Introduction
The tigerfish Hydrocynus vittatus Castelnau, 1861, is a freshwater piscivorous and pelagic predator that is widely distributed throughout southern Africa, specifically in the Zambezi River, the Okavango River and Delta, the Limpopo River system and coastal systems south to the Phongolo (Skelton, Reference Skelton2001). Perhaps due to its importance for both commercial and recreational fisheries (Griffith, Reference Griffith1975; Winemiller & Kelso-Winemiller, Reference Winemiller and Kelso-Winemiller1994), not a few studies have investigated the helminth parasites of this species. To date, along with nematodes (Boomker, Reference Boomker1994; Echi & Ezenwaji, Reference Echi and Ezenwaji2010; Moravec & Van As, Reference Moravec and Van As2015; Tavakol et al., Reference Tavakol, Smit, Sara, Halajian and Luus-Powell2015; Mabika et al., Reference Mabika, Barson, Van Dyk and Avenant-Oldewage2016) and cestodes (Mabika et al., Reference Mabika, Barson, Van Dyk and Avenant-Oldewage2016), eight species/subspecies belonging to Annulotrema have been recorded from H. vittatus; these are A. longipenis Paperna, Reference Paperna and Thurston1969 (Mali; Guégan et al., Reference Guégan, Lambert and Birgi1988), A. magna Paperna, 1973 (Tanzania; Paperna, Reference Paperna1973, Reference Paperna1979), A. nili Paperna, 1973 (Mali; Guégan et al., Reference Guégan, Lambert and Birgi1988), A. nili ruahae Paperna, 1979 (Tanzania; Paperna, Reference Paperna1979), A. pikei (Price, Peebles & Bamford, 1969) Paperna, 1979 (South Africa; Price et al., Reference Price, Peebles and Bamford1969; Mali, Guégan et al., Reference Guégan, Lambert and Birgi1988), A. pikei ruahae Paperna, 1979 (Tanzania; Paperna, Reference Paperna1979), A. pikoides Guégan, Lambert & Birgi, 1988 (Mali; Guégan et al., Reference Guégan, Lambert and Birgi1988) and A. ruahae Paperna, 1973 (Tanzania; Paperna, Reference Paperna1973, Reference Paperna1979).
The present study adds two new species to the parasite fauna of this host and includes a new locality record for A. pikoides.
Materials and methods
Specimens of H. vittatus were captured in Lake Kariba (16°4′51.63″S; 28°52′4.98″E), Zimbabwe, during a field campaign in 2011. Fish were transported live to the field laboratory and sacrificed by severing the spinal cord. The methods of parasite collection, and the preparation of monogeneans for morphological study followed Kičinjaová et al. (Reference Kičinjaová, Blažek, Gelnar and Řehulková2015). Briefly, gills of freshly killed fish were extracted and examined in bottled water under a dissecting microscope. Live monogeneans were picked individually from the gills with fine needles and processed immediately. Specimens prepared for morphological study were flattened using coverslip pressure, in order to best expose their hard parts, and fixed with a mixture of glycerine and ammonium picrate (GAP; Malmberg, Reference Malmberg1957). Measurements are all in micrometres and were taken using digital image analysis (Stream Motion, version 1.9.2; Olympus, Prague, Czech Republic); means are followed by the range and the number (n) of specimens measured in parentheses. The schemes of measurement for the hard structures (i.e. haptoral sclerites; male copulatory organ, abbreviated below as MCO; and vagina) are shown in fig. 1. The numbering of hook pairs (in Roman numerals) follows that recommended by Mizelle (Reference Mizelle1936). Note that the authors of the new taxa are different from the authors of this paper; see Article 50.1 and Recommendation 50A of the International Code of Zoological Nomenclature. Type and voucher specimens were deposited in the invertebrate collection of the Royal Museum for Central Africa (RMCA), Tervuren, Belgium. For comparative purposes, the following specimens of previously described species were examined: A. pikoides Guégan, Lambert & Birgi, 1988, MNHN 192 HC (from H. vittatus, Mali); A. nili ruahae Paperna, 1979 and A. ruahae Paperna, 1973, M.T. 35.518 (from H. vittatus, Tanzania) and A. nili Paperna, 1973, IPCAS M-602 (from Hydrocynus forskahlii, Kenya).
