INTRODUCTION
In nature, there are three natural occurring carbon isotopes: 99% of carbon is 12C, 1% is 13C while 14C (radiocarbon) is present in trace amounts equal to about 1 part per trillion (0.0000000001%) compared to total carbon in the atmosphere (Libby Reference Libby1960).
At the beginning of 3rd millennium, all the raw materials used for the synthesis of plastic materials were entirely petroleum-derived and it has only been since the last 15 years that industry started to produce polymers using renewable sources of carbon, such as plants. The first unsaturated polyester resins with a bio-content of 18% appeared on the market in 2003 (Andjelkovic et al. Reference Andjelkovic, Culkin and Loza2009). Since then, large industrial efforts were dedicated to enhancing the bio-fraction, maintaining the same mechanical, physical, and thermal properties. Many biological raw materials can be restored in the short term (less than 10 years) while the materials of fossil origin are renewable in millions of years (Pais et al. Reference Pais, Franco-Duarte, Sampaiop, Carolas, Figueira, Ferreira, Poltronieri and D’Urso2016); biobased products are therefore able to contribute significantly to the emission of anthropogenic/fossil CO2 (Popa Reference Popa2018). Radiocarbon is therefore considered a direct tracer for biogenic sources and it has been used to discriminate and quantify the origin of fuels and to certify the “biobased products” (Dijs et al. Reference Dijs, van der Windt, Kaihola and van der Borg2006; Onishia et al. Reference Onishia, Ninomiya, Kunioka, Funabashi and Ohara2010; Calcagnile et al. Reference Calcagnile, Quarta, D’Elia, Ciceri and Martinotti2011; Quarta et al. Reference Quarta, Calcagnile, Giffoni, Braione and D’Elia2013; Santos et al. Reference Santos, Macario, Jou, Oliveira, Cardoso, Diaz, Anjos and Alves2019). A material is biobased if its radiocarbon content is significantly different from the same fossil-derived materials and it should ideally reflect the present atmospheric 14CO2 activity value, i.e. coeval to the generation of the biological material making up the product.
The European Community has promoted market initiatives for biobased products (such as bio-plastics, bio-lubricants, surfactants, enzymes and pharmaceuticals) as neutral products from the point of view of greenhouse gas emissions and characterized by a lower impact environment in terms of energy consumption and waste generation (European Commission 2017). To obtain significant results at global level, however, it is necessary that the market of these products is strongly promoted, and this is possible only if the cost is competitive and the origin from renewable sources and the life cycle are certified. To this end, the European Commission (2008) set up an “Ad-hoc Advisory Group for Bio-based Products” composed of representatives of government institutions, industries and universities to analyze the market conditions and the legislative situation related to these products. Therefore, technical standards have been defined for radiocarbon analysis procedures in both the USA (ASTM D6866-18) and Europe (UNI CEN/TS 16137:2011), which certify the percentage of bio carbon present in raw materials, components and finished products.
The biopolymer sector is mainly focused in the food-packaging manufacturing, thanks also to their biodegradability or compostability. Recently, the trend has been to develop new biopolymers with enhanced properties (thermal, mechanical, barrier function, etc.) or to synthesize “traditional” polymers starting from monomers from renewable resources in order to expand the biopolymer market to engineering applications (electric/electronic, constructions, etc.) and to durable materials. It has been shown that biopolymers could replace as many as 84% of plastics from oil sources (Shen et al. Reference Shen, Haufe and Patel2009).
To date, the term “biopolymers” does not yet have a precise and unambiguous definition, as it refers to both compostable plastics (certified according to EN 13432:2000 or EN 149955:2006) deriving from renewable and/or non-renewable raw materials, and to plastics from renewable raw materials (certified according to ASTM D6866-18) not necessarily biodegradable or compostable.
The biobased carbon expresses the carbon content or from a biomass and its content is measured to distinguish between fossil and biogenic materials using the 14C method (radiocarbon dating method) (Environmental Communication Guide 2012; UNI EN ISO 14067, 2018; UNI CEN/TR 15932, 2010; UNI CEN/TS 16137, 2011) adding to the analysis of carbon stable isotopes (Berto et al. Reference Berto, Rampazzo, Gion, Noventa, Ronchi, Traldi, Giorgi, Cicero and Giovanardi2017).
The aim of this work was related to the measure of modern carbon percentage by 14C method in different organic polymers for the determination of the bio content of the final plastic products used in the packaging of food.
