Introduction
Bovine raw milk is an important food but its high liability to spoilage limits its shelf life. Some research-works have tried to prolong raw milk shelf life using thio-cyanate or H2O2 to activate the natural lactoperoxidase (Haddadin et al. Reference Haddadin, Ibrahim and Robinson1996). Nitrogen gas (Munsch-Alatossava et al. Reference Munsch-Alatossava, Gursoy and Alatossava2010) and certain lactic acid bacteria (Mufandaedza et al. Reference Mufandaedza, Viljoen, Feresu and Gadaga2006) were also investigated as shelf-life enhancers. Recently, we have demonstrated that supplementation of raw milk with 0·5% (w/v) prepared cationic proteins e.g. methylated legume proteins has considerably reduced the levels of spoilage markers and pathogenic bacteria during cold storage (Mahgoub et al. Reference Mahgoub, Sitohy and Osman2011; Sitohy et al. Reference Sitohy, Mahgoub and Osman2011). The main factor of this preservative action and antibacterial activity is the net positive charge acquired by esterification and some degree of inherent or acquired hydrophobicity. In spite of their effectiveness, esterified proteins may not be allowed for human consumption in many countries due to the unknown effect of chemical modifications. Although native legume proteins were found devoid of antimicrobial activity, some of their components may be biologically active since they are a mixture of polypeptides with different biochemical properties. Some of these fractions may fulfil the requirements of the plant antimicrobial cationic peptides or proteins which represent the components of innate defence system. Based on the positive net charges of some fractions they can interact directly with the negatively charged bacterial membrane phospholipids increasing their permeation thus interfering with the growth, multiplication and spread of microbial organisms (Shai, Reference Shai2002).
Legume globulins represent the majority of seed soybean proteins and can be subdivided into two main types according to their sedimentation coefficients: glycinin (11S) and β-conglycinin (7S). Glycinin has a molecular mass of 350 kDa and is composed of 5 constituent subunits, each of which consists of an acidic (37–42 kDa) and a basic polypeptide (20 kDa), linked together by a disulphide bond (Nielsen, Reference Nielsen1985). β-Conglycinin is a trimeric glycosylated protein of 150–200 kDa molecular mass (Utsumi, Reference Utsumi and Kinsella1992). The antimicrobial activities of these two main soybean protein subunits have been investigated recently (Sitohy et al. Reference Sitohy, Mahgoub and Osman2012) revealing that 11S has an antimicrobial action against pathogenic and spoilage bacteria equivalent to or higher than penicillin by virtue of its cationic and hydrophobic nature (Kuipers & Gruppen, Reference Kuipers and Gruppen2008) enabling it to interact with the bacterial cell wall and membrane. Hence, the objective of the current work was to delineate the potential preservative actions of these two protein fractions during raw milk storage at 4 and 25 °C by specifying their antimicrobial influence against the contaminating bacteria (total viable count, Pseudomonas count and Enterobacteriaceae count) as well as against artificially inoculated bacterial pathogens (Listeria monocytogenes, Salmonella Enteritidis and Escherichia coli O175:H7) as compared with nisin and lysozyme as positive controls. The associated physicochemical and sensorial changes in the stored raw milk were taken into account.
Lysozymes are naturally present in foods such as cow milk (ca. 0·13 mg/ml; Conner, Reference Conner, Davindson and Branen1993) with bacteriostatic and bactericidal properties against both Gram-positive and Gram-negative bacteria (Mathur et al. Reference Mathur, Dwarkadas, Sharma, Saha and Jain1990; Dűring et al. Reference Dűring, Porsch, Mahn, Brinkmann and Gieffers1999). Nisin possess antibacterial activity against foodborne pathogens and it has been applied to the control of List. monocytogenes in cheese (Maisnier-Patin et al. Reference Maisnier-Patin, Deschamps, Tatini and Richard1992; Davies et al. Reference Davies, Bevis and Delves-Broughton1997).
