Introduction
A nearly continuous record of rocks spanning nearly 50 million years of time is preserved in the John Day Basin of Oregon. The paleontology of this region has been studied for nearly 150 years, with hundreds of localities yielding faunas and floras from the middle Eocene through the late Miocene. The Oligocene and Miocene faunas of this region show incredible mammalian diversity, but the older Eocene records are relatively sparse. While there are many Eocene paleobotanical sites in Oregon (Manchester, Reference Manchester1990, Reference Manchester1994; Wheeler and Manchester, Reference Wheeler and Manchester2002; Dilhoff et al., Reference Dillhoff, Dillhoff, Dunn, Myers and Strömberg2009), only two localities have documented mammal faunas (Hanson, Reference Hanson1989, Reference Hanson1996; Fremd, Reference Fremd2010). These localities, the Clarno Nut Beds and the Hancock Mammal Quarry, are within a small area, which is now protected within the Clarno Unit of John Day Fossil Beds National Monument (Fig. 1).

Figure 1 Map showing location of Clarno Unit of John Day Fossil Beds National Monument, Wheeler County, Oregon.
The Clarno Nut Beds are renowned as one of the best paleobotanical sites on the planet, with an incredibly diverse flora preserved in the form of nuts, seeds, and petrified wood (Scott, Reference Scott1954; Manchester, Reference Manchester1981, Reference Manchester1990, Reference Manchester1994; Wheeler and Manchester, Reference Wheeler and Manchester2002; Dillhoff et al., Reference Dillhoff, Dillhoff, Dunn, Myers and Strömberg2009). Fossil plants indicate the region contained a broad-leafed evergreen subtropical forest (Manchester, Reference Manchester1994; Wheeler and Manchester, Reference Wheeler and Manchester2002). Among the Clarno flora are thermophilic plants such as palms, cycads, and even a banana (Ensete), along with more temperate taxa including sycamores, elms, maples, birches, and walnuts (Manchester, Reference Manchester1994, Reference Manchester1995; Wheeler and Manchester, Reference Wheeler and Manchester2002). Several types of floral analyses (leaf physiognomy and leaf margins) suggest mean annual temperatures of 14-21 °C and mean annual precipitation as high as 3000 mm for the Clarno floral assemblages (Wolfe, Reference Wolfe1992; Manchester, Reference Manchester2000; Wheeler and Manchester, Reference Wheeler and Manchester2002; Myers, Reference Myers2003), with an estimated MAT of 17.05±1.84 °C for the Clarno Nut Beds (Manchester, Reference Manchester2000; Dillhoff et al., Reference Dillhoff, Dillhoff, Dunn, Myers and Strömberg2009).
Vertebrate fossils in the Clarno Nut Beds tend to be rare and located in isolated pockets (Stirton, Reference Stirton1944; Hanson, Reference Hanson1989, Reference Hanson1996; Fremd, Reference Fremd2010), with fragile preservation in extremely hard matrix contributing to the scarcity of finds and their fragmentary nature. Among the Nut Beds fauna are stick insects, a crocodile (referred to Pristichampsus), tortoise (Hadrianus), oxyaenid creodont (Patrofelis), early equid (Orohippus), and hyrachyid (Hyrachyus) (Sellick, Reference Sellick1994; Hanson, Reference Hanson1996). These mammal and reptile fossils from Clarno Nut Beds represent the oldest Cenozoic vertebrate locality within the Pacific Northwest (Robinson et al., Reference Robinson, Gunnell, Walsh, Clyde, Storer, Stucky, Froelich, Ferrusquia-Villafranca and McKenna2004; Fremd, Reference Fremd2010). Here we describe the most abundant vertebrate from the Clarno Nut Beds assemblage, a new genus and species of a rather small-bodied brontothere (Brontotheriidae) previously identified as Telmatherium sp. by Hanson (Reference Hanson1996). Although brontotheres are generally known for having evolved very large body size and being among the earliest mammalian megaherbivores, this new species is one of several small brontotheres discovered in recent decades that suggest an understanding of body size evolution in Brontotheriidae warrants reanalysis.
Geological setting
The Clarno Formation consists of volcanic rocks and volcaniclastic sedimentary rocks, ranging in age from approximately 54 to 39 Ma based on radiometric dates throughout the region (Bestland et al., Reference Bestland, Hammond, Blackwell, Kays, Retallack and Stimac1999; Dillhoff et al., Reference Dillhoff, Dillhoff, Dunn, Myers and Strömberg2009; McClaughry et al., Reference McClaughry, Ferns, Streck, Patridge and Gordon2009) (Fig. 2). The Clarno Formation is characterized by lithologically heterogeneous and laterally discontinuous rocks, including lava flows, tuffs, lahars, mudstone, and conglomerates. These rocks formed within an extensional basin or series of basins near sea level in the Eocene (White and Robinson, Reference White and Robinson1992; Dillhoff et al., Reference Dillhoff, Dillhoff, Dunn, Myers and Strömberg2009). The source of the Clarno volcanic rocks is a broad arc of volcanoes formed by flat-slab subduction beneath western North America, from the Late Cretaceous through the middle Eocene (Noblett, Reference Noblett1981; White and Robinson, Reference White and Robinson1992). Among these volcanoes are andesitic stratocones, which produced basalt and andesite flows, as well as laharic breccias that characterize the Clarno Formation (White and Robinson, Reference White and Robinson1992; Dillhoff et al., Reference Dillhoff, Dillhoff, Dunn, Myers and Strömberg2009).

Figure 2 Generalized stratigraphic column for the Clarno Unit of John Day Fossil Beds National Monument, Wheeler County, Oregon. Strata and dates are based on Bestland et al. (Reference Bestland, Hammond, Blackwell, Kays, Retallack and Stimac1999).
