Introduction
Spinocerebellar ataxia type 2 (SCA2) is an autosomal dominant neurodegenerative disease caused by an expanded CAG trinucleotide repeat in the gene ATXN2 encoding the protein ataxin-2 (Pulst et al., Reference Pulst, Nechiporuk, Nechiporuk, Gispert, Chen, Lopes-Cendes and Sahba1996). It is characterized by a progressive cerebellar syndrome including ataxic gait, cerebellar dysarthria, dysmetria, dysdiadochokinesia, and other visuospatial impairments, including saccadic and voluntary eye movements (Auburger 2012; Fernandez-Ruiz et al., Reference Fernandez-Ruiz, Velásquez-Perez, Díaz, Drucker-Colín, Pérez-González, Canales and Auburger2007). Several cognitive tests have shown that people with SCA2 have deficits in various cognitive domains, including visuospatial memory and attention, as well as executive functions (Mercadillo et al., Reference Mercadillo, Galvez, Díaz, Hernández-Castillo, Campos-Romo, Boll and Fernandez-Ruiz2014). A variety of neuropathological studies have revealed an overall reduction of brain size, with significant atrophy of the cerebellum, brainstem and frontal lobe, as well as, a reduction of cerebral and cerebellar white matter (WM) (Mascalchi et al., Reference Mascalchi, Toschi, Giannelli, Ginestroni, Della Nave, Nicolai and Diciotti2015). Furthermore, a recent study found a disruption in functional connectivity in the fronto-cerebellar network (Hernandez-Castillo, Galvez, Mercadillo, Diaz, Yescas, et al., Reference Hernandez-Castillo, Galvez, Mercadillo, Diaz, Yescas, Martinez and Fernandez-Ruiz2015), and WM alterations related to the ataxia severity (Hernandez-Castillo, Galvez, Mercadillo, Diaz, Campos-Romo, & Fernandez-Ruiz, Reference Hernandez-Castillo, Galvez, Mercadillo, Diaz, Campos-Romo and Fernandez-Ruiz2015).
Advances in magnetic resonance imaging, and in diffusion tensor imaging (DTI) in particular, allow the acquisition of detailed structural images in a millimetric resolution, reflecting the tissue microstructure and integrity through the measurement of water diffusion properties. DTI has the potential to enable mapping of the white matter tract changes across aging and different neurological conditions (Smith et al., Reference Smith, Johansen-Berg, Jenkinson, Rueckert, Nichols, Miller and Behrens2007). Measuring the mean diffusivity (MD) (also referred to as apparent diffusion coefficient, ADC) has gained acceptance as a sensitive quantifiable indicator of white matter microstructural damage in neurodegenerative diseases, including SCAs (Guerrini et al., Reference Guerrini, Lolli, Ginestroni, Belli, Della Nave, Tessa and Mascalchi2004). Although several studies have analyzed WM changes using these methods in SCA2 (Mascalchi et al., Reference Mascalchi, Toschi, Giannelli, Ginestroni, Della Nave, Nicolai and Diciotti2015), there is a lack of information about the relationship between white matter integrity and cognitive deficits present in SCA2.
Here, we compared WM integrity measures in a group of 14 participants with SCA2 with a matched group of healthy participants. Statistical comparison was assessed using a voxel-wise whole-brain analysis of multi-subject diffusion tensor data. To explore the relationship between cognition and WM integrity in SCA2, the MDs of the damaged areas were then correlated with the SCA2 participants’ cognitive scores.
Methodology
Subjects
Fourteen right handed patients with a molecular diagnosis of SCA2 participated in this study (9 women). Fourteen right handed healthy volunteers with no reported history of neurological or psychiatric disorders also participated (8 women). Participants’ demographic data are presented in Table 1. (Extended demographic information of the patient’s group can be found in the Supplementary Table 1.) Ataxia severity was measured using the Scale for the Assessment and Rating of Ataxia (SARA). The SARA is comprised of eight items, including tests of gait, stance, sitting, and speech, as well as the finger-chase test, finger-nose test, fast alternating movements, and heel-shin test (Schmitz-Hübsch et al., Reference Schmitz-Hübsch, Du Montcel, Baliko, Berciano, Boesch, Depondt and Klockgether2006). All participants gave written informed consent before entering the study. All procedures in this study were conducted in accordance with the international standards of the 1964 Helsinki Declaration and its subsequent revisions. The research protocol was approved by the committee on research and ethics of the Faculty of Medicine of the Universidad Nacional Autónoma de Mexico.
