1. Introduction
Soft-bodied fossil biota (Konservat-Lagerstätten) such as the famous Chengjiang (Cambrian Series 2, Stage 3) and Burgess Shale (Miaolingian, Wuliuan) offer an unrivalled opportunity to study assemblages of animals at snapshots in time. Konservat-Lagerstätten are not distributed evenly through time, but instead are most common in the Cambrian Series 2 and Miaolingian (Allison & Briggs Reference Allison and Briggs1993; Gaines, Reference Gaines2014). The Kinzers Formation (Pennsylvania, USA) has been recognized as a Konservat-Lagerstätten for nearly 100 years, since the discovery of antennae in Olenellus getzi (Dunbar, Reference Dunbar1925) and the subsequent description of soft-bodied animals (e.g. Resser, Reference Resser1929; Resser & Howell, Reference Resser and Howell1938). The exceptionally preserved fauna is confined to the Dyeran (Cambrian Series 2, Stage 4) Fine Pelitic Facies of the Emigsville Member (Skinner, Reference Skinner2005). Preservation at different localities varies in iron content, which sometimes overlays and obscures parts of the fossils, owing to differences in the concentration of algae and cyanobacteria of the original sediment (Skinner, Reference Skinner2005). The currently known soft-bodied fauna of the Kinzers Formation includes the worms Selkirkia (Conway Morris, Reference Conway Morris1977), Kinzeria crinita and Atalotaenia adela (Garcia-Bellido & Conway Morris, Reference García-Bellido and Conway Morris1999), the sponge Hazelia walcotti (Rigby, Reference Rigby1987), arthropods such as Serracaris lineata (Briggs, Reference Briggs1978), Isoxys (Campbell & Kauffman Reference Campbell and Kauffman1969), Sidneyia (Campbell & Kauffman Reference Campbell and Kauffman1969), Protocaris (Campbell & Kauffman Reference Campbell and Kauffman1969) and Tuzoia (Resser, Reference Resser1929; Campbell & Kauffman, Reference Campbell and Kauffman1969; Vannier et al. Reference Vannier, Caron, Yuan, Briggs, Collins, Zhao and Zhu2007), and stem-group euarthropod radiodonts (Resser, Reference Resser1929; Briggs, Reference Briggs1979), which are the focus of this study.
Since the original description of radiodont material from the Kinzers Formation, including Anomalocaris pennsylvanica (Resser, Reference Resser1929), and subsequent redescription of material following the recognition that Anomalocaris was the appendage of a euarthropod and not the body of a shrimp (Briggs, Reference Briggs1979), the number of known species, genera and families of Radiodonta described from other localities has increased substantially. Complete Anomalocaris body fossils were discovered and described (Whittington & Briggs, Reference Whittington and Briggs1985), Radiodonta were shown to be stem-group euarthropods (Daley et al. Reference Daley, Budd, Caron, Edgecombe and Collins2009) and what began as three species described from isolated appendages (Anomalocaris canadensis, A. pennsylvanica and ‘appendage F’ – Briggs, Reference Briggs1979) has exploded into a diverse group of apex-predators, many known from complete specimens (Whittington & Briggs, Reference Whittington and Briggs1985; Chen, Ramsköld & Zhou, Reference Chen, Ramsköld and Zhou1994; Hou, Bergström & Ahlberg, Reference Hou, Bergström and Ahlberg1995; Collins, Reference Collins1996; Daley et al. Reference Daley, Budd, Caron, Edgecombe and Collins2009), with diverse feeding strategies representing over ten genera (Daley et al. Reference Daley, Budd, Caron, Edgecombe and Collins2009; Daley & Budd, Reference Daley and Budd2010; Cong et al. Reference Cong, Ma, Hou, Edgecombe and Strausfield2014, Reference Cong, Daley, Edgecombe, Hou and Chen2016, Reference Cong, Daley, Edgecombe and Hou2017, Reference Cong, Edgecombe, Daley, Guo, Pates and Hou2018; Vinther et al. Reference Vinther, Stein, Longrich and Harper2014; Pates & Daley, Reference Pates and Daley2017; Pates, Daley & Ortega-Hernández, Reference Pates, Daley and Ortega-Hernández2017; Pates, Daley & Ortega-Hernández, Reference Pates, Daley and Ortega-Hernández2018b). Radiodonta fossils have been identified worldwide, from the Great Basin, USA (e.g. Briggs & Mount Reference Briggs and Mount1982; Lieberman, Reference Lieberman2003; Briggs et al. Reference Briggs, Lieberman, Hendricks, Halgedahl and Jarrard2008; Lerosey-Aubril et al. Reference Lerosey-Aubril, Hegna, Babcock, Bonino and Kier2014; Pates, Daley & Lieberman, Reference Pates, Daley and Lieberman2018a); Iberian Chains, Spain (Pates & Daley, Reference Pates and Daley2017); Holy Cross Mountains, Poland (Daley & Legg, Reference Daley and Legg2015); Bohemia, Czech Republic (Chlupáč & Kordule, Reference Chlupáč and Kordule2002; Daley, Budd & Caron, Reference Daley, Budd and Caron2013a); Hunsrück, Germany (Kühl, Briggs & Rust, Reference Kühl, Briggs and Rust2009); as well as more famous sites such as the Burgess Shale, Canada (e.g. Whittington & Briggs, Reference Whittington and Briggs1985; Collins, Reference Collins1996; Daley & Budd Reference Daley and Budd2010), Chengjiang and Haiku biotas, China (e.g. Chen, Ramsköld & Zhou, Reference Chen, Ramsköld and Zhou1994; Hou, Bergström & Ahlberg, Reference Hou, Bergström and Ahlberg1995; Cong et al. Reference Cong, Ma, Hou, Edgecombe and Strausfield2014, Reference Cong, Daley, Edgecombe, Hou and Chen2016, Reference Cong, Daley, Edgecombe and Hou2017, Reference Cong, Edgecombe, Daley, Guo, Pates and Hou2018; Guo et al. in press), and Emu Bay Shale, Australia (Nedin, Reference Nedin1995; Daley et al. Reference Daley, Paterson, Edgecombe, García-Bellido and Jago2013b).
In this study, the known Radiodonta material from the Kinzers Formation is reassessed in light of the substantial increase in knowledge of these iconic Cambrian animals. Significantly for this study, two genera, Tamisiocaris Daley & Peel, Reference Daley and Peel2010 and Amplectobelua Hou, Bergström & Ahlberg, Reference Hou, Bergström and Ahlberg1995, with frontal appendages superficially similar to Anomalocaris, have been described since the last review of the Kinzers Radiodonta by Briggs (Reference Briggs1979). Our restudy of the material ascribed to Anomalocaris pennsylvanica and Anomalocaris? cf. pennsylvanica shows that it in fact belongs to at least four different taxa, and confirms that A. pennsylvanica is a distinct species from A. canadensis.