Both the Eocene Clarno Formation and the lower part of the Oligocene John Day Formation are preserved within the Clarno Unit of John Day Fossil Beds National Monument. Hanson (Reference Hanson1996) and Bestland et al. (Reference Bestland, Hammond, Blackwell, Kays, Retallack and Stimac1999) both provide geologic and stratigraphic frameworks for the deposits within this unit, and provide a set of radiometric dates that bracket the faunal assemblage. There is some contention as to how the deposits formed; here we follow the nomenclature and dates provided by Bestland et al. (Reference Bestland, Hammond, Blackwell, Kays, Retallack and Stimac1999) (Fig. 2). The base of the section in the Clarno Unit is composed of the andesite of Pine Creek, dated at 51.2±0.5 Ma (Bestland et al., Reference Bestland, Hammond, Blackwell, Kays, Retallack and Stimac1999). Above the andesite is a sequence of debris-flow conglomerate deposits, the conglomerate of the Palisades and the conglomerates of Hancock Canyon (Bestland et al., Reference Bestland, Hammond, Blackwell, Kays, Retallack and Stimac1999). The Clarno Nut Beds locality itself is a silica-cemented conglomerate and sandstone portion of the conglomerates of Hancock Canyon. This Nut Beds unit is dominated by debris-flow deposits, with interspersed tuffaceous beds including one directly associated with some of the vertebrate fossils (Hanson, Reference Hanson1996; Bestland et al., Reference Bestland, Hammond, Blackwell, Kays, Retallack and Stimac1999). Bestland et al. (Reference Bestland, Hammond, Blackwell, Kays, Retallack and Stimac1999) reported a 40Ar/39Ar date of 44 Ma (provided by C.C. Swisher; no error reported) for the Nut Beds and Muddy Ranch tuff (or tuff of Currant Creek), while Manchester (Reference Manchester1994) reported a separate date of 43.76±0.29 Ma provided by B. Turrin for a plagioclase from the Nut Beds. An amygdaloid basalt unit also within the conglomerates of Hancock Canyon was 40Ar/39Ar dated at 43.8±0.5 Ma (Bestland et al., Reference Bestland, Hammond, Blackwell, Kays, Retallack and Stimac1999). Above the Nut Beds are red and lavender claystone units including a sequence of deeply weathered paleosols and a stony tuff bed dated 42.7±0.3 Ma (Bestland et al., Reference Bestland, Hammond, Blackwell, Kays, Retallack and Stimac1999). On top of the Clarno Formation deposits in this area is the John Day Formation Member A ash-flow tuff (Peck, Reference Peck1964; Robinson, Reference Robinson1975), which has been 40Ar/39Ar dated at 39.22±0.03 Ma in the Clarno area and 39.72±0.03 Ma near the Painted Hills (Bestland and Retallack, Reference Bestland and Retallack1994; Retallack et al., Reference Retallack, Bestland and Fremd2000; McClaughry et al., Reference McClaughry, Ferns, Streck, Patridge and Gordon2009).
Stirton (Reference Stirton1944) initially concluded that the Clarno Nut Beds fauna was Bridgerian in age, based upon the occurrence of Hyrachyus. Hanson (Reference Hanson1996) came to a similar conclusion, interpreting the presence of Orohippus major Marsh, Reference Marsh1874, Hyrachyus eximus Leidy, Reference Leidy1871, and Patriofelis ferox (Marsh, Reference Marsh1872b) as supporting a Bridgerian age for the fauna. A single radiometric date of 48.32±0.11 Ma (Swisher, Reference Swisher1992) was presented to support this interpretation, but multiple dates close to 44 Ma have been published for the Nut Beds itself (Manchester, Reference Manchester1994; Bestland et al., Reference Bestland, Hammond, Blackwell, Kays, Retallack and Stimac1999). Robinson et al. (Reference Robinson, Gunnell, Walsh, Clyde, Storer, Stucky, Froelich, Ferrusquia-Villafranca and McKenna2004) followed Hanson in this Bridgerian interpretation, indicating a late Bridgerian age for the Nut Beds fauna. However, the date of 43.76±0.29 Ma for the Nut Beds presented in Manchester (Reference Manchester1994) and the dates around 44 Ma provided by Bestland et al. (Reference Bestland, Hammond, Blackwell, Kays, Retallack and Stimac1999) would clearly place this locality in the mid Uintan NALMA.
The presence of apparently Bridgerian taxa at a Uintan aged locality in Oregon is not surprising. The Eocene North American Land Mammal Ages are typified based on sites within Wyoming and Utah, and not the Pacific Northwest (Robinson et al., Reference Robinson, Gunnell, Walsh, Clyde, Storer, Stucky, Froelich, Ferrusquia-Villafranca and McKenna2004). The biostratigraphy of the Eocene through Miocene interval in Oregon has not been studied in great detail, except for the Arikareean NALMA (Albright et al., Reference Albright, Woodburne, Fremd, Swisher, MacFadden and Scott2008). Strong concordance between Oregon faunas and those in Wyoming and Utah or the Great Plains is not necessarily expected, given the boundaries to dispersal between them and the general Asian affinities of some Oregon faunas (Hanson, Reference Hanson1996; Lucas, Reference Lucas2006). Both of the Clarno Formation vertebrate sites have well-constrained ages based on radiometric dates, but referring either to a NALMA is problematic. The Nut Beds show little faunal similarity to contemporaneous sites, while the Hancock Quarry shares taxa considered characteristic of multiple land mammal ages (Robinson et al., Reference Robinson, Gunnell, Walsh, Clyde, Storer, Stucky, Froelich, Ferrusquia-Villafranca and McKenna2004). However, as we report below, the overall morphology and phylogenetic affinities of the new species described below are consistent with a Uintan-aged brontothere.