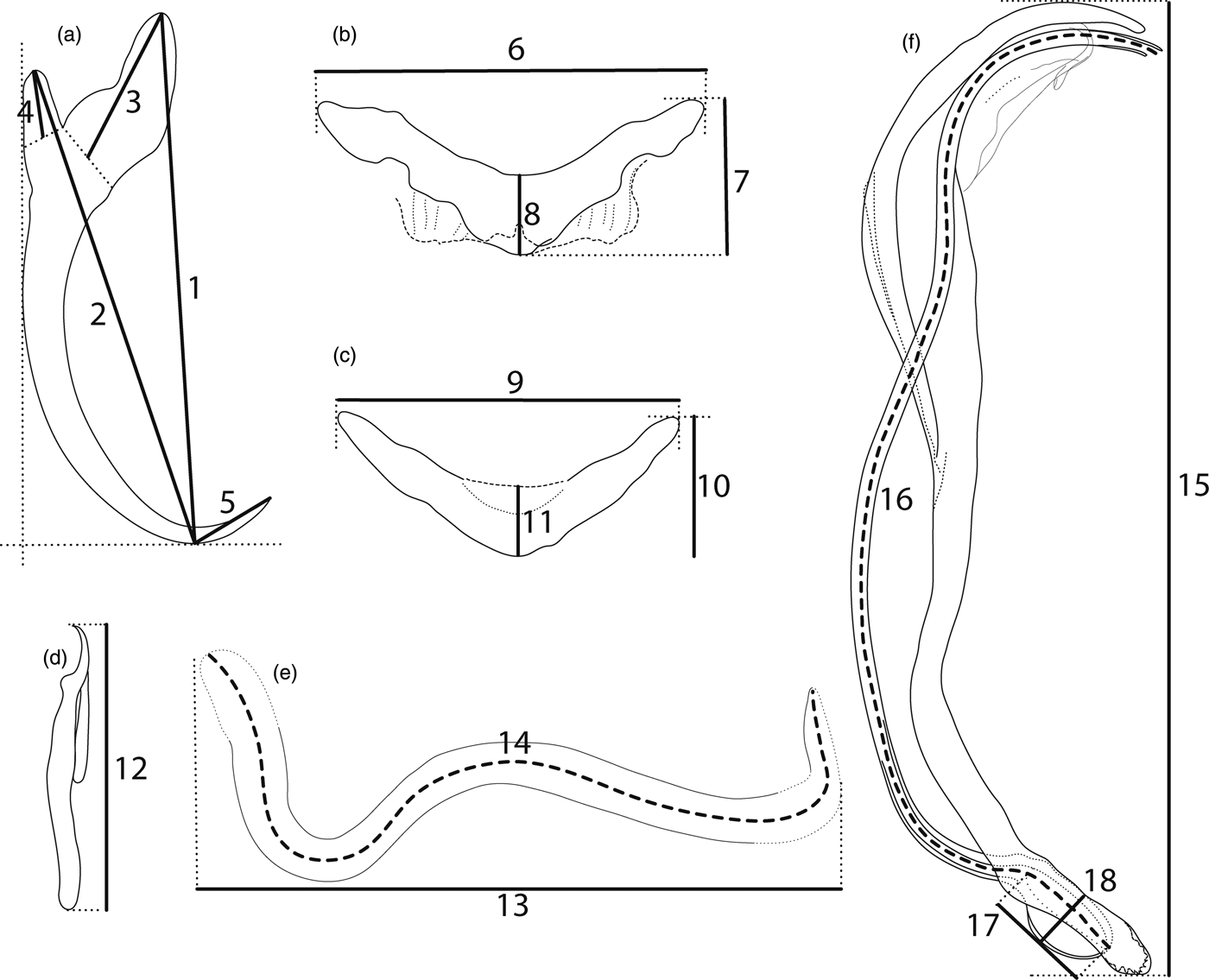
Fig. 1. Scheme of measurements for sclerotized structures of haptor and reproductive organs. (a) anchor: 1 = inner length, 2 = outer length, 3 = inner root length, 4 = outer root length, 5 = point length; (b) ventral bar: 6 = total length, 7 = total width, 8 = median width; (c) dorsal bar: 9 = total length, 10 = total width, 11 = median width; (d) hook: 12 = total length; (e) vagina: 13 = total length, 14 = trace length; (f) MCO: 15 = total length, 16 = tube-trace length, 17 = base length, 18 = base width.