2. Identifying Radiodonta using frontal appendages
Although rare complete body fossils are known, Radiodonta are most commonly found as isolated elements of the body plan: frontal appendages, mouthparts, carapace elements, or flaps. Of these, isolated frontal appendages are the most common radiodont body element found outside of Tier 1 Burgess Shale-type deposits (BSTs) deposits with greater than 100 taxa known, preserved in high fidelity and high abundance (Gaines, Reference Gaines2014), and a number of species are known only from frontal appendages, especially from Tier 2 (10–100 taxa, intermediate fidelity and abundance) and Tier 3 (<10 taxa, low fidelity and abundance) BSTs. Frontal appendages are taxonomically informative, and even partial specimens often provide enough evidence to recognize families, genera or species of radiodonts.
Radiodont frontal appendages consist of a series of podomeres bearing a diverse morphology of spines and/or endites along their length. Radiodont frontal appendages can be separated into the ‘shaft’ (sensu Hou, Bergström & Ahlberg, Reference Hou, Bergström and Ahlberg1995, sometimes referred to as the ‘peduncle’) and the ‘distal articulated region’ (sensu Cong et al. Reference Cong, Edgecombe, Daley, Guo, Pates and Hou2018). The shaft normally has more weakly defined boundaries between podomeres and is less sclerotized than the rest of the appendage, and accordingly it is not always preserved or is only incompletely preserved. It joins the appendage to the body and is joined to the distal articulated region at an angle of 100–180° on the dorsal surface (Fig. 1a: ϑ). The shaft often bears an endite at the distalmost point of the ventral surface (Fig. 1a, shaft endite), and rarely other ventral endites are present on the shaft (e.g. Ramskoeldia platycantha, Cong et al. Reference Cong, Edgecombe, Daley, Guo, Pates and Hou2018). The boundary between the shaft and the distal articulated region can be further identified by the presence, in most taxa, of an enlarged or morphologically differentiated ventral endite on the most proximal podomere of the distal articulated region (e.g. Amplectobelua, Lyrarapax) (Fig. 1a, hypertrophied endite). The distal articulated region can itself often be separated into two parts. The proximal part bears ventral endites of the same morphology, differentiating it from the distal part, which often bears either no ventral endites, or reduced and simplified endites, together with dorsal spines (Fig. 1a, dorsal spines, reduced endites).

Figure 1. Schematic radiodont frontal appendage showing terminology used. Podomeres in dark grey, ventral endites in light grey. (a) Sagittal view. Abbreviations: ϑ, angle between shaft and distal articulated region on dorsal surface; 1, post-shaft podomere; 3 and 5, third and fifth podomere in the distal articulated region. For anomalocaridids, the ventral endites on 3 are longer than those on 5; for amplectobeluids the ventral endites on 5 are longer than those on 3. In hurdiids all podomeres from 1 to 5 will have enlarged endites of the same morphology and subequal length. (b) Frontal view of non-hurdiid podomere, showing relative width and separate attachment of two ventral endites to one podomere. (c) Frontal view of hurdiid podomere showing relative width and attachment of one ventral endite to one podomere.
The morphology and pattern of ventral endites in particular have been used to identify isolated appendages (Table 1). In Hurdiidae, blade-like ventral endites are the same width (frontal view) as the podomere, and only one ventral endite projects from each podomere (Fig. 1c). Hurdiids always have five of these large blade-like ventral endites in the distal articulated region (Fig. 1a: 1–5). For all other families, the ventral endites are paired on each podomere, and are less than half the width (frontal view) of the podomere to which they attach (Fig. 1b). Families can be differentiated using the relative lengths of ventral endites, the presence of enlarged ventral endites on the post-shaft podomere, and/or the morphology of dorsal spines in the distal articulated region. Anomalocarididae (a clade that includes all species of Anomalocaris except for Anomalocaris briggsi) and Amplectobeluidae both have enlarged spine-like ventral endites in the distal articulated region, which alternate in length long/short on odd/even podomeres, with a general reduction in the length of ventral endites over the length of the appendage. Amplectobleuidae differ from Anomalocarididae, as the ventral endite on the fifth podomere in the distal articulated region is longer than that on the third, and the distal region often bears thickened dorsal spines, in contrast to Anomalocarididae where they are thinner. Tamisiocarididae (‘Cetiocaridae’ of Vinther et al. Reference Vinther, Stein, Longrich and Harper2014), a clade comprising Tamisiocaris borealis and Anomalocaris briggsi (Cong et al. Reference Cong, Ma, Hou, Edgecombe and Strausfield2014; Vinther et al. Reference Vinther, Stein, Longrich and Harper2014; Van Roy, Daley & Briggs, Reference Van Roy, Daley and Briggs2015), have a pair of elongated and slender ventral endites that have a length at least 1.5× the height (sag) of the podomere to which they are attached. The appendage is simple, with the same morphology of ventral endite along the distal articulated region. There is no differentiated post-shaft podomere in Tamisiocaris appendages, although Anomalocaris briggsi does have an enlarged post-shaft ventral endite and an additional endite of similar morphology on the distalmost position of the shaft. There is no alternation of long/short ventral endites in these taxa, with the overall length of ventral endites decreasing along the length of the appendage. Caryosyntrips, Laminacaris and Lyrarapax have an uncertain placement at family level, but can be distinguished based on features of the frontal appendages. Caryosyntrips appendages taper distally with an approximately triangular outline. Similar to tamisiocaridids, the appendages are simple, with no alternation of long/short ventral endites, and no enlarged endite distal to the shaft. Laminacaris, a recently described monospecific genus from the Chengjiang biota, bears a ventral endite on the most proximal podomere in the distal articulated region similar in morphology to Hurdia ventral endites, but the remainder of the distal articulated region is similar to Anomalocarididae and Amplectobeluidae. Lyrarapax appendages superficially resemble those of Amplectobeluids, as appendages have an enlarged ventral endite on the post-shaft podomere, and simple ventral endites alternating long/short or present/absent in the distal articulated region. Appendages differ in podomere shape (near square in Lyrarapax and rectangular in Amplectobelua), but the major differences are in the oral cone morphology (tetraradial arrangement of large plates with smaller plates between in Lyrarapax, an association of tuberculate and smooth plates in Amplectobluidae) and gnathobase-like structures present in Amplectobeluidae but not Lyrarapax (Cong et al. Reference Cong, Edgecombe, Daley, Guo, Pates and Hou2018; Liu et al. Reference Liu, Lerosey-Aubril, Steiner, Dunlop, Shu and Paterson2018).
Table 1. Frontal appendage characters used to distinguish radiodont families

*A. briggsi bears an enlarged post-shaft endite, and Tamisiocaris does not. Hence all characters referring to the morphology of the large ventral endite for this family refer to A. briggsi only. Abbreviations: as, auxiliary spine; d.a.r., distal articulated region; en, ventral endite; pd, podomere; tm, triangular membrane.
3. Materials and methods
Eight specimens of Radiodonta from the Kinzers Formation are known. Material is held at the National Museum of Natural History, Washington DC, USA (USNM), North Museum, Franklin and Marshall College, Lancaster, Pennsylvania, USA (PA), and Yale Peabody Museum, New Haven, Connecticut, USA (YPM).