Materials and methods
The following diagnosis, description, and phylogenetic analysis of the Nut Beds brontothere follow methods used for other brontotheriids in Mihlbachler (Reference Mihlbachler2007, Reference Mihlbachler2008, Reference Mihlbachler2011), Mihlbachler and Deméré (Reference Mihlbachler and Deméré2009, Reference Mihlbachler and Deméré2010), and Mihlbachler et al. (Reference Mihlbachler, Lucas, Emry and Bayshashov2004a). To maintain equivalency with the taxonomy in these works, the delimitation of this new species is based on the phylogenetic species concept where species are partitioned into the smallest diagnosable clusters of specimens (Cracraft, Reference Cracraft1989; Nixon and Wheeler, Reference Nixon and Wheeler1990; Wheeler and Platnick, Reference Wheeler and Platnick2000) using a form of population aggregation analysis (Davis and Nixon, Reference Davis and Nixon1992; Sites and Marshall, Reference Sites and Marshall2004), modified for dealing with fossil samples rather than extant populations (Mihlbachler, Reference Mihlbachler2008).
Measurements of all fossil specimens referred to the new Nut Beds brontothere are provided in Supplementary Data 1, using landmarks as defined in Mihlbachler (Reference Mihlbachler2008). To examine the phylogenetic position of the Nut Beds brontothere within Brontotheriidae, a phylogenetic analysis was run using the matrix of Mihlbachler (Reference Mihlbachler2011) with additional characters and taxa as explained below (Supplementary Data 2 and 3). The present matrix consists of 239 character states distributed among 92 characters, and 57 taxa including two outgroups, “Hyracotherium” (=Xenocohippus osborni sensu Froehlich, Reference Froehlich2002), Pachynolophus livinierensis Savage, Russell, and Louis, Reference Savage, Russell and Louis1965. Also included are two early brontotherioids Danjiangia pingi Wang, Reference Wang1995, and Lambdotherium popoagicum Cope, Reference Cope1880, and 53 brontotheriids, including every known member of Brontotheriidae that produce non-equivalent combinations of character data (Mihlbachler, Reference Mihlbachler2007, Reference Mihlbachler2008, Reference Mihlbachler2011; Mihlbachler et al., Reference Mihlbachler, Lucas, Emry and Bayshashov2004a, Reference Mihlbachler, Lucas and Emryb; Mihlbachler and Deméré, Reference Mihlbachler and Deméré2009, Reference Mihlbachler and Deméré2010) (Supplementary Data, Table 1S). All brontotheres were included in the analysis at the species level, except Palaeosyops, which was included as a genus due to ambiguities in species-level taxonomy (Gunnell and Yarbrough, Reference Gunnell and Yarborough2000; Mihlbachler, Reference Mihlbachler2008; Mader, Reference Mader2009). Two additional species, Eotitanops pakistanensis and Balochititanops haqi, recently described by Missiaen et al. (Reference Missiaen, Gunnell and Gingerich2011) from Pakistan were added. Eotitanops minimus Osborn, Reference Osborn1919 was excluded because its character data is redundant with Eotitanops borealis (Cope, Reference Cope1880) and therefore would have no effect on the analysis (Wilkinson, Reference Wilkinson1995). To ensure that the character data account for all of the variability observed in this species, all specimens referred to the new taxon were examined when coding phylogenetic characters. Finally, additional character data were added to Megacerops kuwagatarhinus Mader and Alexander, Reference Mader and Alexander1995 based on new observations of fossils referable to that species in the Royal Saskatchewan Museum, Regina (unpublished data, Mihlbachler, 2016).
Detailed descriptions of characters 1–87 are found in Mihlbachler (Reference Mihlbachler2008) and 88–90 in Mihlbachler (Reference Mihlbachler2011). Two additional characters were added from Missiaen et al. (Reference Missiaen, Gunnell and Gingerich2011).
Character 91
(character 11 in Missiaen et al., Reference Missiaen, Gunnell and Gingerich2011); M1-2 mesostyle: 0: extends not as far labially as the parstyle; 1: parastyle and mesostyle extend an equal distance labially. This character relates to the highly oblique angle of the ectoloph in brontotherioids Lambdotherium and Danjiangia. All brontotheriids were assigned state 1. This character was scored with a “?” for outgroups lacking molar mesostyles.
Character 92
(character 14 in Missiaen et al., Reference Missiaen, Gunnell and Gingerich2011); Upper molar protocone: 0: subequal to hypocone or moderately larger: 1: distinctly larger than the hypocone, at least 50% on M2. Basal brontotherioids and brontotheriids, Eotitanops Osborn, Reference Osborn1908, Palaeosyops Leidy, Reference Leidy1870b, Balochititanops haqi, and Bunobrontops savagei Holroyd and Ciochon, Reference Holroyd and Ciochon2000 have smaller molar hypocones in comparison to more derived brontotheriids.
Other characters from Missiaen et al. (Reference Missiaen, Gunnell and Gingerich2011) were not incorporated because: (1) the character has an autapomorphic distribution in our data matrix, (2) the character requires more explanation to code additional taxa, or (3) the character data were non-repeatable. A phylogenetic analysis of the entire data matrix was run in TNT software (http://www.cladistics.com/aboutTNT.html) Goloboff et al. (Reference Goloboff, Farris and Nixon2008). Multistate characters were ordered as described in Mihlbachler (Reference Mihlbachler2008, Reference Mihlbachler2011). For 1000 replicates, heuristic searches were used to find the most parsimonious tree (MPT) using the default “New Technology Search” settings.
Like prior analyses (e.g., Mihlbachler, Reference Mihlbachler2008), the dataset produced an excessive number of MPTs, mostly due to a small number of wildcard taxa whose positions in the MPTs are unstable, thereby collapsing numerous branches within the strict consensus. Wildcards were identified and pruned from the 2566 trees by calculating a strict reduced consensus (SRC) (Wilkinson, Reference Wilkinson1994, Reference Wilkinson1995) with RadCon software (Thorley and Page, Reference Thorley and Page2000) using procedures described in Mihlbachler (Reference Mihlbachler2008).