MATERIALS AND METHODS
Sample Description
A total of seven samples corresponding to different production phases (semi-finished product: Sbio_01, Sbio_02, Sbio_04, Sbio_05 and Sbio_06; finished product: Sbio_03 and Sbio_07) and in different shapes (granules: Sbio_01 and Sbio_02; flakes: Sbio_04, Sbio_05 and Sbio_06; finished product: Sbio_03 and Sbio_07) were analyzed.
The detailed information on the chemical composition was not available, due to industrial protective rights, but the client knows the value of the biobased content value, as it was declared in the information provided by the raw material vendors. The vendors declared that the bio content was obtained from C4 plants. There is also no information about the presence or absence of carbonates in the material, which in this case would represent a fraction of carbon low in 14C.
Radiometric Method
The liquid scintillation measurement was carried out on the benzene samples that were synthetized (Polach and Stipp Reference Polach and Stipp1967) from the original sample of bioplastics. The benzene synthesis pipeline, made by boron-silicate glass, was completely set up under walk-in fume-hoods, ensuring a safe conduction of all the combustion and synthesis steps. A double chamber combustion cell has been added within the pipeline of the existing combustion system so the possibility to have pyrolysis and/or oxidation has been completely embedded in the original design of the ENEA combustion system. The procedure for radiocarbon analysis on bioplastic materials follows the “C” method provided for by ASTM 6866: the radiometric method by means of the synthesis of benzene, which includes the following steps: CO2 production and purification, synthesis of acetylene, synthesis and collection of benzene.
CO2 Production and Purification
The pretreated organic samples are placed inside a quartz barrel (organic sample combustion reactor) sealed tightly with Viton O-rings on a stainless-steel flange equipped with a safety valve set at 1.5 bar. Heating is conducted by means of externally shielded infrared irradiators, to a variable temperature in the 500–1000°C range, depending on the sample type. The combustion phase is carried out by a flux of pure oxygen gas at pressure lower than 0.8 bar and the gases are pumped continuously by a pumping system using several scroll pumps. In case of overpressure due to highly exothermic reactions or obstruction of the tubing, a safety valve will open passively, ensuring the safety of the operators. The gases produced by combustion fluxes along the tubing and bubble passing through chemical traps containing reagents for the selective removal of possible interferents:
KI/I2 for oxidation/decomposition of nitrates, phosphates and sulphates;
K2Cr2O7 + H2SO4 for complete oxidation of possible interferents and carbon monoxide;
AgNO3 in 0.1 N concentration to removal chlorine by precipitation of AgCl and related compounds;
K2Cr2O7 + H2SO4 for final oxidation of possible interferents.
The produced CO2 undergoes a further purification step by means of cryogenic traps cooled by a refrigerant mixture of dry ice and ethyl alcohol (–78°C) for the capture of humidity and liquid nitrogen (–196°C) to collect CO2 in solid phase. The purified CO2 is then directed to a collection system.
Synthesis of Acetylene
The purified CO2 from the previous step is collected in a cryogenic trap (CF1) and then is let to expand into 5-liter collection glass volumes. From this collection system, the CO2 is transferred into an austenitic super alloy reactor with low carbon content (Inconel) containing pure lithium beads (in Argon atmosphere for all the chemical synthesis of the lithium carbide (reaction intermediate for the subsequent synthesis of benzene). The reactor is then brought to the temperature of 600°C until the solid lithium is completely melted and it begins to react with gaseous CO2 for the production of lithium carbide (Li2C2) according to Equation (1):
The reaction is exothermic and it is controlled by fluxing CO2 over the melted surface of lithium gradually and slowly. At the end of the reaction between CO2 and metallic Li, pure double-distilled water is added. The lithium carbide reacts with the water producing acetylene (C2H2) and hydrogen (H2) as described by the Equation (2):
The produced acetylene is then fluxed to the purification system made by cryogenic traps for the condensation of H2O and the solidification of acetylene.
Synthesis and Collection of Benzene
From cryogenic traps, acetylene is expanded into the CF2 collection flask and progressively transferred to a Pyrex glass barrel, filled with chromium catalyst supported on silica beads, previously activated in an oven under vacuum at 350°C for 2 hr. Within this barrel, the reaction of tri-merization (Equation 3) of acetylene runs leading to the synthesis of benzene (C6H6) that remains adsorbed onto the silica beads:
After 1 hr, the catalyst beads are heated progressively under vacuum to allow desorption of the benzene. The collection of benzene takes place in the cryogenic trap consisting of a Pyrex glass ampoule cooled to the temperature of the dry ice-ethanol refrigerant mixture (–78°C).