Materials and methods
Isolation and characterization of 11S and 7S globulins
Defatted soybean flour was used for the isolation of 11S (glycinin) and 7S (β-conglycinin) according to Nagano et al. (Reference Nagano, Hirotsuka, Mori, Kohyama and Nishinari1992). Hydrophobic amino acid residues; leucine, isoleucine, phenylalanine, valine, methionine, tryptophan, cysteine, tyrosine and alanine (L, I, F, V, M, W, C, Y & A) were counted and related to the total number of amino acids in each subunit to give the hydrophobicity index (% of hydrophobic amino acid residues). The net charge of each domain was calculated by counting the basic amino acid residues; arginine, lysine and histidine (R, K & H) residues as +1 for each one and the acidic amino acid residues; aspartic and glutamic acids (D & E) as −1 for each one then adding the two values algebraically to give the net charge. The sequences of each domain (basic and acidic) in each subunit (G1, G2, G3, G4 & G5) were imported from the Expasy data base (http://www.uniprot.org/uniprot/?query=glycinin&sort=score). Nisin was obtained from Santa Cruz, Biotechnology, Inc. 2145 Delaware Avenue Santa Cruz, California 95060 U.S.A. Lysozyme was obtained from Mallinckrodt. Inc. Paris, Kentucky 40361.
Bacterial strains and inoculum preparation
List. monocytogenes ScottA and Sal. enterica subsp. enterica serovar Enteritidis PT4 strains were kindly obtained from Prof. George John Nychas, Department of Food Science and Technology, Laboratory of Food Microbiology and Biotechnology, Agricultural University of Athens, Greece. Esch. coli O157:H7 was obtained from Microbiology laboratory, Faculty of Agriculture, Zagazig University, Egypt and maintained in 20% glycerin at −20 °C. The three pathogens were activated by three successive transfers in tryptic soy broth (Biolife, Italy) at 37 °C for 24 h prior to use. Bacterial cells were separated by centrifugation (10 000 g for 10 min at 4 °C), washed three times with Ringer's solution (Lab M, Bury, UK), re-centrifuged, and then re-suspended in the same solution to a final volume of 10 ml. Inocula were prepared by serially diluting in sterilized Ringer's solution to a final level of 5 log CFU/ml as estimated by optical density at 600 nm and confirmed by plate counting on selective media.
Ordinary raw milk storage
Raw bovine milk was collected directly after milking from a private farm in Sharkia governorate, Egypt. It was kept in closed stainless steel container, examined for its freedom from bacterial pathogens, gently agitated on a shaker for homogenization (10 min) and maintained at 4 °C for 1 h before use. If the microbiological analysis of the starting milk sample showed some pathogenic contamination, all samples would be discarded and the experiment was repeated with another milk sample. A preliminary experiment was conducted to define the effective level of substance supplementation. Raw milk was supplemented with different concentrations of 11S (0·0, 0·01, 0·10, 0·25, 0·50, 0·75 and 1·0% w/v) and maintained at room temperature for 1 d (∼25–28 °C) before determining total bacterial count in all samples.
An amount of 3 l raw milk was divided into 30 portions of milk (100 ml) in sterile screw-capped bottles classified into 5 groups, 6 bottles in each. The first group did not receive any treatment and served as negative control. The second, third, fourth and fifth groups received supplementation with 0·5% (w/v) 7S, 11S, nisin and lysozyme respectively. Every group was subdivided into two subgroups, three bottles of each. One sub-group of each group was stored at 4 °C for 30 d and the second subgroup was stored at 25±3 °C for 48 h. Samples were withdrawn for analysis under aseptic conditions every 2 d from milk stored at 4 °C and every 6 h from milk stored at 25 °C. This experiment was exactly repeated with same design during the following month and the final recorded results were the average of the two experiments. Statistical analysis was performed among the replicates of each treatment within each experiment. Then a statistical analysis between the two experiments was performed showing no significant differences. The average of the two experiments was calculated and taken as the final results.
Artificially inoculated milk storage
Another 3 l raw bovine milk were similarly divided into 30 portions of milk (100 ml) and handled exactly as in the previous section except that all bottles were firstly inoculated with a 0·1 ml mixed culture of 3 bacterial pathogens so that the final count of each became ca. 4 log CFU/ml just before the supplementation and storage treatments. All milk samples were either stored at 4 °C for 8 d or 25±3 °C for 24 h where samples were withdrawn as previously outlined.