Table 1 General characteristics of the participants

*Significant difference between groups were found in the years of education (t=5.5; p<.05), no significance difference were found in age (t=0.77; p=.44).
M=mean; SD=standard deviation; SARA=Scale for the Assessment and Rating of Ataxia.
Cognitive Assessment
The Cambridge Neuropsychological Test Automated Battery (CANTAB) (Sahakian and Owen, Reference Sahakian and Owen1992) is a computerized testing system used to assess cognitive performance in a variety of populations, including aging, as well as patients with neurological conditions like dementia. The CANTAB comprises a variety of executive and memory tasks administered through a touch-sensitive screen where feedback is given in a standardized manner for all participants. Taking into account that previous studies of SCA2 have reported significant cognitive deficits in spatial working memory and attention shifting (Mercadillo et al., Reference Mercadillo, Galvez, Díaz, Hernández-Castillo, Campos-Romo, Boll and Fernandez-Ruiz2014), in this study, we used the Spatial Span, and the Intra/Extra-Dimensional (IED) Shift tests.
The Spatial Span (SSP) evaluates working memory. Each trial starts with nine white boxes displayed on the screen, some of which change in color in a variable sequence. The participant must touch the boxes that changed color in the same order as they were displayed by the computer. At the beginning, the participant has to choose between two boxes, but with successive trials, the number of boxes increases up to nine. The test is terminated if the participant fails three consecutive trials. The outcome measure used for our study was the span length (the maximum span or box sequence successfully recalled).
The IED is analogous to the Wisconsin Card Sorting Test. It assesses visual discrimination, attentional maintenance, shifting and flexibility of attention. The participant starts watching two simple color-filled shapes, and must learn which one is correct by touching it. After completing this stage, a second dimension of stimuli consisting of white lines, is added to the task. Feedback informs the participant if they have chosen the correct stimuli. Stimuli shifts are initially intra-dimensional (e.g., the color filled shapes remain the only relevant dimension) but then the rules change and they become extra-dimensional (e.g., the lines become the only relevant dimension). Participants have to perform six consecutive correct responses before advancing to the next stage. The measure considered for our study was the number of errors during the test.
Statistical Analysis
Statistical analyses were performed using SPSS 17.0 software (IBM corporation). Since demographic information, disease characteristics, and scores for the cognitive test are variable in SCA2 and control participants, we used the Shapiro-Wilk test to verify that the data is normally distributed. Only the scores for the SARA ratings and CAG repetitions for the SCA2 participants presented a p>.05, indicating a normal distribution. A Mann-Whitney U test was used to compare the SCA2 and control groups’ scores for the CANTAB tasks. The Spearman’s coefficient was used to correlate the CAG repetitions with disease progression and scores of the CANTAB tests. Spearman’s coefficient was also used to analyze the correlations between the SARA ratings and the SCA2 participant’s age, the time of disease evolution, and the scores for the CANTAB test.
Image Acquisition
Images were acquired using a 3 Tesla Discovery MR750 General Electric (GE Medical Systems, Waukesha, WI) scanner with a 32-channel head coil. The study included the acquisition of a high resolution T1 three-dimensional (3D) volume and DTI. The T1 3D acquisition consisted of a T1 fast field-echo sequence, with repetition time/echo time (TR/TE)=8/3.7 ms, field of view (FOV) 256×256 mm2 and an acquisition and reconstruction matrix of 256×256, resulting in an isometric resolution of 1×1×1 mm3. The DTI sequences consisted of single shot echo planar Imaging sequences, acquiring 33 volumes of 70 axial slices (2 mm slice thickness and no separation), one for each of the 32 independent directions of diffusion with b=800 s/mm2 and one corresponding to b=0 s/mm2, TR/TE=8467/60 ms, FOV 256×256 mm2 and an acquisition and reconstruction matrix of 128×128, resulting in an isometric resolution of 2×2×2 mm3.
