Background
Cannabis is a substance that has been consumed in various formulations and for different purposes over many centuries.Reference Ayonrinde1 There is a changing legislative landscape from decriminalization to the legalization of cannabis, primarily for medical purposes, and in some jurisdictions for recreational use. Despite the rising enthusiasm for cannabis use as a panacea for varied conditions, there are conflicting views and relatively few high-quality studies regarding the health benefits and harms of more liberal cannabis consumption.Reference Hall, Stjepanović and Caulkins2,Reference Andrews, Devlin and Le Foll3 Unlike tobacco and alcohol, which have known dose-related adverse health outcomes,Reference Nelson, Van Ryzin and Dishion4,Reference Silins, Horwood and Najman5 there is inadequate knowledge regarding the varied properties of different cannabis products. Recreational cannabis users claim to use it for relaxation, for stress release, during social activities, and to improve concentration.Reference Hyman and Sinha6 Quantification of cannabis usage is typically obtained through self-reporting or from mandated testing due to clinical presentation or risk of toxicity. In youth, cannabis use has been associated with an increased risk of psychosis, but associations with physical health are less clear.Reference Bahji and Stephenson7 The legalization of recreational cannabis in Canada, several states of the USA, and the Australian Capital Territory, and increased availability of medicinal cannabis in various countries, emphasizes the importance of understanding the properties and adverse effects of cannabis use. Therefore, this review aims to summarize data regarding the epidemiology and clinical significance of gestational cannabis consumption for the mother, her developing fetus, neonate, and placenta.
Cannabis consumption in reproductive age adolescents and adults
In Australia, cannabis use or misuse frequently begins during adolescence.8 Approximately 35% of Australians aged 14 years and over have used cannabis during their lifetime. In adolescents and young adults aged 14–24 years, the average age for first cannabis use was 17 years. Among young adults aged in their 20s, 21% had used cannabis, making it the most commonly used recreational drug.9 Adolescents with early trajectories of cannabis consumption are more vulnerable to problematic substance use in early adulthood, plus lower educational and employment achievements.Reference Nelson, Van Ryzin and Dishion4,Reference Silins, Horwood and Najman5 In Canada, about 49% of males aged 15 years and above and 41% of females of the same age have used cannabis in their lifetime, while 44% of 15–19-year olds and 51% of 20–24-year olds report cannabis use in the past 12 months.10 In females, cannabis use beginning prior to or during early reproductive life could be associated with an unplanned pregnancy or inadvertent fetal exposure to cannabis during unrecognized pregnancy. Ceasing or reducing cannabis use after discovering unplanned pregnancy could be challenging for women who are dependent on cannabis. Consequently, at least one-third of cannabis users continue consuming it as a cheaper or “safer” option than tobacco during pregnancy.11 Though males consume larger quantities of cannabis than females and tend to initiate use at younger ages, females increase their rate of cannabis use more rapidly than males.12 International population surveys have described varied prevalence data for cannabis consumption in female adolescents and young adults, including 21% in Canada,10 20% in the USA,13 16% in Australia,9 and a wide range in Europe; from 0.2% in Turkey to 17.5% in Italy.14 Comparative data from these geographical jurisdictions are summarized in Fig. 1.
Perinatal cannabis consumption and maternal and perinatal outcomes
Cannabis is the most common recreational drug used during pregnancy, often in combination with alcohol or tobacco. The American College of Obstetricians and Gynecologists (ACOG) guidance reports a 2%–5% self-reported prevalence of gestational cannabis use that increased to up to 15%–28% in young, urban, socioeconomically disadvantaged women with an increased likelihood of concomitant tobacco and alcohol consumption.11 Other studies from various countries have identified indigenous ethnicity, young maternal age, and lower socioeconomic status to be risk factors for gestational cannabis use.Reference Passey, Sanson-Fisher, D’Este and Stirling15–Reference Grant, Petroff, Isoherranen, Stella and Burbacher17 In a study of Australian women from an Aboriginal birth cohort, 21% used cannabis during pregnancy.Reference Brown, Mensah and Ah Kit18 This associates with increasing cannabis consumption by unemployed or Indigenous Australians.9 Another study found gestational cannabis users, when compared with nonusers, had a lower level of education, lower household income, and lower likelihood of perinatal folic acid use.Reference van Gelder, Reefhuis and Caton19 Contrasting with the perception of sociodemographic disadvantage in women who consume cannabis during pregnancy, a large British study from nearly two decades ago described a 5% prevalence of perinatal cannabis smoking, predominantly in women who were younger, of lower parity, and better educated but more likely to smoke, use alcohol, and other substances.Reference Fergusson, Horwood and Northstone20 In that study, gestational cannabis was not associated with adverse maternal or perinatal outcomes. In the USA, gestational cannabis use doubled between the first and second decades of the twenty-first century, though reported to reduce as the pregnancy progressed from the first to the third trimester.Reference Volkow, Han, Compton and McCance-Katz21,Reference Young-Wolff, Sarovar and Tucker22 The ACOG report, however, described higher rates of cannabis consumption at the time of delivery compared to reports at prenatal visits, and up to 60% of gestational cannabis consumption continuing through pregnancy, suggesting earlier underreporting.11 Therefore, accurate and objective statistics on cannabis use in pregnant women, though important, are often elusive.