For historical reasons, the part and counterpart of most of the specimens are held at two institutions, the USNM and PA. Some material was not available for study by Briggs (Reference Briggs1979), namely the part of the Holotype of Anomalocaris pennsylvanica, and USNM 90827 (Resser & Howell, Reference Resser and Howell1938, pl. 13, fig. 5). As another specimen was also labelled USNM 90827 that was available for study by Briggs (Reference Briggs1979), there is confusion in the literature about the specimens labelled with this number. The USNM 90827 specimen figured in Resser & Howell (Reference Resser and Howell1938, pl. 13, fig. 5) is different to that figured by Briggs (Reference Briggs1979, pl. 81, fig. 11, text-fig. 34), and Briggs (Reference Briggs1979) noted that the specimen figured by Resser & Howell (Reference Resser and Howell1938) was different to those in the collection. Both specimens labelled USNM 90827 were available for this study, but are not part and counterpart. The specimen figured first, by Resser & Howell (Reference Resser and Howell1938), retained the number USNM 90827, and the specimen figured second, by Briggs (Reference Briggs1979), has been given the altered number USNM 90827A. Both specimens are radiodonts, described herein.
Specimens were photographed wet and dry under polarized and non-polarized light using a Canon EOS 500D digital SLR Camera with a Canon EF-S 60 mm Macro Lens, controlled for remote shooting using EOS Utility 2. Digital measurements were made using ImageJ (Schneider, Rasband & Eliceiri, Reference Schneider, Rasband and Eliceiri2012). Modern longitude and latitude coordinates were reconstructed to 510 Ma using GPlates (Scotese, Reference Scotese2016). Coordinates were obtained from the literature. Where it was not possible to obtain the exact coordinates of the fossil locality, the nearest town was used in its place.
4. Systematic palaeontology
Total-group EUARTHROPODA Lankester, Reference Lankester1904
Order RADIODONTA Collins, Reference Collins1996
?Laminacaris sp.
Figure 2
v. 1979 Anomalocaris? cf. pennsylvanica; Briggs, pl. 81, figs 9–11, text-figs 33, 34
Material. Two partial isolated frontal appendages collected from the Kinzers Formation, Pennsylvania, USA, are known from part and counterpart: USNM 213993, PA 394 (counterpart), locality 22L; USNM 90827A, PA 393 (counterpart), locality 12x.
Description. USNM 213993/PA 394 (Fig. 2a, b) is a partial appendage, with one shaft podomere (Fig. 2b: pd1) and six podomeres in the distal articulated region preserved (Fig. 2b: pd2–7). A pair of thickened recurved ventral endites with distally pointing auxiliary spines is present on the shaft podomere, at the distalmost ventral point (Fig. 2b: pd1). Ventral endites attach to the midpoint of the other podomeres. The ventral endite on the podomere immediately distal to the shaft is curved distally, and approximately the same length and the height (sag) as the podomere, and bears a distally pointing auxiliary spine two-thirds of the way down the spine (Fig. 2b: en2). The other ventral endites in the distal articulated region are paired and approximately the height (sag) of the podomere to which they are attached. Distally pointing auxiliary spines are present along the length, and rarely a proximally pointing spine is visible (Fig. 2b: en6). Auxiliary spines point slightly ventrally. Podomeres are tall (sag) rectangles, approximately twice as tall as wide, decreasing in size distally. No dorsal spines are visible.

Figure 2. ?Laminacaris sp. from the Kinzers Formation. (a, b) USNM 213693; (c, d) USNM 90827A. Scale bars 10 mm. Abbreviations: enX, ventral endite X; pdX, podomere X.
USNM 90827A (Fig. 2c, d) preserves six rectangular podomeres approximately twice as high (sag) as wide (trans) with ventral endites attached, and traces of two ventral endites that have been prepared out of the matrix (Fig. 2d: en9? & en10?). Just as for USNM213993/PA394, USNM 90827A ventral endites are straight and approximately the same height as the podomere (e.g. Fig. 2d: en5), and an enlarged endite is present with a single auxiliary spine two-thirds of the way along its length at the proximal end (Fig. 2d: en2). One endite (Fig. 2d: en5) is longer than the others. Auxiliary spines project both distally and proximally, with a slight ventral tilt.
Remarks. This taxon is similar to Lamincaris chimera from the Chengjiang biota (Guo et al. in press). This animal, known only from frontal appendages, has straight ventral endites in the distal articulated region with distally pointing auxiliary spines, and enlarged ventral endites on the distalmost point of the shaft and proximal-most post-shaft podomere. The distally pointing auxiliary spines along the length of the endite in Laminacaris chimera from Chengjiang are similar to the arrangement of auxiliary spines in the Kinzers material. The ventral endites are shorter than the height of the podomere to which they attach in both the Chengjiang and Kinzers material, but the alternating long and short endites of Laminacaris chimera are not present in the incompletely preserved Kinzers specimens where endites are approximately the same length. The enlarged ventral endite in the Kinzers material does not have the distinctive blade-like morphology with a strong similarity to Hurdia ventral endites that is seen in Laminacaris chimera, however it is enlarged relative to the other endites and recurved. This enlarged endite in the Kinzers material bears more similarity to the post-shaft ventral endite of Lyrarapax trilobus or Amplectobelua stephenensis, although it is not as thickened as these two taxa. The endite on the shaft is similar in Laminacaris and this Kinzers taxon, as it bears a single distally facing auxiliary spine approximately two-thirds of the distance from the base to the tip. The Kinzers Laminacaris sp. material could also be compared to Anomalocaris briggsi from the Emu Bay Shale, particularly the shape of the podomeres and the possible lack of alternating long/short ventral spines. However, the auxiliary spines point both distally and proximally in A. briggsi, in contrast to the distally pointing spines of the Kinzers specimens, the ventral endites are shorter relative to podomere height in the Kinzers material than in A. briggsi, and the enlarged ventral endites on the shaft and proximal podomere in the distal articulated region are of a different morphology.
Family ANOMALOCARIDIDAE Raymond, Reference Raymond1935
Genus ANOMALOCARIS Whiteaves, Reference Whiteaves1892
Type species. Anomalocaris canadensis Whiteaves, Reference Whiteaves1892 from the Stephen Formation (Miaolingian, Wuliuan) of British Columbia, Canada.
Anomalocaris pennsylvanica Resser, Reference Resser1929
Figure 3
v. 1929 Anomalocaris pennsylvanica; Resser, pl. 5, fig. 5, pl. 79, fig. 5
v. 1938 Anomalocaris pennsylvanica; Resser & Howell, pl. 10, fig. 4
v. 1979 Anomalocaris pennsylvanica; Briggs, pl. 79, fig. 5, text-fig. 18, p. 641
Holotype. Isolated appendage, Kinzers Formation, Pennsylvania, USA, USNM 80487 (part and counterpart), locality 12x.
Paratype. Isolated appendage, Kinzers Formation, Pennsylvania, USA, YPM 10425 (part only), locality 12x.
Other material. Partial isolated appendage, Kinzers Formation, Pennsylvania, USA, USNM 255611, locality 22L.