Microbiological analysis
The analytical samples were prepared by aseptically mixing 10 ml milk samples with 90 ml peptone saline diluent (1 g peptone, 8·5 g NaCl in 1 l distilled water). Decimal dilutions in peptone saline were prepared and duplicate 0·1 ml samples of appropriate dilutions were spread on Petri dishes containing the suitable solidified culture media. The TVC (total viable count) was determined on plate count agar (Merck 1·05463) after incubation at 25 °C for 2 d (Frank et al. Reference Frank, Christen, Bullerman and Marshall1992). PSC (Pseudomonas spp. count) was counted on Pseudomonas agar base (PAB) supplemented with 10 mg cetrimide fucidin/ml (Oxoid), incubated aerobically at 25 °C for 2 d. Enterobacteriaceae count (ENC) was determined by pouring 1·0 ml milk into 10 ml molten (45 °C) violet red bile glucose agar (VRBG, Biolife, Italy). After setting, a 10 ml overlay of molten medium VRBG was added before incubation at 37 °C for 24 h and counting the large colonies with purple haloes. List. monocytogenes was counted on polymyxin-acriflavin-lithium chloride-ceftazidime-aesculin-mannitol agar (PALCAM agar, Biolife, 401604, Italy) after incubation for 24 h at 37 °C and confirmed according to the guidelines of ISO 11290. In case of Salmonella, the analytical sample was incubated on Xylose Lysine Deoxycholate (XLD agar, Merck, 1·05287, Ger.) for 24 h at 37 °C. Esch. coli O157:H7 was counted on Tryptone Bile X-Glucuronide agar (TBX, Biolife, Italy) after incubation for 4 h at 37 °C followed by incubation at 44 °C for 20 h. All plates were visually examined for typical characteristic colony types.
Chemical analysis
Titratable acidity (as lactic acid%) of stored raw milk was assessed after different intervals of storage at 4 °C (0–30 d) or 25 °C (0–48 h) according to the standard methods (AOAC, 1997). Milk pH was assessed in the same samples by pH meter (pH 211 HANNA instruments Inc. Woonsocket- USA made in Romania). Lactose was determined according to Dubois et al. (Reference Dubois, Gilles, Hamilton, Rebers and Smith1956). Coagulation boiling test was carried out by heating 5 ml aliquots of milk sample in a glass test tube for 5 min in a water bath at 100 °C followed by rapid cooling in an ice-water bath before pouring into a glass plate. Coagulation was judged by optical observation and expressed by + and – signs according to its intensity. SDS–PAGE was performed on a discontinuous buffered system according to Laemmli (Reference Laemmli1970) in 3 and 15% acrylamide for the stacking and principal gels, respectively.
Sensorial evaluation
A trained panelist group of 10 members were given different raw milk samples to evaluate their sensorial acceptability based on three attributes; colour, taste and odour. Every attribute was given a maximum mark of 10 and every evaluation was related to it based on the panelist's judgment. This evaluation was repeated three times on the same samples (without informing the panelists) on three different days and the final results were the average of the three values.
Statistical analysis
All experiments were performed in three replicates and each experiment was conducted twice. The results were expressed by the mean if the two experiments plus the standard error. Data were statistically analysed using ANOVA variance analysis through the general linear models (GLM) procedure of the statistical analysis system software (SAS version 9.1, SAS Institute, Inc., 2003). Least significant differences were used to separate means at P<0·05. Pearson correlation coefficient (r) between the bacterial counts (ENC and PSC) and some chemical variables (acidity, pH and lactose) was calculated using Microsoft Office Excel 2007.
Results and discussion
Preservative action of 11S and 7S in milk
In a preliminary experiment, the concentration 0·5% (w/v) of 11S gave a maximum inhibitory effect on total bacterial count while higher increases in concentration did not produce any significant changes (P<0·05). So this concentration (0·5%) was selected in all the subsequent experiments. This concentration is much higher than the MIC level of 11S (100 μg/ml) against many bacteria grown in Mueller Hinton broth (Sitohy et al. Reference Sitohy, Mahgoub and Osman2012). On milk agar, MIC of 11S was around 200 μg/ml against 150 μg/ml for nisin. The complex mixture of milk food system may require higher concentrations since some milk components may interact with considerable amounts of the substance reducing or partially concealing a part of its action. However, it can be concluded that the added substance can be targeting the contaminating bacteria in milk despite its complex composition.