Analytical Procedure
The measurement of 14C activity was performed using the Liquid Scintillation Counting (LSC) technique by a QuantulusTM 1220 low-background counter (Perkin Elmer, USA). The background of the instrument and the counting efficiency were determined using the CO2 derived from acid hydrolysis of Carrara marble and from the oxidative combustion of a certified reference material (IAEA C6 sucrose) as background and modern standards respectively. The measuring time per sample is about 500 min.
Sample Analysis
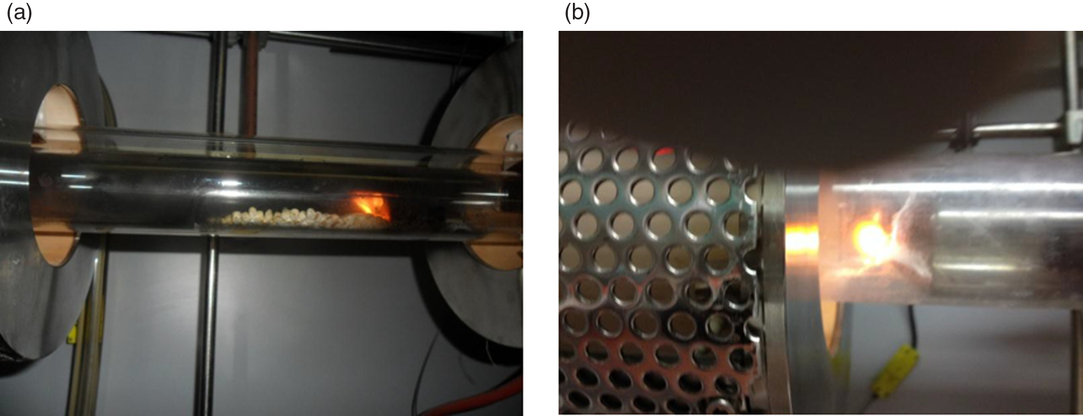
Combustion was the most critical phase of the whole process (Figure 1). The temperature was controlled to not exceed 700°C inside the sample combustion chamber (Figure 1a) to avoid the dissociation of any carbonates present in the matrix, which would affect the quantity of 14C measured at the end of the process. However, it was also necessary to guarantee the complete oxidation to CO2 of all the organic components present whether synthetically derived or natural.
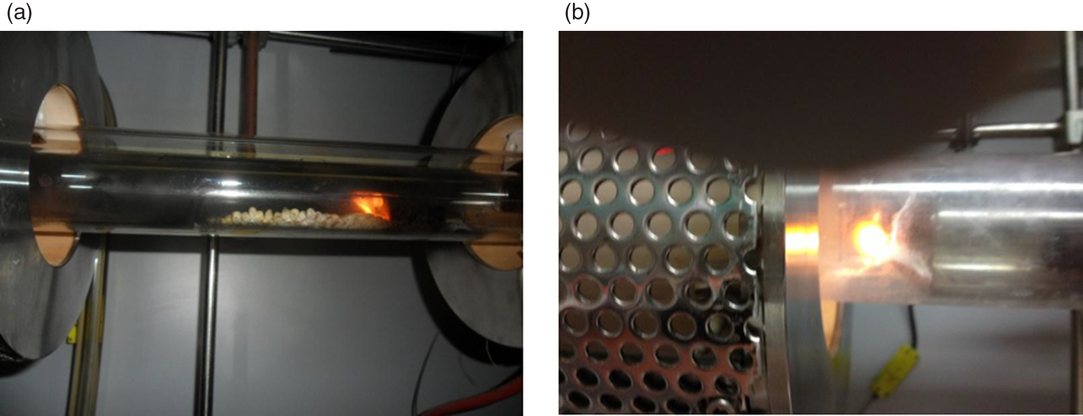
Figure 1 (a) Biopolymer combustion in oxygen flow furnace; (b) post-combustion.
