Introduction
Demodex that reside on humans can be divided into two species Demodex folliculorum and Demodex brevis. Demodex folliculorum, which is approximately 0.3–0.4 mm long, has a longer opisthosoma and usually resides in the hair follicles, and D. brevis, which is approximately 0.2–0.3 mm long, has a shorter opisthosoma and usually resides in sebaceous and meibomian glands (Cheng et al., Reference Cheng, Sheha and Tseng2015). They are mainly found on the forehead, cheek, nose and nasolabial folds. The immune system of the host seems to tolerate Demodex without inflammation as a commensal when the numbers of Demodex are low. However, when the number of Demodex increases, Demodex might cause dermatological or ophthalmological diseases, especially rosacea and blepharitis (Chang and Huang, Reference Chang and Huang2017; Sędzikowska et al., Reference Sędzikowska, Osęka and Skopiński2018).
Demodex infection can occur in some clinical settings, especially in the status of immunodeficiency and the use of immunosuppressant (Yamaoka et al., Reference Yamaoka, Murota, Tani and Katayama2014; Chovatiya and Colegio, Reference Chovatiya and Colegio2016; Arli et al., Reference Arli, Ozsan, Gurkan, Kaya and Kokacya2019). The cytokine tumour necrosis factor-α (TNF-α) plays crucial roles in the pathogenic mechanism and development of ankylosing spondylitis (AS) and TNF-α inhibitors have been recommended as a first-line treatment for AS (Ward et al., Reference Ward, Deodhar, Gensler, Dubreuil, Yu, Khan, Haroon, Borenstein, Wang, Biehl, Fang, Louie, Majithia, Ng, Bigham, Pianin, Shah, Sullivan, Turgunbaev, Oristaglio, Turner, Maksymowych and Caplan2019). However, whether the use of TNF-α inhibitors can cause changes in the Demodex density and dermatological diseases in AS remains unknown. The aim of this study was to investigate changes in the Demodex density by standardized skin surface biopsy (SSSB) before and after therapy with the TNF-α inhibitor adalimumab in patients with AS.
Materials and methods
Study population
Seventy-three patients with definitive AS were enrolled in this study. The diagnosis of AS was established by the Modified New York Criteria (van der Linden et al., Reference van der Linden, Valkenburg and Cats1984). All the patients were initially diagnosed and did not receive specific treatment before this study. Patients who were pregnant; had a history of heart failure, dermatological diseases, neoplasm, diabetes mellitus, infection or tuberculosis; and those using immunosuppressive drugs were excluded.
Therapy
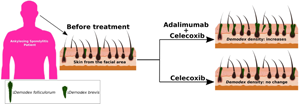
All the patients with AS were classified into two subtype groups (Group A and Group B) according to whether the patients accepted adalimumab treatment due to their willingness and financial situation. Forty patients in Group A were treated with adalimumab 40 mg once every two weeks subcutaneously and celecoxib 200 mg twice each day orally. Thirty-three patients in Group B were only administered 200 mg celecoxib twice each day orally.
Sample collection
The method of SSSB (Aşkin and Seçkin, Reference Aşkin and Seçkin2010) was used to collect Demodex samples from patients at the Department of Rheumatology of the Fifth Affiliated Hospital of Sun Yat-Sen University from April 2020 to February 2021. Five standardized skin surface biopsies were taken from the cheeks, forehead, nose and chin of the patients at the initiation and after three-month adalimumab treatment. For SSSB, a drop of cyanoacrylate adhesive (Aron Alpha Glue, Loctite Corporation, Yantai, China) was placed on a 1 cm2 marked area of a microscope slide. The adhesive-bearing surface was pressed over the skin for 1 min, the slide was gently removed from the skin, covered with 2–3 drops of glycerin and then covered with a coverslip. The slides were assessed for parasites by light microscopy at a magnification of ×10. The average value of five sites was considered the Demodex count. Demodicosis was defined as the identification of five or more Demodex mites in a 1 cm2 area (Baima and Sticherling, Reference Baima and Sticherling2002).
Clinical variables
All demographic data were gathered from the medical records of the patients. Clinical characteristics, including duration, smoking, body mass index (BMI), human leucocyte antigen (HLA)-B27, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), were also collected.
Statistical analysis
The results are described as the mean ± standard deviation (s.d.). The Kolmogorov–Smirnov test was used to determine whether continuous variables were normally distributed. Normally distributed variables were compared using independent and randomized paired t-tests. Non-normally distributed variables were compared using Wilcoxon matched-pairs signed-ranks sum test. Categorical variables were assessed by the χ 2 test. Pearson's rank correlation coefficient was used to examine the relationships between two continuous variables. A P value <0.05 was considered to indicate statistical significance. Statistical analysis was carried out using Statistical Package of Social Science (SPSS) version 24.0.