Diagnosis. Anomalocaris appendage composed of 14 podomeres; one podomere in the shaft wider than tall; 13 podomeres in the distal articulated region taller than wide; all podomeres in the shaft and the distal articulated region bear a pair of ventral endites projecting from the midpoint of the ventral surface; ventral endites are present on pd1–13, lack auxiliary spines, and alternate long/short on even/odd numbered podomeres; ventral endites on proximal podomeres at least as long as the height of the podomere they attach to, and decrease in length relative to podomere height distally; ventral endite of the shaft is not distinct from the ventral endites of the distal articulated region; thin straight dorsal spines project forward from distal dorsal margin of pd9–14; single robust terminal spine.
Description. Anomalocaris pennsylvanica is known from three specimens: the holotype (part and counterpart) measuring 23 mm (Fig. 3a, b); a partial small appendage (USNM 255611) (Fig. 3e, f); and a large complete appendage (YPM 10425) measuring 75 mm (Fig. 3c, d). Complete specimens have 14 podomeres, one in the shaft and 13 in the post-shaft region. The presence of a podomere boundary between pd13 and pd14 is confirmed in the holotype by counting the dorsal spines in the distal region, where the podomere boundaries are not well preserved (see Fig. 3b: ds13, ds14). The holotype also potentially shows a second shaft podomere, an approximately square feature preserved as a faint outline; however, it joins at an oblique angle to the ventral surface, not the dorsal surface like shaft podomeres in other species (Fig. 3b: ?S). YPM 10425 shows that the shaft bears a large ventral endite at the distal margin (Fig. 3d: en1). YPM 10425 also appears to have a longer ventral endite on pd6 than pd4 (compare Fig. 3d en4 and en6); however, the overprint of iron minerals in the rock means that this cannot be confirmed. This is similar to what is seen in Amplectobleuidae; however, the number of podomeres (14) is consistent with Anomalocaris.
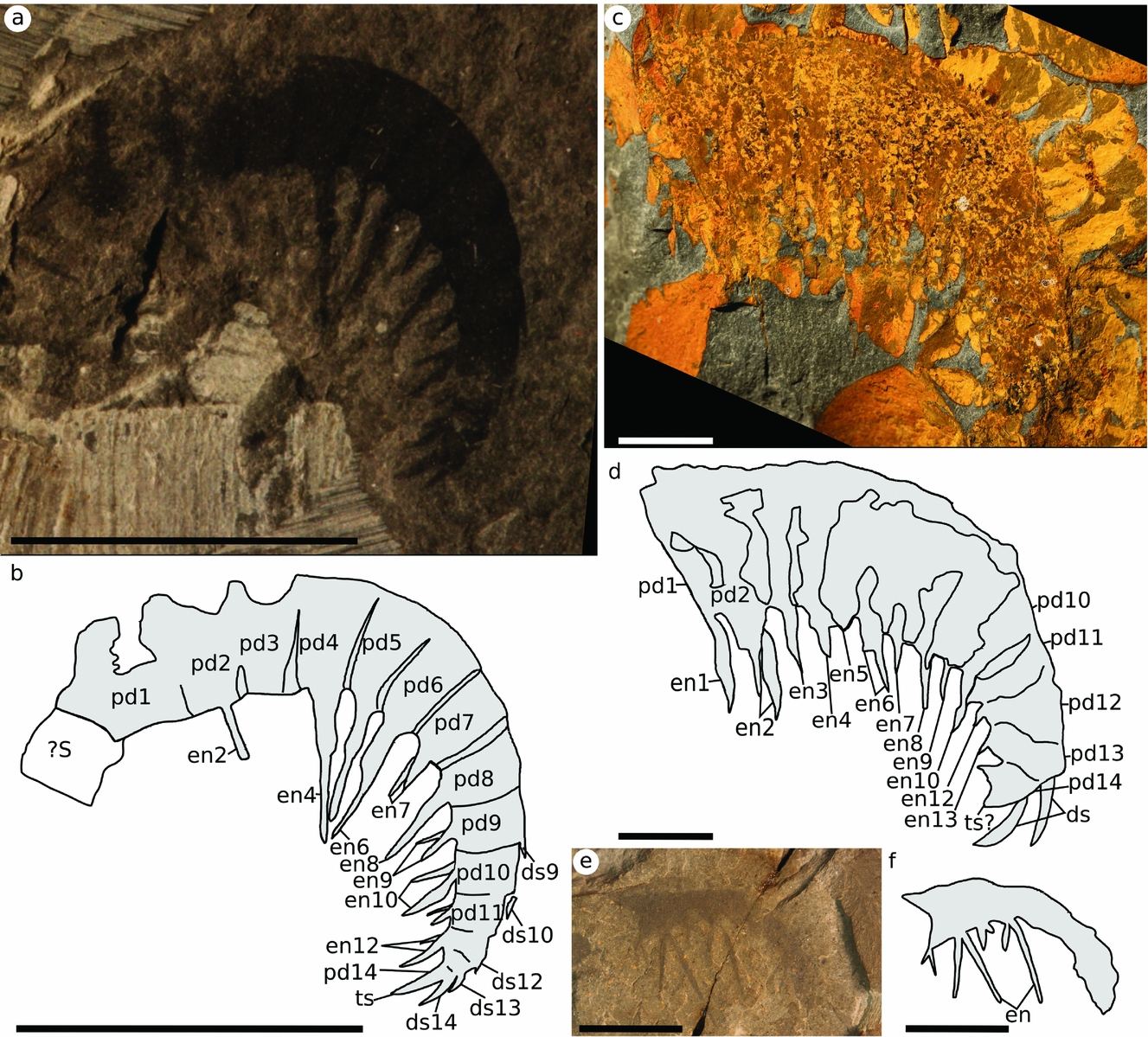
Figure 3. Anomalocaris pennsylvanica from the Kinzers Formation. (a, b) Holotype USNM 80487; (c, d) YPM 10425; (e, f) USNM 255611. Scale bars 10 mm. Abbreviations: dsX, dorsal spine X; enX, ventral endite X; pdX, podomere X; ?S, putative shaft podomere; ts, terminal spine.
Although the material is limited, the three specimens of different size suggest that the podomeres grew taller relative to the width of podomeres and length of ventral endites through ontogeny, and so larger appendages have smaller ventral endites relative to podomere height. The smallest of the specimens (USNM 255611) has the longest endite to podomere height ratio, at 2:1 at the proximal end. The largest specimen (YPM 10425) has the lowest ratio, at close to 1:1, with the ratio in the holotype approximately 1.5:1. Similarly the podomere height:width ratio is higher (2.5:1) in YPM 10425 than the holotype (1.5:1). Podomere boundaries are not preserved in USNM 255611.
Remarks. Much of the material previously assigned to Anomalocaris pennsylvanica is here reassigned to other radiodont genera (see below). PA 389, PA 395A and YPM 63295 are removed from Radiodonta altogether because these likely represent partial bodies and appendages of non-radiodont euarthropods.
The counterpart to the holotype was not available for study by Briggs (Reference Briggs1979); however, the interpretation of this specimen, and the description of this species has not changed significantly. Briggs (Reference Briggs1979) also noted that larger Anomalocaris pennsylvanica appendages had shorter ventral endites relative to podomere height than smaller appendages of this species. Although the material with the shortest ventral endites is here reassigned to other genera, this observation still holds.