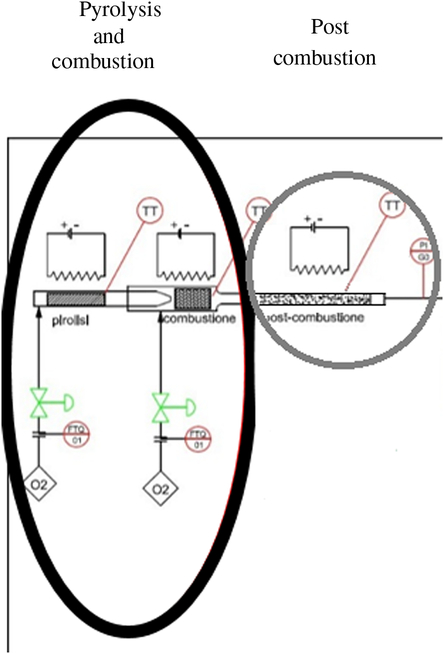
After several tests, the procedure that was found to be preferential and adequate to the polymer matrices was the introduction of a post-combustion chamber (Figure 1b) at 1000°C to guarantee a more complete oxidation of the gaseous components that otherwise would be lost in the subsequent phases of the process. The modification of the combustion system is shown in the layout of Figure 2.
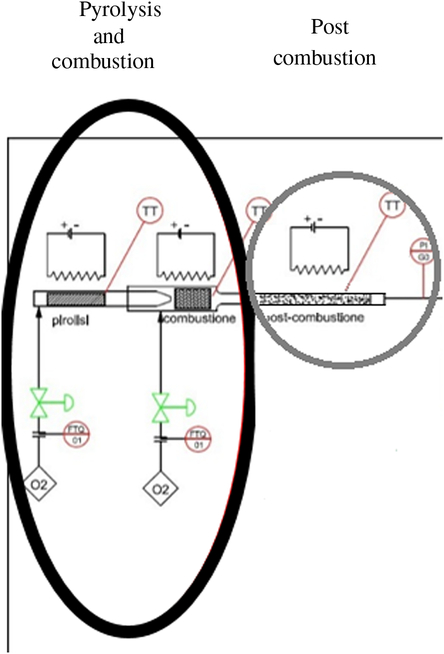
Figure 2 Scheme of the modified combustion system for the radiocarbon analysis on bioplastic materials. Double-step oxidation: pyrolysis and combustion (black circle) and the post combustion phase (gray circle).
We identify this new methodology as “double step oxidation” (pyrolysis-combustion) with two post-combustion stages. This new methodology is described below:
The sample was introduced into a first chamber where it was subjected to pyrolysis with a mixture of nitrogen and low concentration of oxygen (200 mL/min). In this way only the most volatile components were extracted which were subsequently oxidized in a post-combustion chamber at 1000°C.
Another oxidation chamber was added, consisting of a catalytic furnace containing copper oxide at 800°C.
Once the pyrolysis phase was complete, the oxygen content was increased to burn the carbonized residue.
In this way, the process was carried out in two phases introducing a redundancy that lengthens the measurement times but allows the complete oxidizing of the sample, avoiding the chemical speciation and the consequent isotopic fractionation.
pMC Calculation
Both for the dating and for the determination of the biobased content (Li et al. Reference Li, He and Chen2018), the reference value is the concentration of radiocarbon relative to 1950 AD. In the case of dating, 1950 is considered as a reference (year “zero”) while in the case of “biobased” the reference is the radiometric activity of that year which is defined as a reference standard or “modern standard”. The percent Modern Carbon (pMC) expresses the value as the concentration with modern or present defined as 1950. For biobased materials, the percentage of modern carbon (determined by the radiocarbon concentration) is compared with the actual composition of the atmospheric carbon at the time of growth of the biogenic material. It is therefore important to consider also this correction obtained by the pMC calculation, that allows to correct the value percentage of modern carbon.
δ13C Abundance
Stable isotope abundances are expressed in “delta” (δ) notation as values in ‰ (per mil), where δ = (RA/RStd−1) × 103 (‰) and RA and RStd are the ratios of the rare to abundant isotope (e.g. 13C/12C) in the sample and the standard respectively (Libes Reference Libes1992). The international standard used for 13C is Vienna Pee Dee Belemnite (VPDB). In the case of bioplastics, δ13C abundance can be used to identify the origin of the raw material (Suzuki et al. Reference Suzuki, Kobe and Nakashita2010) and to see if corn or maize or millet was used (plant C4, δ13C values ranging from –11‰ to –15‰) or other types of plants as wheat or rice (plant C3, δ13C values ranging from –24‰ to –38‰) (Martinelli et al. Reference Martinelli, Ometto and Ferraz2009; Santos et al. Reference Santos, Macario, Jou, Oliveira, Cardoso, Diaz, Anjos and Alves2019). The measurement of δ13C abundance was performed using isotope ratio mass spectrometry (IRMS). The analysis of each sample was repeated several times to have a relevant statistical information.