Results
The two examined specimens of H. vittatus were infected by 12 and 24 monogeneans, respectively, representing three species of Annulotrema Paperna & Thurston, 1969 (Dactylogyridae). Two of them are described below as species new to science.
Dactylogyridae Bychowsky, 1933, Annulotrema Paperna & Thurston, 1969
Annulotrema pikoides Guégan, Lambert & Birgi, 1988
Type host and locality
Hydrocynus vittatus, Mali.
Present record
Hydrocynus vittatus, Lake Kariba, Zimbabwe.
Site
Gill lamellae.
Comparative material examined
Holotype, paratypes (MNHN 192 HC) of A. pikoides from H. vittatus (Mali).
Material deposited
Vouchers MRAC MT.38190, MRAC MT.38192, MRAC MT.38193, MRAC MT.38194.
Measurements
Based on five unflattened and ten flattened specimens in GAP. The sclerotized structures are illustrated in fig. 2. Body length 682 (633–734; n = 5); greatest width 97 (84–109; n = 5). Ventral anchors: inner length 43 (42–46; n = 10); outer length 41 (36–45; n = 10); inner root 13 (12–13; n = 10); outer root 10 (6–8; n = 10); point 7 (6–8; n = 10). Dorsal anchors: inner length 44 (43–46; n = 10); outer length 40 (38–41; n = 10); inner root 13 (12–15; n = 10); outer root 6 (5–8; n = 10); point 7 (5–8; n = 10). Ventral bar: total length 32 (31–35; n = 10); median width 8 (7–9; n = 10); total width 14 (12–16; n = 10). Dorsal bar: total length 29 (27–32; n = 10); median width 8 (6–10; n = 10); total width 12 (11–14; n = 10). Hooks: seven pairs, dissimilar in size; hook lengths (n = 10): pair I 17 (15–19); pair II 22 (20–23); pair III 29 (26–31); pair IV 31 (24–34); pair V 13 (12–14); pair VI 28 (26–30); pair VII 23 (21–24). Vagina (weakly sclerotized): total length 51 (38–60; n = 10); trace length 68 (47–76; n = 10). MCO: total length 78 (72–87; n = 10); tube-trace length 100 (95–105; n = 10); base length 11 (9–11; n = 10); base width 5 (4–5; n = 10).