Anomalocaris pennsylvanica Resser, Reference Resser1929 was the second radiodont species to be described, and is only known from isolated frontal appendages. It can be distinguished from Anomalocaris canadensis, the type species, based on the morphology of its ventral endites. In A. pennsylvanica these are simple, whereas for A. canadensis each ventral endite bears two auxiliary spines, creating a trident shape (Briggs, Reference Briggs1979). It has been suggested that the lack of auxiliary spines on ventral endites could be taphonomic, in which case A. pennsylvanica could be synonymized with A. canadensis; however, it was retained as a valid taxon based on the limited material available (Briggs, Reference Briggs1979; Lieberman, Reference Lieberman2003; Daley & Peel, Reference Daley and Peel2010). Anomalocaris pennsylvanica is here confirmed as a valid taxon. The ventral endites have smooth margins and show no evidence of taphonomically removed auxiliary spines. Furthermore, the three known specimens of A. pennsylvanica show other differences that distinguish it from other Anomalocaris species. It can be differentiated from the most similar species, A. canadensis, not only by the lack of auxiliary spines on the ventral endites, but also by the ventral endites being longer relative to podomere height towards the proximal end of the appendage, and by the distal end of the appendage not having differentiated shorter and simplified ventral endites, as is seen in A. canadensis. A. canadensis also has a reduced ventral endite on the distalmost point of the shaft, whereas A. pennsylvanica has a long ventral endite similar to those of the distal articulated region.
Anomalocaris pennsylvanica is endemic to the Kinzers Formation, as other specimens attributed to this species from the Pioche Formation (Lieberman, Reference Lieberman2003) bear auxiliary spines and will be described as a new species of Anomalocaris in an upcoming review of Southern Great Basin material.
Family TAMISIOCARIDIDAE nov.
Type genus. Tamisiocaris Daley & Peel, Reference Daley and Peel2010
Diagnosis. Radiodont with a frontal appendage bearing slender blade-like ventral endites longer than the podomere to which they are attached; ventral endites do not alternate long/short.
Remarks. The family ‘Cetiocaridae’ was named by Vinther et al. Reference Vinther, Stein, Longrich and Harper2014; however, this name is invalid by International Code of Zoological Nomenclature (ICZN) conventions (see Van Roy, Daley & Briggs, Reference Van Roy, Daley and Briggs2015). The family comprises Tamisiocaris and Anomalocaris briggsi, which were recovered together by a phylogeny of radiodonts (Vinther et al. Reference Vinther, Stein, Longrich and Harper2014, fig. 3), and subsequently by Cong et al. (Reference Cong, Ma, Hou, Edgecombe and Strausfield2014) and Van Roy, Daley & Briggs, (Reference Van Roy, Daley and Briggs2015).
Genus TAMISIOCARIS Daley & Peel, Reference Daley and Peel2010
Type species. Tamisiocaris borealis Daley & Peel, Reference Daley and Peel2010.
Diagnosis. Radiodont with paired frontal appendages adjacent to an oval-shaped central carapace element; appendage has at least one podomere in the shaft, with 17 podomeres in the distal articulated region separated by a triangular membrane; shaft podomere is wider (trans) than tall (sag) and bears a straight proximally pointing endite at the distal ventral margin approximately the same length as the height (sag) of the podomere; podomeres in the distal articulated region reduce in height towards the distal end and bear paired slender ventral endites at least twice the length (sag) of the height (sag) of the podomere; length of ventral endites decreases distally, forming a straight line between the distal ends of the ventral endites (emended from Daley & Peel, Reference Daley and Peel2010).
Remarks. Fine auxiliary spines were not preserved in the holotype of Tamisiocaris, and so not included in the original diagnosis. They were subsequently recognized in new material described by Vinther et al. (Reference Vinther, Stein, Longrich and Harper2014), although the diagnosis of genus and species was not updated in that study. As the specimen from the Kinzers Formation assigned to this genus does not have apparent auxiliary spines (see below), the presence of fine auxiliary spines is not included in the diagnosis of this genus.
Tamisiocaris borealis Daley & Peel, Reference Daley and Peel2010
2010 Tamisiocaris borealis; Daley & Peel, fig. 1
2014 Tamisiocaris borealis; Vinther et al., figs 1, 2, extended data figs 1–4, 6, 7
Holotype. Isolated frontal appendage, Peary Land, central North Greenland, base of the Buen Formation, MGUH 29154, by original designation.
Paratypes. Isolated frontal appendages, from same locality as holotype. MGUH 30500 (displays articulating triangular membranes); MGUH 30501 (displays fine auxiliary spines), designated herein.
Other material. Three other isolated frontal appendages of this species are known, also from same locality as holotype. MGUH 30502 – 4.
Diagnosis. Tamisiocaris with an appendage that bears fine auxiliary spines along the length of both proximal and distal surfaces of the ventral endites; auxiliary spines are straight, do not change length along the appendage and are separated vertically by less than the width (trans) of the ventral endite (emended from Daley & Peel, Reference Daley and Peel2010).
Remarks. Tamisiocaris borealis was originally described as a radiodont frontal appendage by Daley & Peel (Reference Daley and Peel2010). A subsequent study by Vinther et al. (Reference Vinther, Stein, Longrich and Harper2014) with new material provided new information about the appendage, including the presence of triangular articulating membrane between the podomeres and fine auxiliary spines along the length of the ventral endites. This confirmed the hypothesis that the taxon belongs to Radiodonta, and suggested a sifting/filtering feeding ecology; however, Vinther et al. (Reference Vinther, Stein, Longrich and Harper2014) did not update the diagnosis of the genus or species, nor designate new paratypes with these new features. This has been done here to allow comparison with the Kinzers Formation material.
Tamisiocaris aff. borealis
Figure 4
v. 1938 Anomalocaris pennsylvanica; Resser and Howell, pl. 13, fig. 5
v. 1979 Anomalocaris pennsylvanica; Briggs p. 641
Material. Partial isolated frontal appendage, Kinzers Formation, Pennsylvania, USA, USNM 90827, PA 388 (counterpart).
Description. USNM 90827/PA 388 (Fig. 4) shows a partial appendage of nine podomeres (Fig. 4b: ?pd10–?pd18), each bearing a long and slender ventral endite (Fig. 4b: en). The podomeres are approximately square where boundaries can be discerned (e.g. Fig. 4b: ?pd14) with the ventral endites twice the height (sag) of the podomeres. The podomeres reduce in height towards the distal end, where the shape becomes an elongate rectangle (e.g. Fig. 4b: ?pd18). No auxiliary spines are visible on the ventral endites, and no dorsal spines are visible on the appendage.

Figure 4. Tamisiocaris aff. borealis from the Kinzers Formation. (a, b) PA 388. Scale bars 10 mm. Abbreviations: en, ventral endite; ?pdX, podomere X, inferred from complete T. borealis appendages.
Remarks. This specimen is no longer considered Anomalocaris pennsylvanica as the podomeres are square to elongate rectangular in shape (as opposed to tall rectangles), and the ventral endites, although they lack auxiliary spines, do not alternate long/short on even/odd numbered podomeres and instead reduce in length along the appendage.