Results
Seventy-three patients with a definitive diagnosis of AS were enrolled. The Demodex test was performed in all AS patients (Fig. 1). At least one Demodex mite was detected in 69 (94.52%) out of 73 AS patients. None of the patients had demodicosis before and after treatment. Dermatological symptoms did not occur in patients with AS after adalimumab treatment.

Fig. 1. A Demodex is observed by light microscopy at a magnification of ×40.
Correlation analysis between the Demodex density and clinical characteristics in AS patients
Demodex mites were detected in 96.15% of the male patients (50/52) and 90.48% of the female patients (19/21), but there was no significant difference in terms of sex (P = 0.691). Three of the sixty-five patients (3/65) with positive HLA-B27 and only one of the eight patients (1/8) with negative HLA-B27 had Demodex mites. The incidence of Demodex mite detection was 94.12% (16/17) in patients who smoked and 94.64% (53/56) in patients who did not smoke. No significant difference was found in terms of HLA-B27 and smoking (P = 0.378, P = 1.000, respectively). Table 1 also shows that Demodex density was positively correlated with both age and CRP (P = 0.021, P = 0.019, respectively) but was not correlated with BMI, ESR or duration (P = 0.433, P = 0.244, P = 0.064, respectively).
Table 1. Correlation analysis between Demodex density and clinical characteristics in AS patients

AS, ankylosing spondylitis; HLA, human leucocyte antigen; BMI, body mass index; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
a χ 2 test.
b Fisher's test.
* Statistically significant (P < 0.05).
Comparison of clinical characteristics and the Demodex density between Groups A and B before treatment
Among the AS patients, 40 and 33 patients were placed in Groups A and B, respectively. Clinical characteristics and the Demodex density are shown in Table 2. The male sex rates were 75.00% (30/40) and 66.67% (22/33) in Groups A and B, respectively. The age and symptom duration were 36.08 ± 10.59 and 9.58 ± 7.22 years in Group A and 35.24 ± 11.87 and 7.28 ± 7.18 years in Group B, respectively. There was no significant difference in terms of sex, age or duration between Groups A and B (P = 0.434, P = 0.753, P = 0.179, respectively). Table 2 also shows that there were no significant differences between the two groups regarding the Demodex presence rate and Demodex density before treatment.
Table 2. Comparison of clinical characteristics and Demodex density between Groups A and B before treatment in AS patients

AS, ankylosing spondylitis; HLA, human leucocyte antigen; BMI, body mass index; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
a χ 2 test.
b Fisher's test.
Comparison of the Demodex density before and after treatment
The change in the Demodex density before and after treatment is shown in Fig. 2. Among the patients in Group A, the Demodex densities were 0.44 ± 0.26 and 0.58 ± 0.38 before and after treatment, respectively. The Demodex density increased after adalimumab treatment in Group A (P < 0.001). The Demodex densities before and after treatment were 0.41 ± 0.23 and 0.44 ± 0.28 in Group B, respectively. However, there was no significant difference before and after adalimumab therapy in Group B.

Fig. 2. Increase of the Demodex density after adalimumab treatment in Group A but no change of the Demodex density in Group B.
Discussion
In this study, it has been found that the Demodex density increased in AS patients after receiving adalimumab therapy. Additionally, Demodex density was positively correlated with age and CRP level in AS patients.
Demodex positivity was reported in asymptomatic healthy individuals but at low densities. Using SSSB, a prevalence of approximately 40–50% has been consistently reported in adults aged approximately 50 (Chang and Huang, Reference Chang and Huang2017). Using PCR, a population sample tested positive for Demodex in 100% of adults over 18 (Thoemmes et al., Reference Thoemmes, Fergus, Urban, Trautwein and Dunn2014). In this study, the presence of Demodex was 94.52% (69/73) in patients with AS. Furthermore, there was no significant difference in Demodex presence in regard to the sex of AS patients, and a positive correlation was found between the Demodex density and age. Similar results were also reported: sex had no impact on Demodex infection, but age had a positive correlation with the risk of Demodex infection (Sędzikowska et al., Reference Sędzikowska, Osęka and Skopiński2018; Arli et al., Reference Arli, Ozsan, Gurkan, Kaya and Kokacya2019). In addition, it was also found that there was a positive correlation between the Demodex density and CRP level. The host immune system seems to tolerate Demodex without inflammation as a commensal when they are present at low numbers and it appears that they stimulate inflammation when their number increases (Foley et al., Reference Foley, Kelly, Gatault and Powell2021). The elevated CRP level might be attributed to the increase in the Demodex density.