A proportion of women use cannabis for relief of severe pregnancy-associated nausea, pain, or mental health problems such as anxiety or depressionReference Metz and Borgelt23,Reference Roberson, Patrick and Hurwitz24 and not necessarily addiction or dependence. With the legalization of cannabis in various jurisdictions, a corresponding rise in the reported rates of prenatal cannabis consumption has been observed.Reference Gnofam, Allshouse, Stickrath and Metz25 This may reflect either increased gestational cannabis consumption, or alternatively increased honesty about cannabis use in a decriminalized and more accepting environment. In some instances, cannabis use may explain or worsen nausea during pregnancy. A reduction in cannabis use as the pregnancy progresses is not surprising if hyperemesis improves with the duration of pregnancy and women are repeatedly counseled about potential harms of cannabis for their fetus.
There are conflicting reports regarding associations of gestational cannabis use with adverse perinatal outcomes such as fetal growth restriction, small for gestational age, preterm birth, and admission to the neonatal intensive care unit.Reference Leemaqz, Dekker and McCowan16–Reference Brown, Mensah and Ah Kit18,Reference Kharbanda, Vazquez-Benitez, Kunin-Batson, Nordin, Olsen and Romitti26–Reference Moster, Lie and Markestad28 These are all risk factors for subsequent obesity and cardiometabolic disorders,Reference Calkins and Devaskar29 nonalcoholic fatty liver disease (NAFLD),Reference Cianfarani, Agostoni and Bedogni30 and neurodevelopmental problems.Reference Saenger, Czernichow, Hughes and Reiter31,Reference Moster, Lie and Markestad32 Consequently, unlike the recognized adverse health consequences of heavy gestational alcohol consumption on fetal and child outcomes,Reference Popova, Lange, Probst, Gmel and Rehm33 the longer term effects of gestational cannabis use are relatively unknown. A Canadian cohort study found prenatal cannabis exposure was associated with increased risk of small for gestational age neonates, placental abruption, transfer to neonatal intensive care, and lower 5-minute Apgar score.Reference Corsi, Walsh and Weiss34 Another large study linked gestational cannabis use with a higher prevalence of neonatal neurological morbidity, infection, and mortality.Reference Metz, Allshouse and Hogue35
There is a paucity of high-quality longitudinal studies examining associations between consumption of varied quantities and formulations of cannabis during different pregnancy trimesters, and postnatal physical and mental health outcomes in offspring. Existing data frequently fail to account for confounding effects of maternal gestational smoking, alcohol intake, obesity, gestational diabetes, and pregnancy-associated hypertension. Furthermore, cannabinoids are secreted in breast milk with potential for additional adverse consequences for the neonate or infant resulting from exposure from breastmilk.Reference Metz and Stickrath36–Reference Liston38 Therefore, some mothers may choose not to breastfeed their infant in order to avoid lactational cannabis exposure, following the guidance of authorities such as the ACOG. This may be relevant to intergenerational health, since reduced breastfeeding rates and durations are associated with subsequent adverse cardiometabolic sequelae such as obesity and NAFLD in offspring.Reference Ayonrinde, Oddy and Adams39 Thus, there is a need for a better understanding of the influence of perinatal cannabis use on pregnancy outcomes for the mother, fetus, and placenta, as this may provide insights into early developmental health and intergenerational health.