This specimen is instead considered to belong to the genus Tamisiocaris as it has long, paired, ventral endites which do not alternate long/short, similar to T. borealis from Sirius Passet. The approximately square shape of the podomeres is consistent with the distal end of known Tamisiocaris specimens from Sirius Passet. Just as in Tamisiocaris borealis, this specimen lacks any dorsal spines, which would instead indicate affinities with Anomalocaris briggsi. There is no evidence that this specimen bears auxiliary spines, which are visible in some specimens of T. borealis (although not the type specimen), and it is only known as a partial appendage, so the number of podomeres cannot be ascertained. Because of this the specimen is left in open nomenclature as T. aff. borealis.
This is the youngest Tamisiocaris known (Cambrian Stage 4) and the first from outside the Sirius Passet Lagerstätte (Cambrian Stage 3, Greenland). Tamisiocaris is still only known from Laurentia.
Family AMPLECTOBELUIDAE Vinther et al. Reference Vinther, Stein, Longrich and Harper2014
Genus AMPLECTOBELUA Hou, Bergström & Ahlberg Reference Hou, Bergström and Ahlberg1995
Amplectobelua aff. symbrachiata
Figure 5
1938 Anomalocaris pennsylvanica; Resser & Howell, pl. 13, fig. 6
1953 Anomalocaris pennsylvanica; Roger, pl. 2 fig. 4
1979 Anomalocaris pennsylvanica; Briggs, pl. 79 figs 4, 6, text-figs 17, 19
Material. Isolated frontal appendages, Kinzers Formation, Pennsylvania, USA, YPM 14388 (part) and PA 387 (counterpart) locality 12x; PA390 (part and counterpart) locality 22L.
Description. Two Amplectobelua appendages can be recognized from the Kinzers Formation. YPM 14388 (Fig. 5a, b), with counterpart PA387, is a complete isolated appendage with 12 podomeres. The podomeres are tall and rectangular but become more square towards the distal end (compare pd5 to pd10 in Fig. 5b). A long and stout endite with the base of three spines preserved is present on the most proximal podomere (Fig. 5b: pd1, en1). The central spine base is the thickest, with the proximal-most spine preserved in its entirety. Short and paired spinose endites are present on the remaining podomeres, although most are incomplete, and the endite on pd6 shows that they were simple spines shorter than the podomere to which they attached (Fig. 5b: en6). The broken base on pd5 is wider than the broken base of pd3, implying that the ventral endite on this pd5 was more robust than that of pd3 (Fig. 5b: en3, en5). The distalmost two podomeres (pd11–12) bear robust paired dorsal spines which are recurved following the outline of the appendage and combine with a reduced terminal spine to make a claw-like termination (Fig. 5b: ds & ts). PA 390 (Fig. 5c, d) is a partial appendage with the ten most distal podomeres visible. Podomeres are tall and rectangular, but become more square at the distal end (compare Fig. 5d pd4 to pd9 and pd12). All the ventral endites are short (e.g. Fig. 5b: en6–8), and robust recurved dorsal spines are visible at the distal end (Fig. 5d: ds) alongside a reduced terminal spine (Fig. 5d: ts). In both YPM 14388 and PA 390 some smaller and straight dorsal spines are visible proximal to the robust dorsal spines (e.g. Fig. 5b: ds10).

Figure 5. Amplectobelua aff. symbrachiata from the Kinzers Formation. (a, b) YPM 14388; (c, d) PA 390. Scale bars 10 mm. Abbreviations: ds, dorsal spines; enX, ventral endite X; pdX, podomere X.
Remarks. These specimens are no longer considered Anomalocaris pennsylvanica, as the dorsal spines at the distal end are paired and much more robust, the ventral endites along the appendage are short and the number of podomeres (where they can be counted) is 12 instead of 14. This interpretation differs slightly from the previous descriptions, where both these appendages were interpreted to have 14 podomeres (Briggs, Reference Briggs1979, plate 79 figs 4, 6, text figs 17, 19). The podomere labelled as j6 of PA 390 (Briggs, Reference Briggs1979, plate 79 fig. 4, text fig. 17) is here interpreted as the eighth podomere, j1 and j2 of YPM 14388 (Briggs, Reference Briggs1979, plate 79 fig. 6, text fig. 19) are interpreted as being an enlarged first podomere (Fig. 5b: pd1) as no podomere boundary is visible here (none was drawn in Briggs, Reference Briggs1979, text fig. 19). The structure labelled as j14 is here interpreted as one of a pair of robust dorsal spines (Fig. 5b: ds12).
A number of lines of evidence support the reinterpretation of these two appendages as Amplectobelua, including the presence of paired but short ventral endites along the majority of the appendage, the rectangular podomeres, and paired robust dorsal spines at the distal end. In addition, YPM 14388 has an enlarged ventral endite on pd1 (pd1 is not preserved in PA 390), and the base of the endite on pd5 appears more robust than that of pd3. These specimens are most similar to Amplectobelua symbrachiata, as they both bear short ventral endites along the appendage alternating long/short and the morphology of the enlarged ventral endite is constructed of three spines, with the thickest in the centre, unlike in Amplectobelua stephenensis where there is only one very enlarged and thickened spine on the most proximal podomere. Amplectobelua symbrachiata also bears smaller dorsal spines proximally to the enlarged recurved dorsal spines, as is also seen in both the specimens from the Kinzers Formation. The material from the Kinzers however does not have exactly the same morphology as Amplectobelua symbrachiata, because it has a reduced terminal spine, not present in the Chengjiang species, and evidence for paired dorsal spines not reported from the Chinese animal, but a feature known from Amplectobelua stephenensis. The podomeres become more square towards the distal end in the Kinzers species, a feature not seen in either Amplectobelua symbrachiata or Amplectobelua stephenensis.
The preservation does not allow all the anatomical features to be recognized. For example the morphology of the enlarged ventral endite is not completely known, specifically the length of the central thickened spine. In addition there is no evidence of the shaft, which is expected to be of three podomeres in Amplectobleua. For these reasons this species is left in open nomenclature.
These are the first Amplectobelua from Cambrian Stage 4, the first from the USA, and the oldest in Laurentia. Amplectobelua was previously known from Stage 3 of China and Wuliuan of Canada. They bear more similarity to the Chinese species Amplectobelua symbrachiata than the Canadian Amplectobelua stephenensis, perhaps indicating an invasion by the Chinese species at the end of Stage 3 followed by a subsequent radiation in Laurentia.