RESULTS
The measured data of pMC and δ13C in the biobased materials are shown in Table 1. The values obtained are in accordance with the information that the client has received form the raw materials vendors, excluding the case of Sbio_06 for which the result of the “biobased” content is lower than declared.
Table 1 pMC and δ13C percentage of the biobased materials analyzed.
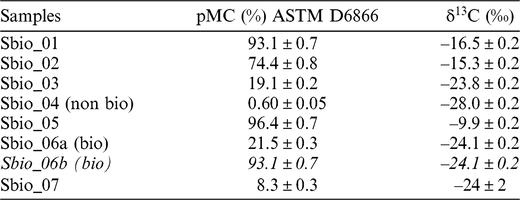
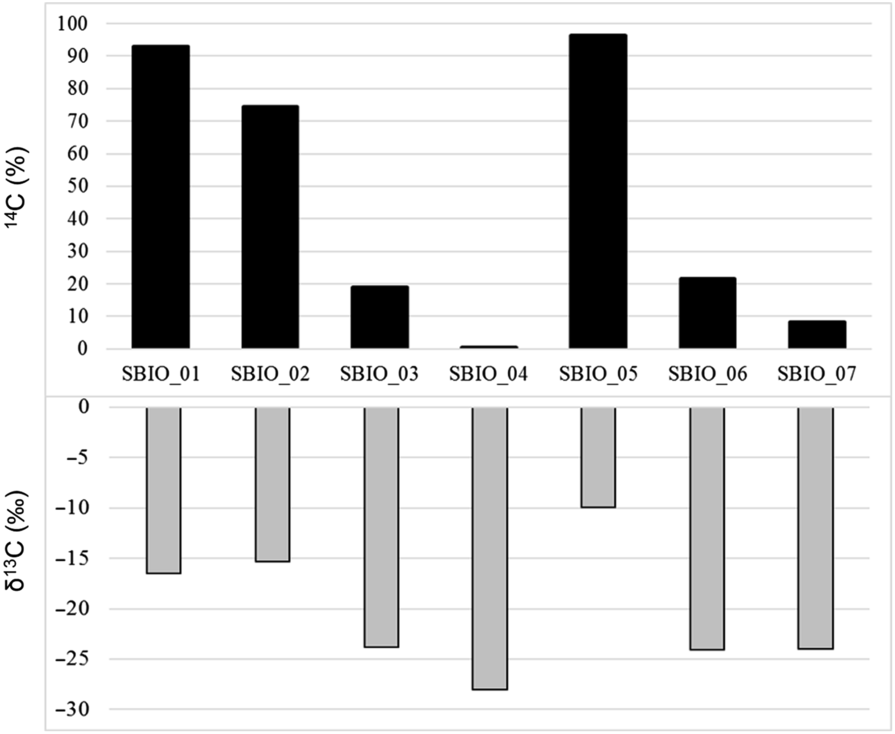
Figure 3 shows values of both pMC (activities of 14C) and δ13C (values of isotopic abundances of 13C) of the samples being analyzed and of one non-bio material (Sbio_04), as declared by the manufacturer. On vertical axis, 14C is expressed in percentage (%) and the δ13C in per mil (‰).
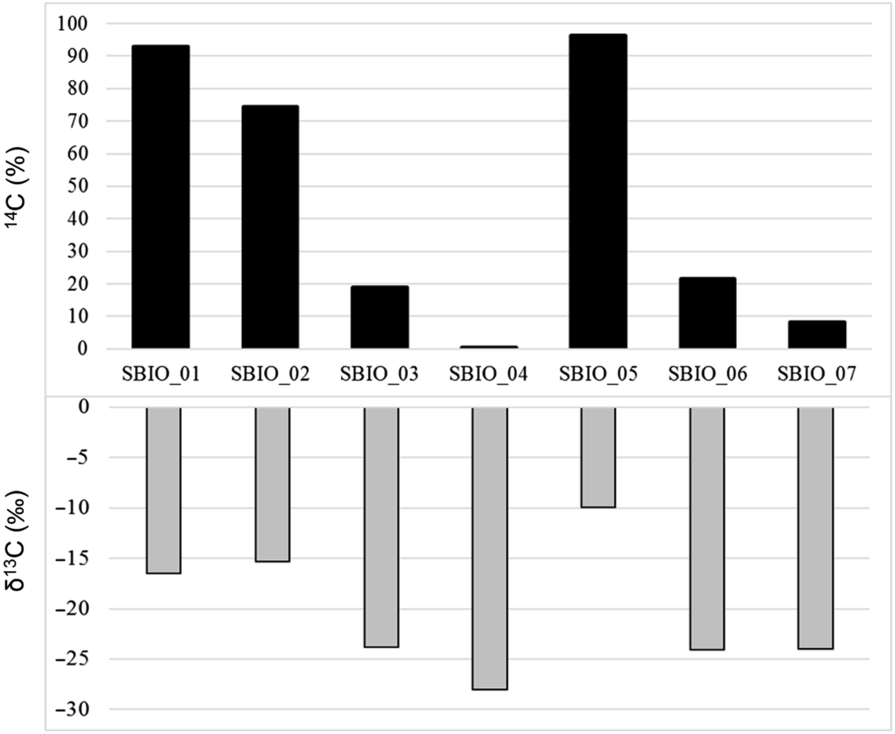
Figure 3 14C (%) and δ13C (‰) of the biobased materials analyzed, according to ASTM D6866.