In the USA, a comprehensive research agenda was proposed to address research gaps related to cannabis,40 incorporating: (a) “basic science studies to help inform efforts to minimize harms and maximize benefits of acute and chronic use of cannabis and cannabinoids”; (b) “health policy and public health research to examine health effects of broader social and behavioral changes associated with the legalization of recreational and/or medical cannabis and other changes in cannabis policy”; (c) “research that identifies plausible mechanisms by which cannabis affects specific health endpoints”; and (d) translational research “to ensure that research findings will be of practical use to help inform health care practices, public health priorities, national and state policy, and public safety standards”. Similar research priorities have been made in Canada.41 From the evidence documented in these reviews, a critical component should be the effects of gestational cannabis exposure.
A systematic review and meta-analysis of maternal and perinatal outcomes associated with prenatal exposure to cannabis concluded that “use of cannabis during pregnancy may increase adverse outcomes for women and their neonates. As use of cannabis gains social acceptance, pregnant women and their medical providers could benefit from health education on potential adverse effects of use of cannabis during pregnancy”.Reference Gunn, Rosales and Center42 Consistent with this, in 2020, the UK Food and Safety Authority recently advised pregnant and breastfeeding women not to consume foods containing chemicals from the cannabis plant.43
Cannabinoids and receptor signaling
To date over 100 different cannabinoids have been isolated, with these molecules broadly classified according to their source (Fig. 2). Endogenously produced endocannabinoids (ECs) are further separated into two distinct types of fatty acid members, the first being anandamide (also known as N-arachidonoylethanolamine; AEA) (Fig. 2), a product formed from arachidonic acid and ethanolamine conjugation. The second EC member, 2-arachidonyl glycerol (2-AG) is formed from glycerol esterification of arachidonic acid. Both of these ECs act as ligands for two separate G protein-coupled (GPCRs) cannabinoid receptors, CB1 and CB2 (Fig. 3a, b). Under physiological conditions, CB1 is expressed predominantly in the central nervous system (CNS) and to a lesser degree the peripheral nervous system and several peripheral organs. CNS CB1 receptors are expressed from early in the first trimester of gestation,Reference Zurolo, Iyer and Spliet44 and are concentrated within the striatum, basal nuclei, hippocampus, cingulate gyrus, cortex, and cerebellum structures, and their embryonic precursors. On an ultrastructural level, CB1 localizes to the plasma membranes of presynaptic neurons where it modulates neurotransmitter release, thereby regulating synaptic signaling and protecting against glutamate excitotoxicityReference Marsicano, Goodenough and Monory45,Reference Oka, Ishima, Waku, Sugiura, Onaivi, Sugiura and Di Marzo46 (Fig. 3a). In contrast, CB2 shows limited neuronal expression but strong localization to various cells of the immune system, including monocytes/macrophages, dendritic cells, B- and T cellsReference Matsuda, Lolait, Brownstein, Young and Bonner47,Reference Klein, Newton and Larsen48 (Fig. 3b). CB2 expression is also identified within the spleen, thymus, and gastrointestinal system.Reference Lombard, Nagarkatti and Nagarkatti49 Cannabinoid binding to CB2 causes potassium and calcium channel gating changes and altered gene expression through inhibition of adenylyl cyclase (AC) and activation of the mitogen-activated protein kinase (MAPK) pathways. This, in turn, increases anti-inflammatory cytokine IL-10 expression and modulation of pro-inflammatory genesReference Tomar, Zumbrun, Nagarkatti and Nagarkatti50 (Fig. 3b).