5. Discussion
5.a. Diverse feeding strategies of Kinzers Formation Radiodonta
The Kinzers Formation contains one species of Anomalocaris, Amplectobelua, Tamisiocaris and tentatively Laminacaris (Fig. 6; Table 2). The presence of a number of different radiodonts from the same site is not unusual for Cambrian Lagerstätten, and supports previous suggestions that these stem-group euarthropods employed a number of different feeding strategies (Daley & Budd, Reference Daley and Budd2010; Vinther et al. Reference Vinther, Stein, Longrich and Harper2014; Van Roy, Daley & Briggs, Reference Van Roy, Daley and Briggs2015). Anomalocaris pennsylvanica would have used its flexible appendages to actively grab likely soft-bodied prey and slice it with the simple elongate ventral endites. Amplectobelua aff. symbrachiata would have used its more robust proximal ventral endite and thickened distal dorsal spines to more tightly hold and slice or potentially crush prey. The presence of reduced ventral endites between the proximal and distal end would have allowed the appendage to coil more tightly, allowing the distal and proximal robust spines to come together more easily in a slicing or crushing motion. Tamisiocaris aff. borealis may not have been a filter feeder targeting plankton like the Sirius Passet species if the lack of fine auxiliary spines is not an artefact of preservation, and so instead may have sifted or raked through the sediment. It is also possible that it filtered larger particles than the Greenland species, and so did not require auxiliary spines. ?Laminacaris sp. likely used its straight ventral endites with auxiliary spines to function as a net-like apparatus, potentially similar to some hurdiids, and as suggested for Anomalocaris briggsi from the Emu Bay Shale (Daley et al. Reference Daley, Paterson, Edgecombe, García-Bellido and Jago2013b). Its longer ventral spines and lack of clear alternating long/short ventral endites make a raptorial feeding strategy, similar to that inferred for Laminacaris chimera, less likely.
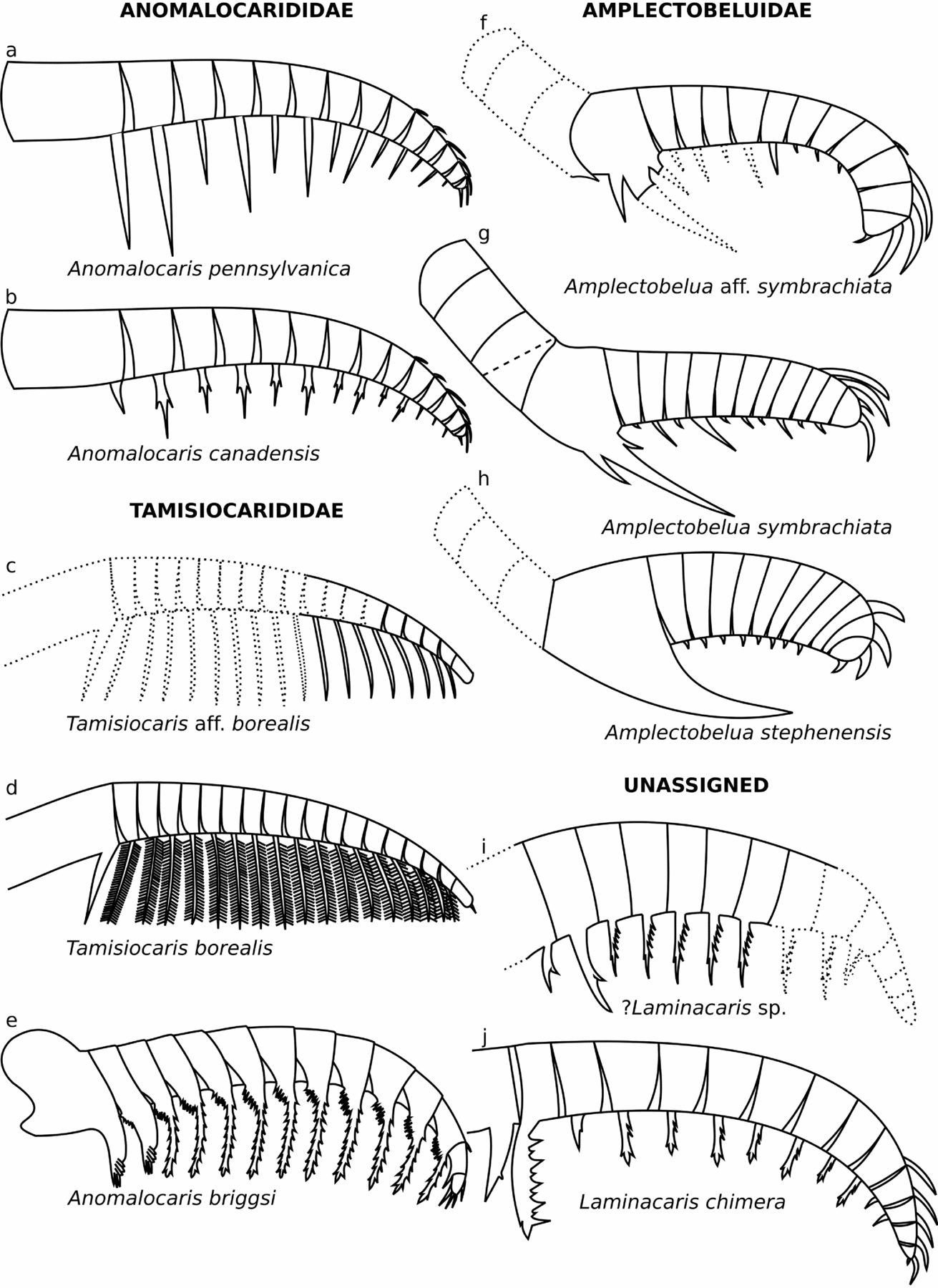
Figure 6. Reconstructions of Radiodonta from the Kinzers Formation, and other frontal appendages with similar morphology. (a) Anomalocaris pennsylvanica; (b) Anomalocaris canadensis; (c) Tamisiocaris aff. borealis; (d) Tamisiocaris borealis; (e) Anomalocaris briggsi; (f) Amplectobelua aff. symbrachiata; (g) Amplectobelua symbrachiata; (h) Amplectobelua stephenensis; (i) ?Laminacaris sp; (j) Laminacaris chimera. Dotted lines show parts of appendage that are not preserved. Line drawings (b), (g) and (h) adapted from Daley & Budd (Reference Daley and Budd2010, text fig. 1A, F, G), (d) adapted from Vinther et al. (Reference Vinther, Stein, Longrich and Harper2014, extended data fig. 6a), (e) adapted from Daley et al. (Reference Daley, Paterson, Edgecombe, García-Bellido and Jago2013b, fig. 2), and (j) redrawn from Guo et al. (in press, fig. 3A).
Table 2. Current and previous interpretations of Kinzers radiodont appendages

Site numbers: 12x, one and three-quarter miles north of Rohrerstown, Pennsylvania; 22L, half a mile south of East Petersburg, Pennsylvania (Briggs, Reference Briggs1979). References refer to previous interpretation.
* Briggs (Reference Briggs1979) only had PA 388 available, and was not able to study USNM 90827.
5.b. Importance of the Kinzers Formation
Radiodonta are known from other Cambrian Series 2, Stage 4, Konservat-Lagerstätten; however, the Kinzers Formation (30° S palaeolatitude, Laurentia) is uniquely important for understanding the palaeogeographic and palaeolatitudinal distribution of these animals (Fig. 7) because of the high diversity of taxa found there. Radiodonta are known from four other broadly coeval formations in North America from Laurentia (all equatorial): Latham Shale (Briggs & Mount Reference Briggs and Mount1982), Comet Shale Member, Pioche Formation (Lieberman, Reference Lieberman2003), Eagar Formation, Cranbrook Shale (Briggs, Reference Briggs1979), and Pyramid Shale Member, Carrara Formation (unpublished material). Radiodonta have also been reported from two formations of this age on the South China palaeocontinent (c. 30° N palaeolatitude): the Balang Formation (Liu, Reference Liu2013), and Wulongqing Formation (Wang, Huang & Hu, Reference Wang, Huang and Hu2013); and from two Gondwanan sites: the Emu Bay Shale in Australia (c. 15° N palaeolatitude) (Nedin, Reference Nedin1995; Daley et al. Reference Daley, Paterson, Edgecombe, García-Bellido and Jago2013b) and Valdemiedes Formation in Spain (c. 60° S palaeolatitude) (Pates & Daley, Reference Pates and Daley2017) (Table 3; Fig. 7).