Repositories and institutional abbreviations
AMNH: Division of Vertebrate Paleontology, American Museum of Natural History, New York; CM: Carnegie Museum of Natural History, Pittsburgh; DMNH: Denver Museum of Nature and Science, Denver; JODA: John Day Fossil Beds National Monument, Thomas Condon Paleontology Center; UCM; Museum of Natural History, University of Colorado, Boulder; UCMP: Museum of Paleontology, University of California, Berkeley.
Systematic paleontology
Class Mammalia Linnaeus, Reference Linnaeus1758
Order Perissodactyla Owen, Reference Owen1848
Superfamily Brontotherioidea Marsh, Reference Marsh1873
Family Brontotheriidae Marsh, Reference Marsh1873
Genus Xylotitan new genus
Type species
Xylotitan cenosus Mihlbachler and Samuels by monotypy.
Diagnosis
As for type species by monotypy.
Etymology
The genus name Xylotitan combines “xylo”, from Greek, meaning ‘wood’, with “titan”, meaning giant. The name refers to the abundance of fossilized wood remains at the Clarno Nut Beds and the forest paleoenvironment in which this animal is found. “Titan” follows a naming convention for genera of Brontotheriidae, which are often also referred to as titanotheres.
Occurrence
As for type species, by monotypy.
Remarks
A genus may be monotypic or include any number of species (Winston, Reference Winston1999). In this specific instance, our decision to erect a new genus is born out of necessity due to ambiguity in the phylogenetic analysis presented below, in which the sister taxon (either Wickia or Metatelmatherium) is ambiguous. It was therefore not possible for us to include the new species into a preexisting genus and satisfy, with certainty, the criterion of monophyly required of polytypic genera. At present the genus and species diagnoses are identical.
Xylotitan cenosus new species
Figures 3.1–3.3, 5.1–5.12, 6.1–6.6

Figure 3 The holotype of Xylotitan cenosus n. sp. (UCMP 121825), left maxilla fragment with P1-P4: (1) lateral view; (2) lingual view; (3) occlusal view. Scale bar=2 cm.
Holotype
UCMP 121825, a left partial maxilla with P1, P2, P3, P4 (Fig. 3) from Clarno Formation, Nut Bed 1, UCMP locality V78127.
Diagnosis
Xylotitan cenosus n. sp. has a brief postcanine diastema, a simple P1 crown, a distinct P2 metacone, and weak premolar preprotocristae. Premolar hypocones are absent. Central molar fossae, a cingular parastyle shelf, and well-developed anterolingual cingular cusps are absent. Metalophs and vestigial paraconules are absent. The lower dentition includes large incisors that are all of similar size, a p1–p2 diastema (variably present), a postcanine diastema, an unreduced canine, elongate p2 trigonid, a metaconid on p4 but not on p2 or p3, shallow molar basins, and a slender m3. The molars of X. cenosus are typical for members of Brontotheriinae with tall, lingually angled ectolophs with weak labial ribs, and thinned lingual ectoloph enamel with wedge-shaped paracones and metacones, thereby differentiating it from Palaeosyops, Eotitanops, and other basal brontothere taxa.
Xylotitan cenosus n. sp. is most readily recognized by the combination of its small size and craniocaudally short nasal incision. X. cenosus is similar in size to Mesatirhinus junius (Leidy, Reference Leidy1872). However, compared to M. junius and most other Brontotheriinae, the nasal incision of X. cenosus is craniocaudally shortened, extending posteriorly to a point above the P1. Sthenodectes incisivum, Metatelmatherium ultimum, and Wickia brevirhinus have similarly short nasal incisions. However X. cenosus is smaller than any of these species.
Other fragmentary species of similar body size, but whose nasal incisions are not preserved, are differentiated from X. cenosus n. sp. in the following ways. Pygmaetitan panxienensis Miao, Reference Miao1982 has taller molars with large central molar fossae. Microtitan mongoliensis (Osborn, Reference Osborn1925) has antero-posteriorly longer molars. Acrotitan ulanshirehensis Ye, Reference Ye1983 has fewer lower incisors with an additional diastema between p1 and p2.
Occurrence
From the Nut Beds of the Clarno Formation, Central Oregon, middle Uintan North American Land Mammal Age, 43.76±0.29 Ma (Manchester, Reference Manchester1994).
Description
Xylotitan cenosus n. sp. is a relatively small brontothere whose holotype (UCMP 121825) is a maxillary fragment with premolars (Fig. 3) and a variety of referred specimens, mostly upper and lower dental specimens, all from the conglomerate of Hancock Canyon unit of the Clarno Formation of Oregon. From what can be determined of the skull from the partial holotype, Xylotitan cenosus bears special resemblance to other Uintan brontotheres Sthenodectes incisivum (Douglass, Reference Douglass1909), Wickia brevirhinus Mihlbachler, Reference Mihlbachler2008, and Metatelmatherium ultimum (Osborn, Reference Osborn1908) due to its craniocaudally short nasal incision. Therefore, the following description includes extensive comparison to these three larger species, in addition to brontotheres of similar size to X. cenosus, which include Fossendorhinus diploconus (Osborn, Reference Osborn1895), Mesatirhinus junius, Metarhinus Osborn, Reference Osborn1908, Microtitan mongoliensis, Pygmaetitan panxienensis Miao, Reference Miao1982, and others. Comparative descriptions and measurements of these other species are based on referrals, descriptions, and data found in Mihlbachler (Reference Mihlbachler2008) and Mihlbachler and Démére (Reference Mihlbachler and Deméré2010).
The most distinctive feature of the holotype (UCMP 121825) is its dorsoventrally tall and craniocaudally short nasal incision (Figs. 3, 4). Although the nasal incision is incompletely preserved, enough of it is present to determine that the upper margin of the maxilla is concave and strongly angled posterodorsally with the rostrum deepening posteriorly. This margin becomes nearly vertical above the P1, therefore the nasal incision would not have extended more posteriorly. This cranial fragment indicates a relatively tall face with craniocaudally shallow and dorsoventrally deep nasal incision, similar to the larger Sthenodectes, Wickia, and Metatelmatherium (Fig. 4). The rostrum also lacks the specialized dorsal bony cover seen in Metarhinus and Dolichorhinus hyognathus (Osborn, Reference Osborn1889).