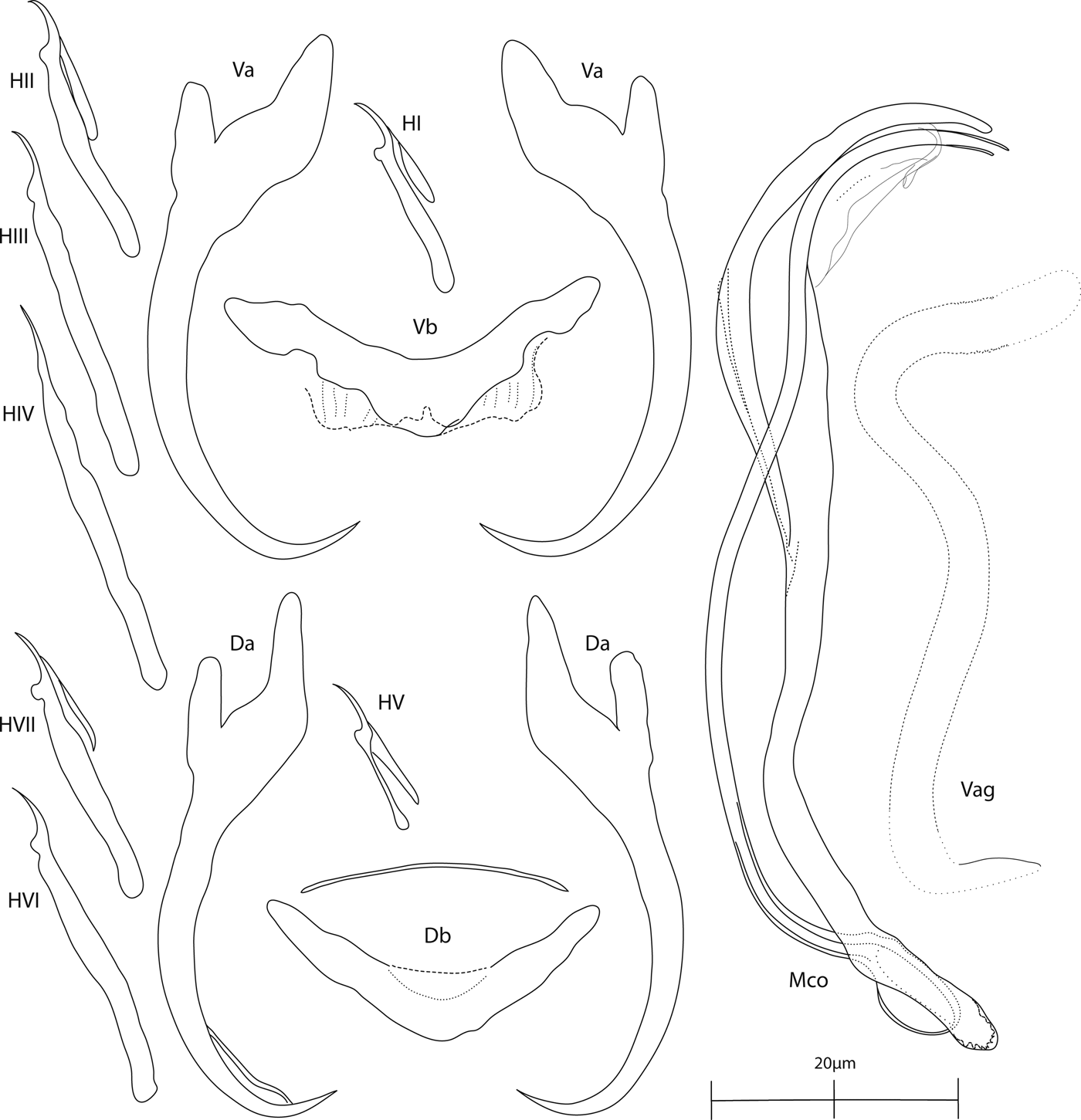
Fig. 2. Annulotrema pikoides Guégan, Lambert & Birgi, 1988. Sclerotized structures: Va, ventral anchor; Vb, ventral bar; Da, dorsal anchor; Db, dorsal bar; H, hooks (pair I–VII); Vag, vagina; Mco, male copulatory organ.
Remarks
The present specimens of A. pikoides correspond in most morphological and metrical characters to those described originally by Guégan et al. (Reference Guégan, Lambert and Birgi1988) from the same host species in Mali. The comparison of our specimens from Zimbabwe with the type material (MNHN 192 HC) of A. pikoides revealed only a minor difference in the length of the copulatory tube, which is slightly larger in specimens from Zimbabwe (i.e. 95–105 vs. 74–101 in the type specimens). Annulotrema pikoides differs from all other congeners by having an MCO markedly large in relation to the size of the haptoral structures. This species is well differentiated even under a low magnification on the basis of the presence of the big MCO, resembling open handcuffs (fig. 3).

Fig. 3. Annulotrema pikoides Guégan, Lambert & Birgi, 1988. Phase contrast micrograph of the MCO to display its resemblance to open handcuffs. Scale bar: 20 μm.
Annulotrema bracteatum Kičinjaová & Řehulková n. sp.
Type host and locality
Hydrocynus vittatus, Lake Kariba, Zimbabwe.
Site: Gill lamellae.
Type specimens
Holotype, paratypes MRAC MT.38195; paratypes MRAC MT.38189.
Comparative material examined
Holotype (M.T. 35.518C) of A. ruahae from H. vittatus (Tanzania).
ZooBank registration
To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for A. bracteatum n. sp. is urn:lsid:zoobank.org:act:5D4077CD-3D30-4D50-9103-5F4D7E90D4BC.
Etymology
The specific name ‘bracteatum’ refers to the accessory piece provided with bract-like structures.
