and defects of the eardrum prior to infection are not mandatory, as opposed to cases of rapidly growing non-tuberculous mycobacteria. Soft tissue and lymphatic engagement is also far more common in M avium complex infections.
Infection with non-tuberculous mycobacteria should be suspected in patients with a defect of the eardrum (ventilation tube or perforation) when secretion and granulation tissue resistant to standard treatment develop, and in patients who develop insidious mastoiditis. Histopathological analysis can provide a rapid, presumptive diagnosis. Mycobacterial culture is necessary for confirmation, species identification and susceptibility testing. Computed tomography scans and repeated MRI studies should be conducted because intracranial engagement is not uncommon. Repeated culture is valuable given the risk of change in susceptibility pattern.
• Non-tuberculous mycobacteria infection should be suspected in therapy-resistant otitis media cases with polyps, granulation tissue and insidious mastoiditis
• Histopathological analysis gives a rapid, presumptive diagnosis; culture is necessary for confirmation, species identification and susceptibility testing
• Repeated culture and susceptibility testing are important because bacterial susceptibility may change
• Magnetic resonance imaging should be conducted because of the risk of intracranial spread
• Mastoid surgery is usually necessary
• Antibiotic treatment length should be six months minimum; antibiotic monotherapy should be avoided
The aim of surgical treatment should be to diminish local bacterial load with as little destructive effect on the ear and hearing as possible. The choice of antibiotics is complicated because of difficulties in interpreting susceptibility patterns. Multimodal long-term antibiotic treatment seems to be curative in most cases. However, it normally includes at least one drug administered intravenously. In addition, several of the drugs have severe side effects that hamper acceptance for long-term treatment and put a great deal of stress on the affected families. Medical treatment should therefore be directed by infectious diseases specialists who are well versed in assessing treatment of non-tuberculous mycobacteria infections. In cases not responding to treatment, immunodeficiency, especially that hampering the interferon-gamma axis, should be ruled out.
This study showed that intracranial spread of non-tuberculous mycobacteria infection from the ear is not uncommon. Because treatment recommendations are not addressed systematically in the literature, it is important that such cases are published in detail in the future.
Acknowledgements
We would like to acknowledge our Swedish colleagues in otosurgery for help with access to patient records, and Mrs Hanna Fransson at the Department of Pathology, Central Hospital in Karlstad, for assisting in collecting the histopathological samples.