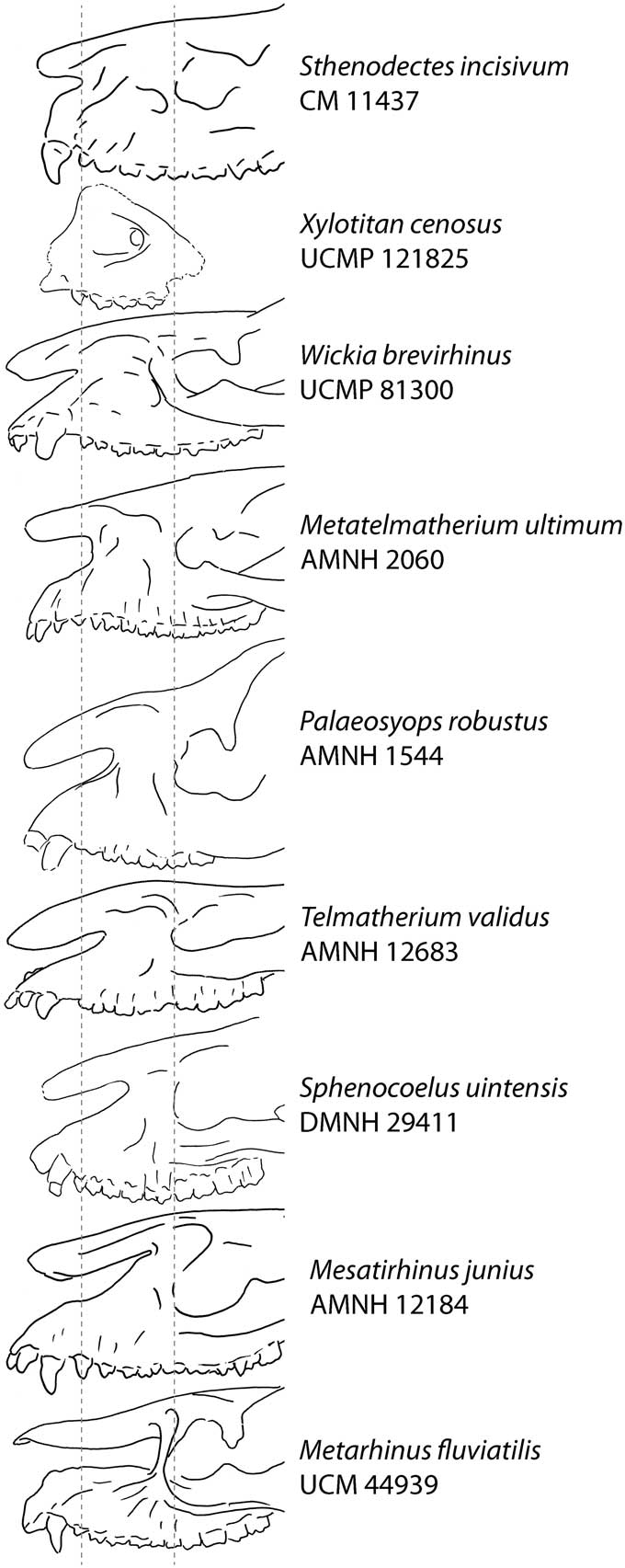
Figure 4 Lateral view of selected Bridgerian and Uintan brontotheres showing proportions of nasal incisions. All specimens are scaled and aligned so the anterior line passes through P1 and the posterior line passes through the anterior orbital margin.
The orbit is not preserved, but the infraorbital foramen, which, in brontotheres, is positioned immediately anterior to the anterior orbital rim, is located above the anterior root of P4. A preserved remnant of the anteroventral margin of the orbital rim is positioned superior to the anterior M1 root. This remnant of the orbital rim indicates that the orbits were not laterally protruding as in Metarhinus. The orbit would have been positioned above the M1 and more anteriorly than the orbits of Mesatirhinus and Microtitan, but similar to Sthenodectes, Wickia, and Metatelmatherium.
The holotype includes a complete left upper premolar row (P1-P4; Fig. 3). These teeth are worn, although the major details of crown morphology can be discerned. Other upper dental specimens referred to Xylotitan cenosus are shown in Fig. 5. A single relatively large incisor (UCMP 121836) of unknown position has a conular subcaniniform crown with a prominent lingual cingulum. Although this incisor is isolated, it resembles most other middle Eocene hornless brontotheres and in comparison to the size of other teeth is not as disproportionately enlarged as are the incisors of Sthenodectes incisivum or Pygmaetitan panxianensis. A number of isolated canines (e.g., UCMP 121835), on the other hand, are proportionately quite large. Many brontotheres have canines of variable size, and were probably sexually dimorphic (Mihlbachler, Reference Mihlbachler2011). If the canines of X. cenosus followed a pattern of sexual dimorphism similar to other brontotheres, the known canines are “male-like” in terms of their size. Although the canine of the holotype is not preserved, the remaining remnant of the postcanine diastema indicates its presence, though it is of uncertain length.

Figure 5 Isolated cranial and upper dental elements referred to Xylotitan cenosus: (1) Skull fragment showing occlusal surfaces of left P1, and DP3-DP4 (UCMP 121824) and lateral surface of M1, (2) left M1 (UCMP 121824), (3–4) anterior view and occlusal view of upper incisor (UCMP 121836), (5) canine (UCMP 121835), (6) right P3 (UCMP 121828), (7) right P3 (UCMP 192882), (8) left DP4 (UCMP 121826), (9) maxilla fragment with partial left M1, M2, and M3 (JODA 4771), (10) right M3 (UCMP 121833), (11) right M3 (UCMP 121832), (12–13) occlusal view and oblique view of right M3 (UCMP 121847). Scale bar=2 cm.