Considerable inhibitory action on the proliferation of TVC, PSC and ENC was exerted by 11S subunit and nisin while lysozyme and 7S subunit manifested relatively low inhibitory action (Fig. 1). TVC was reduced by about 2·1 log CFU/ml after 10 d of storage at 4 °C and by 2·4 log CFU/ml after storage at 25 °C for 36 h when raw milk was supplemented with 0·5% (w/v) 11S which is very close to the action of nisin (respective reductions were 2·2 and 2·7 log CFU/ml). After 10 d storage at 4 °C, all TVC reductions were significantly different from control (P<0·05) while TVC reductions by 11S, nisin and lysozyme were not significantly different from each other (P<0·05). After 36 h storage at 25 °C, all treatments were significantly different from control and from each other (P<0·05).

Fig. 1. Total viable count (TVC), Pseudomonas spp count (PSC) and Enterobacteriaceae count (ENC) in raw bovine milk (control) as supplemented with 0·5% (w/v) 7S and 11S soy globulin and kept at 4 °C for 30 d or at 25 °C for 48 h.
The maximum magnitude of PSC reduction by 11S was 1·9 log CFU/ml at 4 °C (10 d) and 2 log CFU ml-1 at 25 °C (36 h) compared with 2·3 log CFU/ml in case of nisin under either condition. All treatments were significantly different from control and from each other at two indicated time points and temperatures (P<0·05). The contents of ENC in raw milk stored at at 4 °C for 10 d or at 25 °C for 36 h did not significantly (P<0·05) change in all treatment from control and from each other. ENC was reduced by 11S by about 4 log CFU/ml at 4 °C (10 d) and 2·4 log CFU/ml at 25 °C (36 h) compared with 4·74 log CFU/ml and 2·6 log CFU/ml, in case of nisin, respectively. Hence, the antibacterial action of 11S against these three groups of contaminating bacteria was very close to that exerted by nisin and much better than that of lysozyme while 7S showed the least effect.
The data in Fig. 2 show high anti-bacterial action of 11S against 3 artificially inoculated pathogenic bacteria (List. monocytogenes, Sal. Enteritidis and Esch. coli 0157:H7) in bovine raw milk during storage, while 7S was much less effective. This antibacterial action seems rather bacteriostatic during early periods (4 d at 4 °C and 12 h at 25 °C) and anti-proliferative during later periods of storage, The three pathogenic bacteria did not grow to any significant level (P<0·05) after 24 h at 25 °C or after 6 d at 4 °C in 11S-supplemented raw milk compared with the starting level of each bacterium. This antibacterial action was generally comparable with the action of nisin and excels that of lysozyme. It is significantly higher than nisin against Esch. coli at either temperature (4 or 25 °C) but not significantly different from nisin against List. monocytogenes (P<0·05). The bacteriostatic and anti-proliferative actions of 11S against the three pathogenic bacteria are probably exerted through targeting the negatively charged components of the bacterial cell membranes and cell walls (Sitohy et al. Reference Sitohy, Mahgoub and Osman2012) by virtue of the positive charges of 11 s leading to their disintegration and finally bacterial death (Shai, Reference Shai2002; Pan et al. Reference Pan, Shiell, Wan, Coventry, Roginski and Lee2007).

Fig. 2. Changes in the counts of pathogens (List. monocytogenes, Sal. Enteritidis and Esch. coli) inoculated into raw bovine milk (Control) as supplemented with 0·5% (w/v) 7S and 11S globulin and kept at 4 °C for 8 d or at 25 °C for 24 h.
The chemical structure of 11S fulfills the requirements of antibacterial activity, albeit the real activity may be rather focused in the basic domain of each subunit (Table 1). All the basic domains of each of the subunits (G1–G5) are endowed with both high hydrophobicity and net positive charges where G3, G4 & G5 have the highest net positive charges, +8, +10 and +15 respectively. These two parameters may participate in the antibacterial action where the basic domains of G4 and G5 may be exerting the highest antibacterial action. In conclusion, 11S can help prolong preservation of raw milk through inhibiting the bacterial growth by virtue of its hydrophobic basic domains. Lack of cationic nature from the structure of 7S may be the explanation for its limited action. The chemical structure of 11S, indicating its separation into basic and acidic domains may allow either domain to electrostatically interact with the bacterial cellular components affecting the cells integrity (Kuipers & Gruppen, Reference Kuipers and Gruppen2008). It should be apparent that the observed antimicrobial action did not originate from soybean trypsin inhibitor since pH 6·4, used for 11S isolation, is far from the acidic isoelectric point (pI 4·4) of trypsin inhibitor (Macedo et al. Reference Macedo, Matos, Machado, Marangoni and Novello2000).