Fig. 2. Cannabinoid classes. Cannabinoids are categorized into physiologically available endocannabinoids (ECs), which include anandamide and 2-arachidonoylglycerol (2-AG), plant-derived phytocannabinoids, and fully synthetic cannabinoids. The most common phytocannabinoids are CBD and THC. The synthetic cannabinoid class includes over 100 separate molecules that possess increased affinity for CB1 compared with EC and phyocannabinoids. This class is substratified into several major groups according to their chemical structure. Classical synthetics, such as HU-210, retain the core THC structure, while the THC pyran ring is absent in nonclassical cannabinoids as seen in CP-47497. Hybrid cannabinoids, which include AM-4030 share structure features of both classical and non-classical. Conversely, aminoalkylindoles are chemically distinct molecules, which in addition to CB1 and CB2 receptor activity also inhibit cyclooxygenase activity.Reference Pacheco, Childers, Arnold, Casiano and Ward108
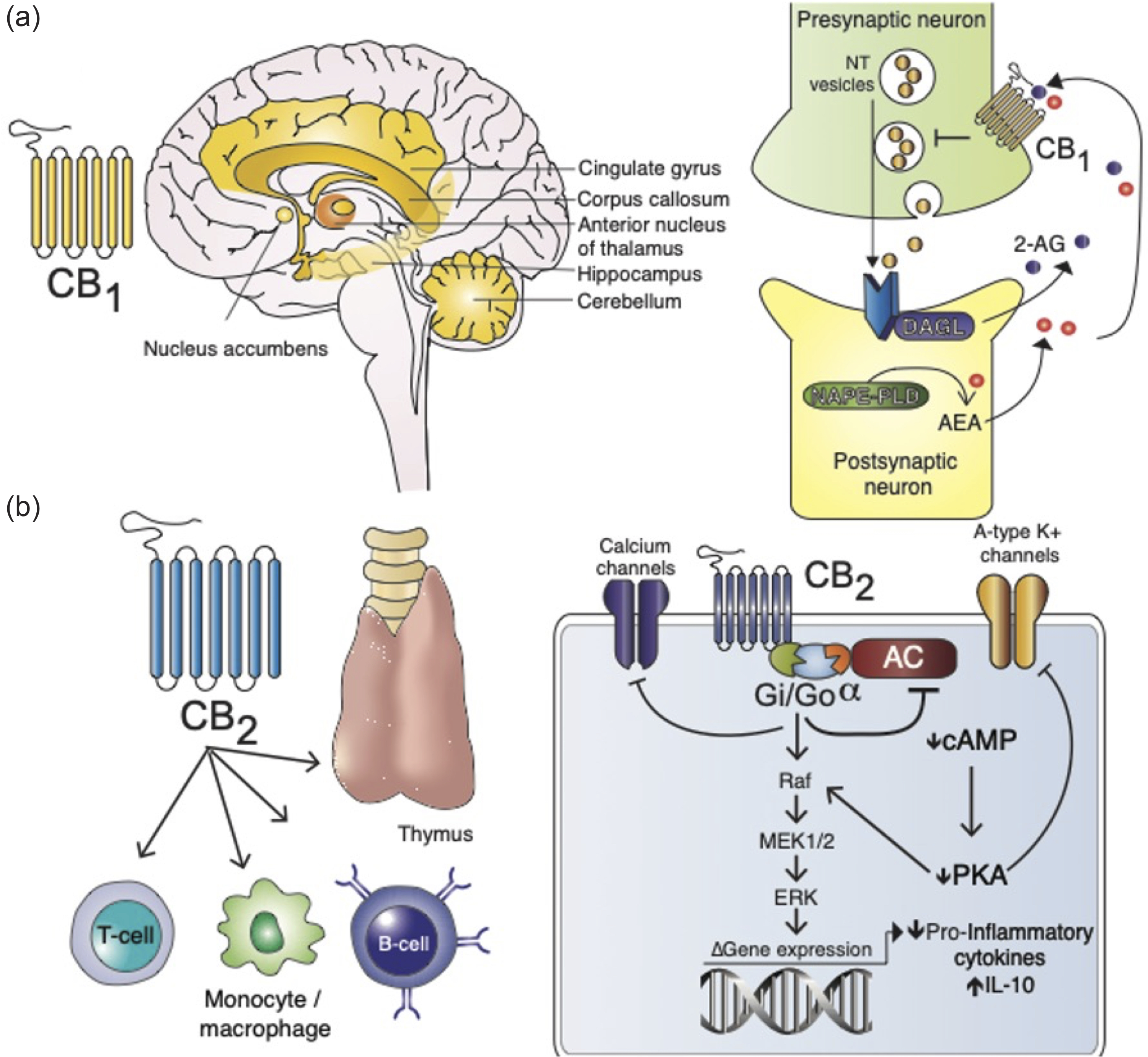
Fig. 3. Physiological localization and function of CB1 and CB2. (a) CB1 is the most abundant transmembrane Gi/Go-protein-coupled receptor (GPCR) expressed in the brain and is highly concentrated in the basal nuclei, cingulate gyrus, nucleus accumbens, hippocampus, cerebellum, cortex, and striatum. Here, CB1 is localized to the presynaptic neurons and is essential in modulating neurotransmitter (NT) signaling.Reference Mackie109 Following NT release from presynaptic neurons, signal activation occurs at the postsynaptic neuron leading to N-acetylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD) synthesis of anandamide (AEA). Similarly, diacylglycerol lipase generates nascent 2-AG. 2-AG and AEA then diffuse into the synaptic space and activate CB1 at the presynaptic neuron which, in turn, inhibits further NT vesicle release. Non-EC cannabinoids potentiate this pathway to induce psychotropic effects. (b) CB2 is selectively expressed on monocytes, B- and T cells, within the thymus, spleen, and gastrointestinal system. Cannabinoid activation of CB2 results in adenylyl cyclase (AC) inhibition and downstream reductions in cyclic adenosine monophosphate (cAMP), which negatively regulates cAMP-dependent protein kinase (PKA). A-type potassium channels are closed as a consequence. CB2 is also capable of directly inhibiting calcium channels, as well as activating the Raf/MEK/ERK pathway leading to suppression of pro-inflammatory and augmentation of anti-inflammatory genes.