The P1 is the smallest premolar with the simplest crown, consisting of a single cusp and a crested posterior heel with a small lingual shelf and a lingual cingulum. There is no diastema between P1 and P2. A P1-P2 diastema is found in some brontotheres, such as Telmatherium validus Marsh, Reference Marsh1872a, but its presence is intraspecifically variable, so it is possible that the absence of a P1-P2 diastema in Xylotitan cenosus was not a fixed trait.
The anterior margin of the P2 is steeply angled posterolingually. There is a marked discontinuity in shape of the premolar crowns from the rather oblique and labiolingually short P2 crown to the more rectangular and labiologially elongate crowns of P3 and P4. Premolar mesostyles are not present. The parastyle of P2 arches slightly lingually, while the parastyle of P3 is nearly straight, and the P4 parastyle is angled labially. The P2 metastyle is slightly angled lingually, while those of P3 and P4 are straight. The labial ribs of the paracones follow the typical pattern for brontotheres of becoming narrower in progressively posterior premolars.
The lingual heel of the P2 is small and posteriorly shifted. Likewise, a small protocone is positioned posteriorly and nearly labial with respect to the metacone. The small preprotocrista of the P2 is angled anteriorly and is continuous with an anterior cingulum. A distinct posterior crest extends posteriorly from the protocone. The lingual heels of P3 and P4 are wider than that of P2, so that the anterior and posterior sides of the crowns are nearly parallel and the protocones are more centrally positioned labially between the paracone and metacone. Paraconules are not present on any of the premolars. A very low preprotocrista connects the protocone to the lingual side of the ectoloph of P3. The preprotocrista on the P4 is slightly weaker in appearance. Likewise, in comparison to the P2, the posterior crest of the protocone is weak on P3 and absent on P4. Premolar hypocones are absent. The lingual cingula of the P2 and P3 are thin but continuous around the lingual margins of these teeth, whereas the lingual cingulum of the P4 is discontinuous. Posterior to the protocone of the P4 there is a relatively broad posterior cingulum along the posterolingual corner of the tooth.
In these above respects, the premolars of Xylotitan cenosus are undifferentiated from other brontotheres to which Xylotitan is being compared in detail. Among these taxa, the premolars of X. cenosus differ mostly from Pygmaetitan panxianensis, which has a more complex semimolariform P1, more rectangular premolars, occasional premolar mesostyle, and small premolar hypocones.
The upper molars of Xylotitan cenosus exhibit numerous derived characteristics that clearly separate it from basal bronototheriids (e.g., Palaeosyops and Eotitanops). These advanced molar features include: (1) tall and lingually angled ectolophs with thin labial ribs, (2) thinned enamel on the lingual side of the ectoloph, and (3) sharp, vertically wedged lingual margins of the paracone and metacone. Basal brontotheres, Palaeosyops and Eotitanops, have a thickened anterior cingulum that forms a prominent parastylar shelf, which is absent in Xylotitan cenosus. The anterior molar cingulum of X. cenosus is thin, passes proximally to the distal peak of the parastyle, and no parastylar shelf is present. In all of these respects, the molars of X. cenosus resemble other brontotheres of the subfamily Brontotheriinae (sensu Mihlbachler, Reference Mihlbachler2008).
The upper molars of Xylotitan cenosus lack autapomorphies and are similar to those of Metatelmatherium ultimum and Wickia brevirhinus. None of the molars referred to X. cenosus have a central molar fossa. Some molar specimens (e.g., UCMP 121847, JODA 4771) have a small anterolingual cusp on the anterior cingulum, but this feature is not seen on the deciduous premolars (UCMP 121826). Paraconules do not occur on any of the molars. Some of the deciduous premolars referred to X. cenosus have a small metaloph-like ridge extending from the lingual margin of the metacone toward the hypocone (e.g., UMCP 121826; Fig. 5.8). However this ridge is absent in molars (e.g., JODA 4771; Fig. 5.9). None of the M3s has a distinct hypocone, although the posterolingual cingulum of the M3 is thickened and raised into a small cusp-like peak. The labial cingula of the molars tend to be thin and discontinuous around the mesostyles. The anterior cingulum of each molar terminates at the lingual base of the protocone and does not continue around the lingual side of the crown.
Relatively complete lower dentitions of Xylotitan cenosus are known from two mandibular specimens, UCMP 121839 and UCMP 121840 (Fig. 6), as well as a few isolated teeth. The shape of the mandibular symphysis (preserved in UCMP 121839) does not differ notably from most other Bridgerian–Uintan taxa. The inferior margin of the symphysis is typically steep (≥ 45°). The posterior margin of the symphysis is positioned between the borders of the p2 and p3.

Figure 6 Lower dentitions referred to Xylotitan cenosus: (1) occlusal view and (2) right lateral view of UCMP 121840, partial mandible with right p1-m3; (3) right lateral view and (4) occlusal view of UCMP 121839, partial mandible with right p1-p4; (5) enlarged labial and (6) lingual views of right p2-p4 (gray-scale cast of UCMP 121839). Scale bars=2 cm.
Although the anterior dentition is not preserved, incompletely preserved alveoli in UCMP 121839 indicate an unreduced lower dental formula (3-1-4-3). The alveolar border indicates the incisor row would have been strongly arched with approximate root diameters (from mesial to distal) not greater than 4.73 mm, 4.71 mm, and 3.98 mm. Judging from these partial alveoli, the i1 and i2 were of similar size, with the i3 being smaller. The incisors are proportionally similar to the incisors of most other Bridgerian–Uintan brontos. The lower incisors were not enlarged as in Sthenodectes incisivum or Pygmaetitan panxienensis, nor were they reduced to a nearly vestigial state as seen in many more advanced horned brontotheres such as the classic late Eocene Megacerops. A precanine diastema appears to have been absent. The canine alveoli of UCMP 121839 indicate a canine root diameter of at least 15 mm. There is a short postcanine diastema (length ~10 mm) and a shorter p1-p2 diastema (length=5 mm).