Description
Based on two unflattened and four flattened specimens in GAP. The sclerotized structures are illustrated in fig. 4. Body length 683 (523–792; n = 2); greatest width 91 (81–108; n = 2). Ventral anchors with inner root recurved along its terminal part, well-developed outer root, elongated bent shaft, and short point; inner length 44 (43– 46; n = 4); outer length 45 (43–46; n = 4); inner root 11 (11–13; n = 4); outer root 6 (5–7; n = 4); point 9 (9–10; n = 4). Dorsal anchors with elongated inner root having recurved terminal half, well-developed outer root, moderately curved shaft, and short point; inner length 47 (42–51; n = 4); outer length 41 (38–42; n = 4); inner root 15 (15–16; n = 4); outer root 5 (n = 4); point 7 (6–8; n = 4). Ventral bar with triangular process arising from its medial part, supporting membrane well defined; total length 31 (29–33; n = 4); median width 12 (11–12; n = 4); total width 15 (15–17; n = 4). Dorsal bar saddle-shaped, supporting membrane weakly sclerotized; total length 27 (24–28; n = 4); median width 9 (8–11; n = 4); total width 15 (13–16; n = 4). Hooks: seven pairs, dissimilar in size; hook lengths (n = 4): pair I 16 (15–16); pair II 22 (21–22); pair III 23 (21–24); pair IV 29 (25–31); pair V 11 (10–12); pair VI 24 (22–26); pair VII 22 (20–25). Vagina a weakly sclerotized short tube; total length 28 (25–31; n = 4); trace length 33 (30–36; n = 4). MCO comprising basally unarticulated copulatory tube, accessory piece; total length 49 (36–56; n = 4). Copulatory tube an elongate slender tube, usually medially looped or meandering, with thick-walled pouch-like base; tube-trace length 113 (108–117; n = 4); base length 12 (11–13; n = 4); base width 8 (7–8; n = 4). Accessory piece comprising leaf-shaped (filamentous) sheath along distal part of the copulatory tube.

Fig. 4. Annulotrema bracteatum n. sp. Sclerotized structures: Va, ventral anchor; Vb, ventral bar; Da, dorsal anchor; Db, dorsal bar; H, hooks (pair I–VII); Vag, vagina; Mco, male copulatory organ.
Remarks
The new species most closely resembles A. ruahae described by Paperna (Reference Paperna1973, Reference Paperna1979) from the gills of H. vittatus in Tanzania. Examination of one of the four syntypes (i.e. specimen circled in C on slide M.T. 35.518; all others were excessively cleared, which precluded information on their MCO) of A. ruahae (fig. 5) showed that both species possess morphologically similar haptoral sclerites and a meandering copulatory tube with a base consisting of a heavily sclerotized neck and bulbous proximal part. However, the two species are clearly differentiated, as A. bracteatum n. sp. has an MCO composed of a longer copulatory tube (108–117 vs. 74 in A. ruahae) and a leaf-shaped accessory piece enveloping the distal part of the tube (accessory piece complex in A. ruahae with noticeable proximal hook and nearly enveloping the medial part of the copulatory tube).

Fig. 5. Annulotrema ruahae Paperna, 1973 (from type material M.T. 35.518). Sclerotized structures: Va, ventral anchor; Vb, ventral bar; Da, dorsal anchor; Db, dorsal bar; H, hooks (pair I–VII); Vag, vagina; Mco, male copulatory organ.
Annulotrema pseudonili Kičinjaová & Řehulková n. sp.
Type host and locality
Hydrocynus vittatus, Lake Kariba, Zimbabwe.
Site: Gill lamellae.
Type specimens
Holotype, paratypes MRAC MT.38194; paratypes MRAC MT.38189, MRAC MT.38191, MRAC MT.38192, MRAC MT.38193, MRAC MT.38195, MRAC MT.38196.
Comparative material examined
Syntypes (M.T. 35.518. B, C) of A. nili ruahae from H. vittatus (Tanzania); voucher (IPCAS M-602) of A. nili from H. forskahlii (Kenya).
ZooBank registration
To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for A. pseudonili n. sp. is urn:lsid: urn:lsid:zoobank.org:act:AEA4DC34-2367-4C84-B5C2-C28E1FFF3780.
Etymology
The specific name is formed from the prefix ‘pseudo’ (Greek pseudo-, comb. form of pseudes = false) and the species name ‘nili’ because of its resemblance to A. nili Paperna, 1973.