Diffusion Tensor Analysis
The DTI images were processed using FSL’s Diffusion Toolbox (Smith et al., Reference Smith, Johansen-Berg, Jenkinson, Rueckert, Nichols, Miller and Behrens2007). Eddy current effects were corrected and the diffusion tensor model was adjusted to generate the mean diffusivity and fractional anisotropy images for each participant. The statistical analysis was done in a voxel-wise manner using the tract-based spatial statistics (TBSS) methodology (Smith et al., Reference Smith, Johansen-Berg, Jenkinson, Rueckert, Nichols, Miller and Behrens2007). TBSS was done using the following steps: identification of a common registration target and alignment of all participants’ fractional anisotropy (FA) images to this target [Montreal Neurological Institute (MNI) template]; creation of the mean of all aligned FA images and of a thresholded skeletonized mean FA image (threshold=0.2); and projection of each participant’s FA image onto the skeleton and voxel-wise statistical analysis across participant on the skeleton-space FA data.
Using the same nonlinear registration, skeleton and skeleton projection vectors derived from the FA analysis, MD data were projected onto the skeleton before voxel-wise statistical analysis across participants (Smith et al., Reference Smith, Johansen-Berg, Jenkinson, Rueckert, Nichols, Miller and Behrens2007). The two-sample t test between SCA2 and control group was performed using FSL’s randomize function, controlling the effect of the age. Correction for multiple comparisons was assessed using randomize Threshold-Free Cluster Enhancement. Only those voxels surviving the familywise error correction (FWE) at a p value <.05 were considered as showing a significant difference between groups. The final parametric maps were parcellated, binarized, and labeled using both the white matter atlas made at Johns Hopkins University (Wakana, Jiang, Nagae-Poetscher, van Zijl, & Mori, Reference Wakana, Jiang, Nagae-Poetscher, Van Zijl and Mori2004) and the automated anatomical labeling atlas (Tzourio-Mazoyer et al., Reference Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard, Delcroix and Joliot2002). They were then used as masks for further analysis.
Correlation Analysis
The normalized and skeletonized MD images were then loaded in MATLAB R2014a (The Mathworks, Inc., Natick, MA), and using the parcellation masks, the individual MD values were extracted. For each white matter region, the mean MD value of non-zero voxels was calculated. Kendall’s correlation between cognitive scores and diffusion measurements were calculated controlling the effect of the years of education. Significant correlations were set at the p value <.05 after correcting for multiple comparisons using the false discovery rate method.
Results
Behavioral Results
Analysis of the CANTAB scores showed that participants with SCA2 made more incorrect responses in the IED (U(28)=−3.28; p≤.001) and had a shorter span in the SSP (U(28)=−3.97; p≤.001). Also, participants with SCA2 took more time to choose the correct response in the IED (U(28)=−3.83; p≤.001) and in the SSP (U(28)=−2.71; p=.007) (see Table 2). Regarding the clinical characteristics of the SCA2 group, Spearman’s rank correlation analyses did not show any significant correlation between the CAG repetitions and any behavioral or cognitive test. However, positive correlations were observed between age and the latency for the Spatial Span task (r(14)=0.592; p=.010), and between the disease duration and the latency in each of the two CANTAB tasks: IED (r(14)=0.611; p=.016), and SSP (r(14)=0.679; p=.005) and between the SARA score and the latency of the two CANTAB tasks: IED (r(14)=0.834; p=.002) and SSP (r(14)=0.541; p=.04).
Table 2 Statistical comparison between patients and control groups for CANTAB scores

*Represents significant statistical differences between patients and controls when applying the nonparametric Mann–Whitney U test at p≤.001.
IED Errors=number of incorrect responses when executing trials in the Intra/Extra Dimensional Shift task; IED Latency (ms)=reaction time in seconds to respond to the trials in the Intra/Extra Dimensional Shift; SSP Span=last passed span for the Spatial Span task; SSP latency (ms)=time in seconds to choose the correct stimuli until the last passed span.