Details of the morphology of the lower premolars are quite clearly preserved in both specimens. However, there is little variation in the premolar morphologies of brontotheres to which Xylotitan cenosus bears close resemblance. The lower premolars therefore have little diagnostic value. The p1 (preserved in UCMP 121840) is a simple tooth with a single cusp with a shortened talonid heel and is anchored vertically in the jaw. The premolars are increasingly molariform posteriorly, although none of the premolars is completely molariform. The trigonids are shorter and broader in more posterior premolars: p2 trigonid is twice the length of the talonid; the p3 trigonid is slightly longer than the talonid, while the p4 trigonid is slightly shorter than the talonid. The p2 trigonid and talonid are of similar width; the p3 trigonid is slightly narrower than the talonid, and the p4 talonid is slightly wider than the trigonid. The paralophid of p2 extends in a directly anterior direction, curving lingually only at the anterior end; the p3 paralophid is slightly lingually directed, while the p4 paralophid is strongly angled lingually. The protolophids of the p2 and p3 are positioned lingually and angled directly posteriorly. Both p2 and p3 lack metaconids. The p4 protolophid arches 90° lingually, its orientation is equal to that of the molars, and it is connected to a large, lingually positioned metaconid. The lingual orientation of the p4 paralophid and protolophid results in a very broad lingual trigonid notch.
The talonid of the p2 is narrow with a weakly developed cristid obliqua and a mesiodistally short hypolophid. The lingual side of the p2 trigonid forms a slightly concave sloped surface. The p3 and p4 have more developed cristids obliqua and longer hypolophids. The lingual-talonid notches of p3 and p4 are broader than that of p2, although they do not form fully molariform basins. Lingual premolar cingulids are absent, while the labial premolar cingulids are weak and discontinuous.
The lower molars of Xylotitan cenosus also closely resemble almost all other brontotheriines, but have some diagnostic value when compared to other small-sized brontotheres. The molars (preserved only in UCMP 121840) have thinner enamel and more double-crescent shaped wear pattern than basal brontotheres, such as Eotitanops and Palaeosyops. The molars have typically shallow talonid and trigonid basins. In this respect, the lower molars are distinct from Pygmatitan panxianensis, whose molars are more unusual in having deeper basins. The length-width proportions of the third molar resemble most other brontotheriines, but are not nearly as elongate as that of Microtitan mongoliensis.
Etymology
The species name cenosus, from Latin, means ‘muddy’, in reference to the volcanic mud flows that preserved the Clarno Nut Beds.
Materials
JDNM-12, Uppermost Central Nut Beds: JODA 4771, partial maxillae with right and left M2 and M3, partial left M1. Locality UCMP V78127, Nut Bed 1: UCMP 121821, right M2 (partial); UCMP 121822, left upper molar fragment; UCMP 121824, fragment of left maxilla with P1, M1 and other partially exposed premolars and left DP2, DP3; UCMP 121826, left DP4; UCMP 121828, right (?)P3; UCMP 121827, right maxillary fragment with premolar fragments; UCMP 121831, left (?)P3; UCMP 121832, right M3; UCMP 121833, right M3; UCMP 121834, canine; UCMP 121835, canine; UCMP 121836, upper incisor; UCMP 121837, canine; UCMP 121838, right dentary fragment w/ p2 and p3 (partial); UCMP 121839, anterior mandible fragment with right p2, p3, p4; UCMP 121840, partial mandible with right p1, p2, p3, p4, m1, m2, m3, and left p1, p1, p3; UCMP 121841, right P2; UCMP 192882, right P3. Locality UCMP V65113, Clarno Nut Bed General: UCMP 121842 right p2; UCMP 121844, right M1 or M2 (partial); UCMP 121845, right M1 or M2 (partial); UCMP 121846, right M1 or M2 (partial); UCMP 121847, right M3; UCMP 121848, right m2; UCMP 121851, right lower molar fragment; UCMP 121852, molar fragment. Locality Nut Bed 2, UCMP V78127: UCMP 121853, partial tibia (provisionally included).
Phylogenetic results
TNT produced 2566 most parsimonious trees (MPTS) with lengths of 409 steps (CI=0.51; RI=0.81). For simplicity, abbreviated results are shown in Fig. 7 where Brontotheriina (the clade containing brontotheres with fronto-nasal horns) is reduced to a single terminal. The complete strict consensus and strict reduced consensus (SRC) trees are shown in Supplementary Data 3 (Figs. 1S, 2S). The strict consensus (Fig. 7.1) places Xylotitan cenosus in a polytomy with Wickia brevirhinus and Metatelmatherium ultimum. However, the position of this clade is uncertain with respect to several other North American and Asian brontotheres of middle Eocene age. Removal of wildcards in the SRC places Epimanteoceras formosus Granger and Gregory, Reference Granger and Gregory1943 with Brontotheriina, but adds no additional resolution (Fig. 7.2).

Figure 7 Summary of phylogenetic results for Brontotherioidea, including Xylotitan cenosus. (1) Summary of strict consensus. For simplicity, all 28 species of the Brontotheriina are collapsed into a single branch. (2) Reduced strict consensus of node marked with an arrow after a posteriori removal of Qufutitan zhoui and Nanotitanops shanghuangensis from all of the MPTs.
Discussion
Size
To gain perspective on its size relative to other brontotheres, the lengths and widths of upper and lower third premolars (P3, p3) of numerous late Bridgerian, Uintan, and similarly sized and aged Asian brontotheres are plotted in Fig. 8. The P3s of X. cenosus plot within the data clusters of Mesatirhinus junius, Metarhinus sp., and among the smallest specimens of Dolichorhinus hyognathus (Osborn, Reference Osborn1889). It is larger than Microtitan mongoliensis and smaller than all other species plotted. The p3s of X. cenosus also plot within the M. junius and Metarhinus sp. clusters, and are smaller than all other species plotted except M. mongoliensis.