Description
Based on three unflattened and 11 flattened specimens in GAP. The sclerotized structures are illustrated in fig. 6. Body length 580 (520–670; n = 3); greatest width 123 (116–126; n = 3). Ventral anchors with short inner root slightly recurved along its terminal part, well-developed outer root, elongated bent shaft and short point: inner length 39 (37–41; n = 11); outer length 43 (41–46; n = 11); inner root 10 (8–12; n = 11); outer root 7 (5–11; n = 11); point 7 (5–8; n = 11). Dorsal anchors with elongated inner root having recurved terminal half, well-developed outer root, slightly curved shaft and short point: inner length 47 (46–50; n = 11); outer length 37 (34–39; n = 11); inner root elongated 18 (16–19; n = 11); outer root 7 (6–7; n = 11); point 8 (5–11; n = 11). Ventral bar with triangular process arising from its medial part, supporting membrane well defined; total length 30 (29–31; n = 11); median width 12 (9–13; n = 11); total width 17 (16–19; n = 11). Dorsal bar saddle-shaped, supporting membrane weakly sclerotized; total length 28 (26–29; n = 11); median width 13 (12–14; n = 11); total width 19 (18–19; n = 11). Hooks: seven pairs, dissimilar in size; hook lengths (n = 11): pair I 19 (17–19); pair II 23 (22–23); pair III 26 (26–27); pair IV 31 (29–32); pair V 14 (13–15); pair VI 26 (25–27); pair VII 32 (31–34). Vagina not observed. MCO comprising basally unarticulated copulatory tube, accessory piece; total length 33 (27–36; n = 11). Copulatory tube an elongate slender tube, usually medially looped or meandering in the shape of a number three (fig. 7), with thin-walled pouch-like base (usually, the proximal part of the base folds back over the more distal part); tube-trace length 86 (75–91; n = 11); base length 9 (6–10; n = 11); base width 5 (4–6; n = 11). Accessory piece a filamentous sheath along the distal part of the copulatory tube.

Fig. 6. Annulotrema pseudonili n. sp. Sclerotized structures: Va, ventral anchor; Vb, ventral bar; Da, dorsal anchor; Db, dorsal bar; H, hooks (pair I–VII); Mco, male copulatory organ.

Fig. 7. Comparison of MCO morphology of two Annulotrema species: (a) A. pseudonili n. sp.; (b) A. nili Paperna, 1973, voucher IPCAS M-602 (Kičinjaová et al., Reference Kičinjaová, Blažek, Gelnar and Řehulková2015). Scale bar: 10 μm.
Remarks
Annulotrema pseudonili n. sp. closely resembles A. nili Paperna, 1973 (see Fig. 10 in Kičinjaová et al., Reference Kičinjaová, Blažek, Gelnar and Řehulková2015) and A. nili ruahae Paperna, 1979, as shown by the comparative morphology of the haptoral armament and MCO. Morphological differences between A. pseudonili n. sp. and A. nili Paperna, 1973 are small, but sufficient for separating these two species. In A. pseudonili n. sp., both anchor–bar complexes are comparatively shorter, the ventral anchor possesses a proximally slightly bent shaft (vs. proximally sharply bent shaft), the inner root of the dorsal anchor is shorter and the MCO is more delicate (with a thinner tube; see fig. 7). Finally, the base of the copulatory tube is thin-walled (a feature which is difficult to observe even under phase-contrast microscopy) in A. pseudonili n. sp., whereas in A. nili the base is formed as a thick-walled, crescent-shaped roll with a fibrous proximal part. A copulatory tube similar to that found in A. pseudonili n. sp. was depicted by Paperna (Reference Paperna1979) in A. nili ruahae (see Plate XXXVII, Figs 2, 3). He described this subspecies on H. vittatus in Tanzania and differentiated it from A. nili by virtue of it having longer anchors with elongated shafts, much longer hooks and a much longer MCO. Our examination of the type material of A. nili ruahae (i.e. three specimens circled in B on slide M.T. 35.518.) showed that our specimens of A. pseudonili n. sp. differ from A. nili ruahae by possessing a smaller ventral anchor (inner length: 37–41 vs. 44–46 in A. nili ruahae) with a shorter shaft, a smaller dorsal anchor (inner length: 46–50 vs. 56–60 in A. nili ruahae) with a shorter inner root (approximately 2 times the length of the outer root vs. 4 times in A. nili ruahae) and an MCO with a markedly longer copulatory tube (81vs. 61 in A. nili ruahae; measured without base). Unfortunately, the base of the copulatory tube and the accessory piece are not completely visible in the excessively cleared specimens mounted in glycerine jelly, and comparison with our specimens was not possible. Annulotrema nili ruahae should probably be elevated to specific rank; however, re-description will depend on collection of new parasite material from H. vittatus, ideally in the type locality (Ruaha River,Tanzania).