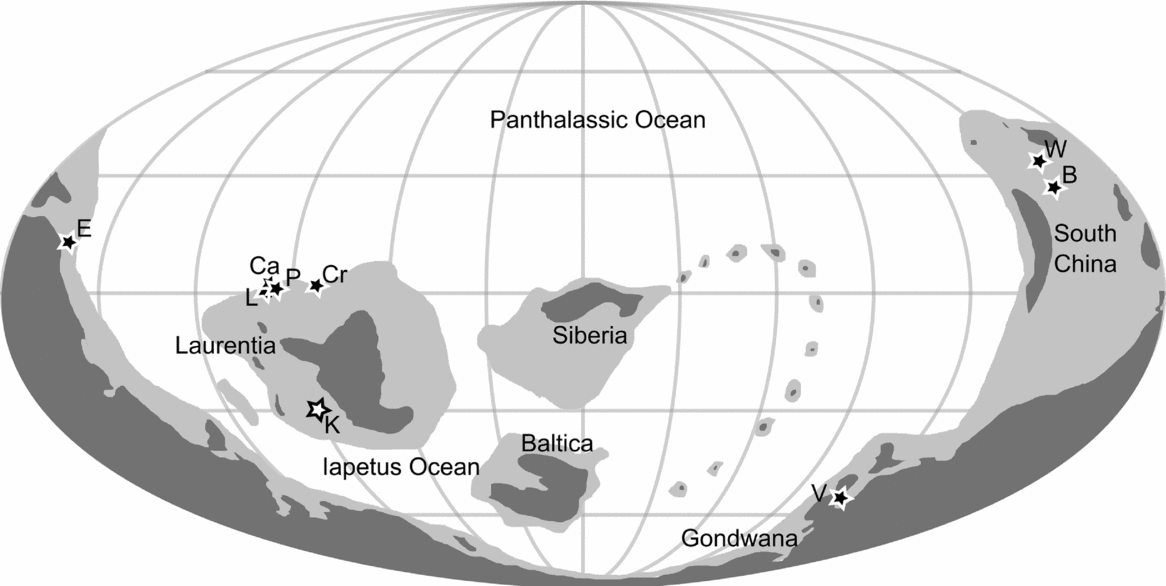
Figure 7. Palaeogeographic reconstruction to 510 Ma from GPlates (Scotese, Reference Scotese2016). White star shows Kinzers Formation; black stars show other Stage 4 sites where radiodont fossils have been found. Abbreviations: B, Balang Formation; Ca, Carrara Formation; Cr, Cranbrook Formation; E, Emu Bay Shale; K, Kinzers Formation; L, Latham Shale; P, Pioche Formation; V, Valdemiedes Formation; W, Wulongqing Formation.
Table 3. Cambrian Series 2, Stage 4 Radiodonta bearing Konservat-Lagerstätten

Number of known specimens and known taxa, with palaeocontinent and palaeolatitude reconstructed using GPlates (Fig. 7; Scotese, Reference Scotese2016) (numbers of unpublished specimens studied by authors in parentheses).
The recognition of four different radiodont taxa, from at least three different families, means that the Kinzers Formation has the most diverse Radiodonta fauna of all the Stage 4 Konservat-Lagerstätten, despite relatively few specimens being known – especially compared to the Emu Bay Shale and Wulongqing Formation (Table 3). The fauna contains links with both the older Sirius Passet (Tamisiocaris) and Chengjiang (Amplectobelua, Anomalocaris and Laminacaris) faunas, as well as the younger Burgess Shale (Amplectobelua and Anomalocaris). It has a named endemic species with Anomalocaris pennsylvanica, with at least two (and potentially all four) of its radiodont species not yet known from other localities. Its unique position at a non-equatorial latitude in Laurentia, as well as the lack of any Tier 1 Burgess Shale-type deposits (sensu Gaines, Reference Gaines2014) in the Cambrian Series 2 Stage 4 highlights the importance of the Kinzers Formation. The previous categorization of the Kinzers Formation as a Tier 3 Lagerstätten was because of limited rock exposure, not fossilization conditions (Gaines, Reference Gaines2014). The recovery of a highly diverse radiodont fauna from a relatively low number of specimens suggests that further exploration of the Kinzers Formation could yield new taxa rapidly. Caryosyntrips and Hurdia could also be present in the Kinzers Formation, as the former is present at the higher-latitude coeval Valdemiedes Formation (Spain) and equatorial Laurentia in the Wuliuan (Pates & Daley, Reference Pates and Daley2017), and the latter present at the coeval equatorial Laurentian Pioche Formation (unpublished material) and higher-latitude Wuliuan Bohemia (Chlupáč & Kordule, Reference Chlupáč and Kordule2002; Daley, Budd & Caron, Reference Daley, Budd and Caron2013a). Their absence might also be due to similar inferred feeding niches beingalready occupied in the Kinzers Formation by Amplectobelua, Tamsiocaris and ?Laminacaris, a result of unfavourable environmental conditions, or reflect the dominance of Amplectobeluidae, Anomalocarididae and Tamisiocarididae in older, Series 2, deposits and later dominance of Hurdiidae in the Miaolingian.
6. Conclusions
The vast progress in our understanding of the diversity of Radiodonta since the previous study of Kinzers Formation radiodonts allowed the identification of Tamisiocaris and Amplectobelua in the USA for the first time. These reinterpretations mean that the Kinzers Formation can now be considered a Tier 2 BST with more than ten soft-bodied taxa (sensu Gaines, Reference Gaines2014), and has the highest known diversity of Radiodonta of any Cambrian Series 2 Stage 4 Konservat-Lagerstätten. The radiodonts found in the Kinzers Formation are potentially all endemic species, and include the youngest Tamisiocaris known, and the oldest Amplectobelua in Laurentia. This highlights the importance of the Kinzers Formation in understanding the diversity and evolution of Cambrian Radiodonta, and supports further exploration of this Lagerstätten.
Acknowledgements
We would like to thank the editor, Paul Upchurch, and two anonymous reviewers for their comments on the manuscript. S.P. is funded by an Oxford-St Catherine's Brade – Natural Motion Scholarship. A Palaeontological Association Sylvester-Bradley Award (PA-SB201503) allowed travel to Franklin Marshall College, USNM and YPM. For facilitating access to specimens, we thank Susan Butts and Jessica Utrup at YPM, Mark Florence at USNM and Roger D. K. Thomas at Franklin Marshall. The manuscript benefited from discussions with P. Cong, G. Edgecombe, R. R. Gaines, B. S. Lieberman, and R. D. K. Thomas.
Declaration of interest
None.