Table 1. Charge and hydrophobic information of glycinin subunits

† Hydrophobic amino acid residues (L, I, F, V, M, W, C, Y &A) were counted and related to the total number of amino acids in each subunit
‡ Basic amino acid (R, K & H) residues were counted as + and acidic amino acid (D & E) residues as -. The two values were added algebraically
Milk physicochemical quality
The development of titratable acidity (Fig. 3) in control raw milk stored at 4 or 25 °C was so rapid that it reached critical acidity level (0·21–0·22% as lactic acid) after 8 d at 4 °C and 18 h at 25 °C. This was coupled with slightly reduced pH of about 6·4 (data not shown). Arrival to this level of acidity was delayed in 11S supplemented (0·5% w/v) raw milk to d 24 at 4 °C and to 36 h at 25 °C. Lower effectiveness was associated with 7S supplementation. The development of acidity was apparently at the expense of milk lactose which was mostly consumed by the contaminating bacteria in control raw milk during storage. About 33% of the original lactose level in the untreated raw milk was consumed after 8 d storage at 4 °C against only 13·5% when supplemented with 0·5% of 11S. After 24 d storage under the same cold conditions lactose consumption increased up to ca. 58% in the untreated raw milk against only 31% in the 11S-supplemented milk while the protective effect of 7S was rather slight. A similar trend was observed when raw milk was maintained at 25 °C, as prolonging the storage period to 36 h increased lactose consumption up to 67% in untreated raw milk against only 21% in the 11S-supplemented milk.

Fig. 3. Development of titratable acidity (% lactic acid) and lactose consumption in raw bovine milk during 30 d storage at 4 °C or 48 h at 25 °C, as supplemented with 0·5% (w/v) 7S and 11S globulin.
The action of 11S was similar to that previously reported for the esterified legume proteins (Sitohy et al. Reference Sitohy, Mahgoub and Osman2011) where the delay in the development of titratable acidity can be attributed to its inhibitory action on the bacterial proliferation usually associated with acid production in milk (Fox et al. Reference Fox, Power, Cogan and McKellar1989). So, 11S can be used to counteract the rapid acidity development in raw milk through its effective antibacterial action.
The increase in lactic acid production is mainly due to the fermentation of lactose by a variety of bacteria e.g. lactic acid bacteria and Enterobacteriaceae (Jay, Reference Jay2000) which includes coliforms (e.g. Esch. coli). Strong and positive correlation (r>0·7) was found between each of the two bacterial counts (ENC and PSC) and acidity of milk kept either at cold or room temperature (Table 2) either in control milk or milk supplemented with 7S or 11S. Likewise, strong negative correlation (r⩾0·9) was noticed between the two studied bacterial counts and pH or lactose level in milk kept either at cold or room temperature supporting the conclusion that most of the chemical changes originate from the activities of the contaminating spoilage bacteria.
Table 2. Correlation coefficient between the bacterial counts and chemical parameters in bovine milk as supplemented with 11S or 7S and compared with control
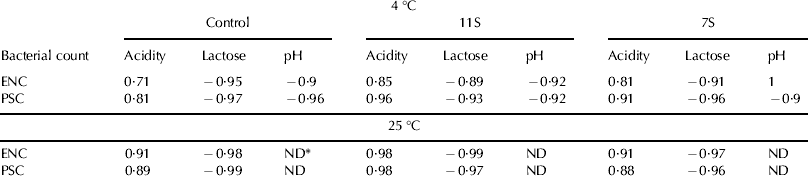
* Not determined
Supplementing raw milk with 11S could maintain caseins in their intact forms for 10 d at 4 °C and for 36 h at 25 °C instead of 2 d and 12 h as in the case of the respective controls based on the milk SDS-PAGE patterns (Fig. 4) probably due to its inhibitory action against the contaminating proteolytic bacteria. Many strains of Pseudomonas and Enterobacteriaceae were reported to have considerable extracellular proteolytic activity capable of hydrolyzing casein (Dogan & Boor, Reference Dogan and Boor2003; Morales et al. Reference Morales, Fernández-García and Nuñez2003), thus reducing their counts by 11S may explain its casein protecting action and assures longer shelf life for raw milk.