Discussion
Hydrocynus is a genus of large characin fish in the family Alestidae, which contains five species, namely H. brevis (Günther, 1864), H. forskahlii (Cuvier, 1819), H. goliath (Boulenger, 1898), H. tanzaniae Brewster, 1986 and H. vittatus Castelnau, 1861 (Froese & Pauly, Reference Froese and Pauly2016). In addition to some unidentified tigerfishes (see Paperna, Reference Paperna1969), three of the five valid species of Hydrocynus have been reported as hosts of dactylogyrids belonging to Annulotrema; namely, H. brevis, H. forskahlii and H. vittatus (Paperna, Reference Paperna1969, Reference Paperna1973, Reference Paperna1979; Guégan et al., Reference Guégan, Lambert and Birgi1988; Řehulková et al., Reference Řehulková, Musilová and Gelnar2014; Kičinjaová et al., Reference Kičinjaová, Blažek, Gelnar and Řehulková2015; see table 1). As indicated in table 1 and the Introduction, six species and two subspecies belonging to Annulotrema have been recorded on H. vittatus. Based on the illustrations given by Paperna (Reference Paperna1979), A. nili ruahae and A. pikei ruahae should be, in our opinion, elevated to specific rank. However, we have not formally made this taxonomic action at this time in order to prevent possible unnecessary synonymy upon completion of revisory work on Annulotrema. Also, Paperna's (Reference Paperna1969) generic assignment of Afrocleidodiscus hydrocynuous Paperna, 1969 from Hydrocynus sp. in Ghana is likely erroneous. This view is supported by Paperna's whole-body illustration (Fig. 29) of this species, which shows an annulated peduncle region, one of the defining characters of Annulotrema. In addition, the MCO of the species (Fig. 27) shares similar morphological patterns with those of A. nili, A. nili ruahae and A. pseudonili n. sp. Thus, A. hydrocynuous should probably be transferred to Annulotrema.
Table 1. Annulotrema species reported from the gills of the three Hydrocynus species (Alestidae).

Beyond corroboration of morphological evidence for five species recognized in the current taxonomy of Hydrocynus, Goodier et al. (Reference Goodier, Cotterill, O'Ryan, Skelton and de Wit2011) discovered five previously unknown mitochondrial DNA (mtDNA) lineages in their phylogenetic tree (cyt b data) in relation to their geographical distributions. Within H. vittatus, three well-supported, previously unrecognized lineages were recovered by these authors. Inasmuch as the natural classification of some parasite groups usually corresponds directly with the natural relationships of their hosts (Eichler, Reference Eichler1948; Mendlová et al., Reference Mendlová, Desdevises, Civáňová, Pariselle and Šimková2012; Šimková et al., Reference Šimková, Serbielle, Pariselle, Vanhove and Morand2013), it will be interesting to see whether and how community structures of the Annulotrema species infesting African tigerfishes vary with geographic location. Thus, broader sampling of tigerfishes and further analysis (utilizing both morphological and molecular data) are required to provide more detailed resolution on the relationships between monogeneans and their Hydrocynus hosts across Africa.
Acknowledgements
We are indebted to Iva Přikrylová (Department of Botany and Zoology, MU Brno, Czech Republic) for help with monogenean collection. We are grateful to the staff of the University of Zimbabwe Lake Kariba Research Station for their field assistance in the collection of samples. We also thank Maarten Vanhove, Didier Van den Spiegel and Christophe Allard (Royal Museum for Central Africa, Tervuren, Belgium) for the kind loan of type specimens.
Financial support
This study was supported by the Czech Science Foundation (project no. P505/12/G112).
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals (Law 246/1992 of the Czech Republic).