SCA2 WM Deterioration
TBSS group comparison revealed significant MD increases in participants with SCA2compared to controls in cerebellar WM, including the medial lemniscus, the superior/middle cerebellar peduncles, the bilateral anterior corona radiata, the posterior limb of the internal capsule, the pontine crossing tract and the bilateral posterior thalamic radiation, bilateral parahippocampal WM, corticostpinal tract, superior fronto-occipital fasciculus, supramarginal WM, and inferior frontal WM (Supplementary Figure S1).
Cognitive Correlations of WM Integrity
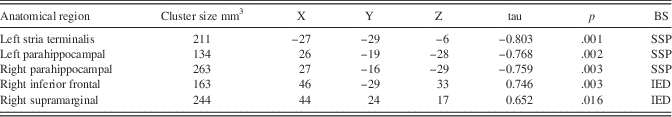
IED and SSP test scores showed significant correlations with diffusion measurements taken from WM tracts with abnormal MD in the SCA2 group. Detailed information can be found in Table 3 and Figure 1.

Fig. 1 Significant correlations between abnormal diffusivity measurements and cognitive scores. (a) WM regions showing a significant correlation with the maximum span in the SSP test, (b) WM regions showing a significant correlation with the number of errors in the IED test. LPH=left parahippocampal WM; LST=left stria Terminalis; RPH=right parahippocampal WM; RIF=right inferior frontal gyrus WM; RSM=right supramarginal gyrus WM.
Table 3 Location of degenerated white matter tracts showing significant correlation with Behavioral scores

Note. Coordinates represent the cluster maxima in MNI space in mm.
BS=behavioral score; IED=number of errors in Intra/Extra Dimensional shift; SSP=maximum span in the Spatial Span Task.
Discussion
Here, we found significant differences in mean diffusivity of participants with SCA2 compared with matched controls. Notably, our analysis also found significant correlations between specific WM changes and the deterioration in the spatial span, and set shifting capacities of the participants with SCA2.
SCA2 WM Deterioration
The significant changes in the MD measures of participants with SCA2 are consistent with previous reports that have shown increased MD in the corticospinal tract, transverse pontine fibers, superior/middle cerebellar peduncles, and cerebellar WM (Mascalchi et al., Reference Mascalchi, Toschi, Giannelli, Ginestroni, Della Nave, Nicolai and Diciotti2015). However, our analyses also showed significant diffusivity increases in other areas, such as the inferior cerebellar peduncle, the anterior corona radiata, and the supramarginal, inferior frontal, and parahippocampal WM. These novel findings could be the result of the inclusion of a larger group of participants, as well as a different DTI acquisition.
Correlations between WM Deterioration and Cognitive Impairment in SCA2.
Specific WM regions showed significant correlations between MD and the cognitive scores in the SCA2 group. A highly significant correlation was found between spatial working memory and the MD of the bilateral parahippocampal WM. Previous studies have reported atrophy in the parahippocampal area, gray matter (Mercadillo et al., Reference Mercadillo, Galvez, Díaz, Hernández-Castillo, Campos-Romo, Boll and Fernandez-Ruiz2014), glucose metabolism deficits (Wang, Liu, Yang, & Soong, Reference Wang, Liu, Yang and Soong2007), and functional connectivity abnormalities (Hernandez-Castillo, Galvez, Mercadillo, Diaz, Yescas, et al., Reference Hernandez-Castillo, Galvez, Mercadillo, Diaz, Yescas, Martinez and Fernandez-Ruiz2015) as a result of the SCA 2 mutation. Since the parahippocampal gyrus has been related to visuospatial processing and episodic learning and memory (Aminoff, Kveraga, & Bar, Reference Aminoff, Kveraga and Bar2013), it is possible that WM deterioration of this area could be impacting these cognitive abilities in the participants with SCA2. We believe these findings support further research in the possible role of the parahippocampal gyrus in the cognitive deterioration of SCA2, and maybe even in other SCAs showing atrophy in this region, including SCA3, SCA6 (Wang et al., Reference Wang, Liu, Yang and Soong2007), SCA7 (Hernandez-Castillo, Galvez, Diaz, & Fernandez-Ruiz, Reference Hernandez-Castillo, Galvez, Diaz and Fernandez-Ruiz2015), and SCA17 (Reetz et al., Reference Reetz, Lencer, Hagenah, Gaser, Tadic, Walter and Binkofski2010).