Fig. 4. Electrophoretic patterns of bovine milk (control) as supplemented with 0·5% (w/v) 7S and 11S soy globulin and kept at 4 °C for 16 d or at 25 °C for 48 h.
Heat coagulation occurred in untreated raw milk after 4 d storage at 4 °C (data not shown) but was delayed to d 8 and d 16 respectively, when supplemented with 7S or 11S. Un-supplemented raw milk stored at 4 °C was spontaneously coagulated (without heat treatment) after 24 d against 30 d in 7S-supplemented milk while no coagulation occurred in case of 11S-supplemented milk. Raw milk stored at room temperature was coagulated by heat after 18 h compared with 42 h in 11S supplemented raw milk. Spontaneous coagulation occurred in untreated raw milk and 7S-supplemented milk after 30 h and 36 h, respectively against 48 h in case of 11S-supplemented raw milk. The observed delay in heat coagulation by supplementing raw milk with 11S can be attributed to the maintained casein moieties (Fig. 4) as well as the observed attenuated acid production. Casein hydrolysis markedly affects the heat stability–pH profile of skim milk by altering the properties of the casein micelles through changing their charges (Crudden et al. Reference Crudden, Afoufa-Bastien, Fox, Brisson and Kelly2005). As supplementation with 11S could maintain casein integrity and restrict milk physicochemical changes, it should enhance heat stability. After 10 d storage at 4 °C, milk heat stability of 11S-supplemented raw milk coincided with intact form of casein as well as low level of titratable acidity (0·19%). Likewise, for milk stored at 25 °C, good heat stability maintained after 36 h, coincided with intact casein as well as low acidity level (0·23%).
Milk sensorial traits
Supplementation with 7S and 11S did not significantly affect the sensorial traits of raw milk directly after application (zero time), i.e. the expected beany flavour was not detected by any panelist. After 5 d storage at 4 °C, non-supplemented raw milk lost about 30–40% of its original sensorial traits and about 50–70% after 10 d against 10% in case of 11S-supplemented raw milk (Fig. 5). Raw milk stored at room temperature and treated likewise showed similar trend. After 36 h at room temperature, 11S-supplemented milk could maintain 90% of the sensorial traits against only 40–50% in case of the untreated raw milk. All treatments were significantly different from control and from each other (P<0·05).

Fig. 5. Sensorial quality of raw bovine milk (Control) as supplemented with 0·5% (w/v) 7S and 11S soy globulin and kept at 4 °C for 10 d or at 25 °C for 36 h. Data are expressed as means±sd Different letters indicate significance difference at P<0·05 when compared with control.
Maintenance of the sensorial properties of milk through storage at 4 or 25 °C by 11S is mainly due to its inhibitory actions against the contaminating bacteria particularly those associated with high proteolytic and acid production activities. The mechanism of the antibacterial and preservation action of 11S may be similar to that reported for the methylated legume proteins (Sitohy et al. Reference Sitohy, Mahgoub and Osman2011) as both have cationic and hydrophobic properties. Although methylated legume proteins have higher isoelectric point (pI 8) than 11S (pI 6·5), the latter contains basic subunits with a higher isoelectric point (pI 8·5) (Kuipers & Gruppen, Reference Kuipers and Gruppen2008).
Conclusions
Supplementation of raw milk with 11S can enhance its keeping quality during storage either at room temperature or cold conditions without significantly affecting its sensorial properties. This action is comparable to that of nisin and exceeds that of lysozyme. It is mainly exerted through limiting the proliferation of the contaminating bacteria restricting their associated activities; lactose fermentation, acid production and caseinolysis. 11S can be utilized as a natural preservative in raw milk enhancing its applications, prolonging its shelf life and avoiding enormous losses caused by spoilage and pathogenic bacteria.