Demodicosis can occur in some clinical settings. Diabetes mellitus, metabolic syndrome and immunodeficiency resulting from active infection with HIV generate an environment that supports Demodex infestations (Annam et al., Reference Annam, Yelikar, Inamadar, Palit and Arathi2010; Keskin et al., Reference Keskin Kurt, Aycan Kaya, Karateke, Benk Silfeler, Soylu Karapınar, Neslin Akkoca and Hakverdi2014; Yamaoka et al., Reference Yamaoka, Murota, Tani and Katayama2014; Arli et al., Reference Arli, Ozsan, Gurkan, Kaya and Kokacya2019; Toka et al., Reference Toka Özer, Akyürek and Durmaz2020). However, there was no correlation between BMI and the Demodex density in this study, which was not in accordance with previous studies (Arli et al., Reference Arli, Ozsan, Gurkan, Kaya and Kokacya2019; Toka et al., Reference Toka Özer, Akyürek and Durmaz2020). Because all the samples of such studies were small, so larger samples should be needed to investigate the real relationship. Demodicosis has also been reported in transplant recipients receiving long-term regimens of immunosuppressants (Chovatiya and Colegio, Reference Chovatiya and Colegio2016) and in cancer patients receiving targeted treatment with EGFR blockers (Chon and Hassler, Reference Chon and Hassler2016). The cytokine TNF-α plays crucial roles in the innate and adaptive immune response, but it also participates in the pathogenic mechanism and development of AS. TNF-α inhibitors have been recommended as the first-line treatment for AS (Ward et al., Reference Ward, Deodhar, Gensler, Dubreuil, Yu, Khan, Haroon, Borenstein, Wang, Biehl, Fang, Louie, Majithia, Ng, Bigham, Pianin, Shah, Sullivan, Turgunbaev, Oristaglio, Turner, Maksymowych and Caplan2019). This study revealed that the Demodex density in patients with AS increased after adalimumab treatment. Immunological studies showed that the Demodex mites were able to modulate the inflammatory response. As a survival mechanism, Demodex mites may suppress the adaptive immune system by downregulating T-cell levels (Akilov and Mumcuoglu, Reference Akilov and Mumcuoglu2004) or by blocking molecules required for an effective antiparasitic Th2 immune response (Liu et al., Reference Liu, Arseculeratne, Liu, Whitmire, Grusby, Finkelman, Darling, Cheever, Swearengen, Urban and Gause2004). Low Demodex mite numbers may also downregulate the host immune TLR signalling pathway to facilitate their survival (Lacey et al., Reference Lacey, Russell-Hallinan, Zouboulis and Powell2018). Moreover, analysis of skin-homing CD4 + T-cell subsets suggested that Demodex infestation induced an increase in the regulatory T-cell subpopulation compared to the Demodex-negative donors (Gazi et al., Reference Gazi, Gureser, Oztekin, Karasartova, Kosar-Acar, Derici, Artuz, Mumcuoglu and Taylan-Ozkan2019). So we speculate that the suppression of TNF-α might create a better environment that promotes the survival of Demodex and the increase in the Demodex density. However, no clinical symptoms or diseases related to Demodex were observed in this study.
There are still limitations to this study. First, though the increase of Demodex density is statistically significant, patients with AS receiving adalimumab treatment did not present with clinical manifestation caused by infestation of Demodex. Thus, patients need to be observed for a longer time to confirm whether adalimumab therapy can cause dermatological or ophthalmological diseases in AS. Second, though Demodex density was positively correlated with both age and CRP level, the correlations were still weak. Moreover, the sample size of this study is relatively small. The change in the Demodex density resulting from adalimumab therapy in AS should be confirmed in larger cohorts.
In conclusion, the changes in the Demodex density before and after adalimumab therapy were observed in AS patients. These results provide important implications for patients with AS and their treating physicians, and also call for the awareness of long-term complication during the adalimumab therapy for AS patients. Future studies should focus on whether early intervention of Demodex infestation could reduce the risk of Demodex-related diseases.
Acknowledgements
The authors thank the staff of the Department of Rheumatology, the Fifth Affiliated Hospital of Sun Yat-sen University for providing the necessary facilities.
Author contributions
All the authors contributed to this work. Xuegang Li and Nelson Siu Kei Lam conceived and designed the study. Xuegang Li and Li Yang took samples and conducted data gathering. Anqi Liang and Xin Xin Long performed statistical analyses. Junli Guo and Shuping Zhong wrote the article.
Financial support
This research received no specific grant from any finding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
Before this study, patients were informed of the clinical requirements and possible risks of all operations and signed informed consent forms were obtained. This study conformed to the Declaration of Helsinki and was approved by the Ethics Committee of the Fifth Affiliated Hospital of Sun Yat-Sen University (Approval number: K48-1).