For samples Sbio_01, Sbio_02 and Sbio_05 high values of 14C pMC associated with low values of δ13C indicate materials with high content of a “bio” component, as long as the bio-fraction is derived from C4 plants, as declared by the vendors. On the other side, for samples Sbio_03, Sbio_06 and Sbio_07 low values of 14C pMC associated with high values of δ13C indicated a lower content of bio component, considering the above-mentioned assumption. Sbio_04 exhibit, as expected, a very low 14C pMC and the highest value of δ13C, being very near to the value of substances of petrochemical origin.
For sample Sbio_06 that was declared by the manufacturer as a high bio content material but it showed lower “biobased” content than expected, it was noted that the CO2 yield obtained from the combustion of this sample was lower than the expected (64% compared to an average of 95% for the other samples). It was therefore decided to reprocess another aliquot of the same sample, with the double step oxidation technique, as described in the sample analysis paragraph. The results obtained are shown in Table 1, with a CO2 yield of 97%. While the δ13C value remains the same, this new methodology allows to better quantify the total 14C concentration that varies from 21.5% with one oxidation chamber to 93% with the double step oxidation chambers.
DISCUSSION
The biobased content in plastics can be investigated by 14C using a liquid scintillation technique that has been proven also as a powerful technique to assess contamination by petrochemical raw materials or carbonates that are not declared in the composition of the bio-plastics. The sample that was declared of non-biogenic origin was actually identified by the proposed analytical method, whereas the declared bioplastics were assessed against their real value of bio-content (i.e. Sbio_01 > Sbio_02). The double step oxidation technique (pyrolysis-combustion) with two post-combustion stages is a promising methodology that could be used to avoid speciation and fractionation during the combustion. The economic impact of this technique compared to the traditional one (one-step combustion) is very small but it should be considered that it is also slightly more time consuming. It should be used whereas the true composition of the sample is not known and/or there are indications of the presence of volatile components (i.e. Sbio_06). The δ13C measurements have shown their impact in addressing the traceability of the raw materials for the bio-plastics and they can provide useful information on the sustainability of the bio-driven process of fabrication. The Sbio_05 sample has exactly the value of the δ13C of the C4 plants, i.e. was produced directly using maize plant, whereas all the other bio-plastics exhibit higher δ13C, that may be due to the addition of synthetic substances (petrochemical raw materials).
CONCLUSIONS
The proposed methodology for bioplastics follows ASTM method D6866-18. The critical phase is the treatment of the sample, which involves combustion. An eventual presence of carbonates or volatile substances in the samples could significantly alter the final measurement of 14C content. The double step oxidation technique (pyrolysis-combustion) with two post-combustion stages seems promising in order to avoid fractionation and loose of volatile components of the bioplastics. The 14C pMC associated with the δ13C value can be used to assess the biobased content of the bioplastic existing on the market and could provide useful information for the traceability and the origin of the respective raw materials. It is worthwhile to consider that during the last few years, less traditional cultivars (such as corn or rice, typical C4 plants) have been used for bioplastics production, because of ethical issues, as they subtract crops from human consumption. This approach is pushing bio-plastic technology toward the use of vegetable wastes. Considering that vegetable wastes can reflect growth over several years, they reflect more the average yearly fluctuations of the radiocarbon content, causing a variability in the modern interval (106–153 pMC, depending on the growth period of the plant). This phenomenon must be evaluated and estimated for the determination of the biogenic content in cellulose or lignin-based composite biopolymers.