Similarly, we found a correlation between the MD of the stria terminalis and the SSP score. The stria terminalis is a group of fibers that connect the amygdala with other brain areas including the hippocampus, enthorinal cortex gyrus, and caudate nucleus. Lesions of the stria terminalis tracts block the effects of electrical stimulation of the amygdala on memory for inhibitory avoidance training, resulting in deficits of memory storage (McGaugh, Reference McGaugh2002). This suggests that the degeneration of the stria terminals in SCA2 could also represent an important component of the cognitive pathology of this disease.
The IED test, which was designed to measure rule acquisition and reversal, is primarily sensitive to changes in the fronto-striatal areas of the brain (Sahakian & Owen, Reference Sahakian and Owen1992). Our results concur with this concept, since we found a significant correlation between the MD in the pars triangularis of the inferior frontal gyrus and the number of errors in the IED test. Converging evidence suggests the right frontal cortex might subserve inhibitory processes underlying switching (Aron, Robbins, & Poldrack, Reference Aron, Robbins and Poldrack2004). Specifically, neuroimaging studies using the Wisconsin sorting card test (analogous to IED), reversal learning and task-set switching have reported activation of the right inferior frontal gyrus (Cools, Clark, Owen, & Robbins, Reference Cools, Clark, Owen and Robbins2002).
This brain region is also affected in other SCAs such as SCA6 (Wang et al., Reference Wang, Liu, Yang and Soong2007), SCA7 (Hernandez-Castillo, Galvez, Morgado-Valle, & Fernandez-Ruiz, Reference Hernandez-Castillo, Galvez, Morgado-Valle and Fernandez-Ruiz2014) and SCA17 (Reetz et al., Reference Reetz, Lencer, Hagenah, Gaser, Tadic, Walter and Binkofski2010). Another region showing a significant correlation between WM degeneration and the number of errors in the IED test was the supramarginal gyrus. This area, which is functionally related to the prefrontal cortex, seems to participate in a distributed network for multiple cognitive tasks, such as rule selection and cognitive set shifting (Konishi et al., Reference Konishi, Nakajima, Uchida, Kameyama, Nakahara, Sekihara and Miyashita1998), both of which are critical to the correct IED test performance. Furthermore, it has been suggested that the inferior prefrontal cortex and the supramarginal gyrus are capable of adapting their function according to the demands of both rule-selection and action-selection (Rowe, Hughes, Eckstein, & Owen, Reference Rowe, Hughes, Eckstein and Owen2008). Therefore, the degeneration of the white matter tracts in the supramarginal gyrus could represents an alteration in this network that may result in the set-shifting deficits observed in people with SCA2.
We also found correlations between the latencies to respond on the two CANTAB tasks and the cerebellar peduncles and pontine crossing tract MDs. However, the correlation between these cerebellar MDs and the behavioral scores could be more related to the ataxia severity than to the cognitive deficit, because these latencies also show significant correlations with age, age at onset, and SARA score. These findings support previous results showing a significant correlation between MD in the cerebellum and the SARA score (Hernandez-Castillo, Galvez, Mercadillo, Diaz, Campos-Romo, et al., Reference Hernandez-Castillo, Galvez, Mercadillo, Diaz, Campos-Romo and Fernandez-Ruiz2015).
Conclusion
Our study shows significant correlations between microstructural integrity changes in specific WM tracts and cognitive deficits in working memory and attention flexibility in a group of SCA2 participants. The parahippocampal WM degeneration showed a close relationship with the working memory deterioration in this group. The inferior frontal and the supramarginal WM deterioration correlated with detrimental changes in flexibility of attention, suggesting a possible disruption in the information flow between cognitive-integration areas. These findings contribute to a better understanding of the neural basis of the cognitive impairment presented by people with SCA2.
Acknowledgments
All authors declare that they do not have a conflict of interest. This work was supported in part by Universidad Nacional Autonoma de Mexico (J.F.-R., grant number UNAM-DGAPA-PAPIIT IN221413); Consejo Nacional de Ciencia y Tecnologia (J.F.-R., grant number 220871); and the National Ataxia Foundation (USA) postdoctoral award to CRHC.
Supplementary Materials
To view Supplementary material for this article, please visit http://dx.doi.org/10.1017/S1355617716000084