Figure. 8 Bivariate plots of (1) upper and (2) lower third premolar dimensions of various middle Eocene brontotheres from North America and Asia.
Although Xylotitan cenosus is a large animal in generalized mammalian standards, it is diminutive among brontotheres. Brontotheres are commonly perceived as graviportal megaherbivores with body sizes roughly between that of modern rhinos and elephants. However, discoveries since the 1980s include several small-bodied species, including Pygmaetitan panxianensis, Nanotitanops shanghuangensis (Qi and Beard, Reference Qi and Beard1996), Acrotitan ulanshirehensis, and Balochititanops haqi (Missiaen, Gunnell, and Gingerich, Reference Missiaen, Gunnell and Gingerich2011). Body size estimates for X. cenosus, using equations based on M2 area (length×width) for hyracoids and perissodactyls provided by Janis (Reference Janis1990), suggest a mass of roughly 430 kg (Mihlbachler and Samuels, Reference Mihlbachler and Samuels2013), similar in size to the larger extant tapirs (Tapirus bairdii and Tapirus indicus). In comparison, the earliest brontotheriid, Eotitanops borealis, yields body mass estimates around 140 kg, while the largest brontotheres to have ever lived from the late Eocene of Asia and North America, Embolotherium andrewsi Osborn, Reference Osborn1929 and Megacerops coloradensis Leidy, Reference Leidy1870a, respectively, yield mass estimates between 3700–8500 kg (using dental measurements published in Mihlbachler, Reference Mihlbachler2008). The sister taxa of Xylotitan, including Wickia and Metatelmatherium, yield mass estimates between 1640–2140 kg. While the accuracy of these mass estimates is certainly questionable in absolute terms (Fortelius, Reference Fortelius1990), the estimates reveal the small size of Xylotitan cenosus in comparison to most brontotheres (Mihlbachler and Samuels, Reference Mihlbachler and Samuels2013) and suggest it is one of a number of examples that potentially represent phyletic nanism (O’Sullivan, Reference O’Sullivan2003; Gould and MacFadden, Reference Gould and MacFadden2004) in the Brontotheriidae. The size of Xylotitan may be a consequence of the environment preserved at the Nut Beds; the floral assemblage includes over 40% vines (lianas and scrabbling climbers) suggesting a densely vegetated habitat characterized by frequent disturbance (Manchester, Reference Manchester1994, Dillhoff et al., Reference Dillhoff, Dillhoff, Dunn, Myers and Strömberg2009). Dwarfism in the largest mammal preserved in the Clarno Nut Beds, and an overall size similar to tapirs inhabiting similar habitats today, may reflect constraints imposed on body mass for ungulates living in relatively dense tropical-forest environments.
Biogeography
Although the early record of brontotheres is more extensive in North America, there is enough fragmentary fossil material from Asia to suggest brontotheres were widespread in Asia and possibly more diverse in Asia than in North America during the early Eocene and early part of the middle Eocene (Holroyd and Ciochon, Reference Holroyd and Ciochon2000; Mihlbachler, Reference Mihlbachler2008; Missiaen et al., Reference Missiaen, Gunnell and Gingerich2011). However, the continent of origin remains in question for brontotheres (Mihlbachler, Reference Mihlbachler2008). By the middle Eocene, brontotheres were the most diverse large mammalian clade on both continents, until their terminal Eocene extinction. Prior phylogenetic analyses indicate that neither the North American nor the Asian brontothere faunas represent independent radiations. Brontothere phylogeny suggests that brontothere faunas on both continents are phylogenetically interwoven with 9-12 intercontinental dispersals having occurred during middle Eocene time, with dispersal in both directions (Mihlbachler, Reference Mihlbachler2008).
Eberle and Storer (Reference Eberle and Storer1999), Eberle (Reference Eberle2006), and Eberle and Eberth (Reference Eberle and Eberth2015) described early and middle Eocene brontothere specimens from within the Arctic Circle, which suggest that brontotheres were adaptable to high latitudes, possibly enabling frequent Beringian dispersal. To further support this hypothesis, some brontothere species have been found on both continents and had pan-Beringian distributions.
The Hancock Quarry, a fossil quarry from the upper Clarno Formation of the John Day Basin, yields a single large horned brontothere, Eubrontotherium clarnoensis Mihlbachler, Reference Mihlbachler2007, which was determined to be conspecific with brontothere remains recovered from the Ergilin Dzo, late Eocene of Mongolia (Mihlbachler, Reference Mihlbachler2008). The earlier John Day Basin species, Xylotitan cenosus, is not closely allied to E. clarnoensis, but has close phylogenetic ties with Metatelmatherium ultimum, another transcontinental species. Metatelmatherium ultimum occurs in the Myton Member of the Uinta Basin, Utah, Adobe Town Member of the Washakie Formation, Washakie Basin and Wind River Basin, both in Wyoming, and has also been identified from a complete skull and mandible from the “Irdin Manha” Formation of China (Nei Mongol) (Mihlbachler, Reference Mihlbachler2008). Its other potential sister taxon, Wickia brevirhinus, occurs only in North America, in the Sandwash Basin, Colorado and Adobe Town Member of the Washakie Formation, Wyoming. Unfortunately, poor phylogenetic resolution below the Xylotitan-Wickia-Metatalmatherium clade limits our understanding of the origins of this mid-Eocene clade of brontotheres. However, close phylogenetic proximity to M. ultimum would reinforce the conclusion that middle Eocene local faunas of central Oregon sustained some ties to Asian faunas, or at least ties to pan-Beringian species (Lucas et al., Reference Lucas, Foss and Mihlbachler2004; Lucas, Reference Lucas2006; Mihlbachler, Reference Mihlbachler2007).
Acknowledgements
We would like to thank P. Holroyd (University of California Museum of Paleontology) for kindly allowing access to specimens. Support for this research was provided in part by the National Park Service and the New York Institute of Technology College of Osteopathic Medicine.
Accessibility of supplemental data
Data available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.gk1s2