Cannabis metabolism
Cannabis is a heterogeneous substance containing numerous different phytocannabinoids. These phytocannabinoids are metabolized by the liver, with the half-life considered to be 1–5 days, depending on the frequency of consumption and exact phytocannabinoid composition.11 Complete excretion may, however, take weeks. The composition of cannabis has changed in recent decades, with a considerable increase of up to 10-fold in the concentration of delta-9-tetrahydrocannabinol (THC), the psychoactive ingredient, compared with 1980.Reference Hall, Stjepanović and Caulkins2,51 This creates challenges in comparison of different effects of cannabis through the life course.
Gestational cannabis consumption and the placenta
Physiologically, the EC system is involved in all stages of ovary and uterus function, embryo development, embryo placentation, as well as maintenance of pregnancy.Reference Taylor, Amoako and Bambang52 These EC pathways are carefully regulated by controlled synthesis and degradation of AEA and 2-AG. Cannabis, however, contains multiple phytocannabinoid molecules with nonphysiological CB1 and CB2 affinities and activities. These phytocannabinoids, along with their respective metabolites, readily cross the placenta and disrupt these physiological EC pathways,Reference Maia, Midão and Cunha53 thereby contributing to several pathological changes within the placenta and developing fetus (Fig. 4a). In a mouse model, fetuses exposed to cannabis in utero had increased placental weight and reduced fetal-to-placental weight ratio, mostly in male pups.Reference Benevenuto, Domenico and Martins54 In humans, maternal cannabis consumption also alters placenta to neonate weight ratios. Following chronic gestational cannabis consumption, placentas are disproportionately larger unless coexistent pathologies develop, such as fetal and maternal vasculopathy.Reference Carter, Wainwright and Molteno55 Furthermore, gestational cannabis consumption is associated with impaired placental blood flow and increased placental vascular resistanceReference El Marroun, Tiemeier and Steegers56,Reference Brar, Patil, Jackson, Gardner, Alexander and Doyle57 . Uteroplacental vascular lesions, in turn, can lead to complications such as trophoblastic necrosis, thereby exacerbating fetal growth restrictionReference Natale, Gustin and Lee58,Reference Roncero, Valriberas-Herrero, Mezzatesta-Gava, Villegas, Aguilar and Grau-López59 (Fig. 4b). Fetal growth restriction has also been demonstrated in a rat model, in which pregnant dams were exposed to THC. Again, in this model. fetuses displayed symmetrical intrauterine growth restriction, small birth weights, and lower brain to body weight parameters. While the placentas from these cannabis-exposed dams were significantly larger with a marked expansion of the labyrinth zone, there was an overall reduction in fetal blood surface area. Moreover, placental trophoblast expression of glucose transporter (GLUT)1 and glucocorticoid receptor (GR) were significantly reduced post-THC exposure; these results were replicated by the authors in the established human BeWo trophoblast cell line (Fig. 4c). It should be noted that placental GLUT expression is regulated by GR.Reference Hahn, Barth and Graf60 These data suggest THC use may decrease the availability of glucose, the primary energy substrate of the fetus, during fetal development.
Interestingly, THC use does not significantly alter CB1 or CB2 expression within placental tissues.Reference Taylor, Amoako and Bambang52 However, it does significantly augment N-arachidonoylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD) activity and suppress fatty acid amide hydrolase (FAAH). An imbalance between these two enzyme profiles results in higher concentrations of ECs within the placenta milieu (Fig. 4) leading to reduced fetal survival, altered development, and neurophysiological abnormalitiesReference Grant, Petroff, Isoherranen, Stella and Burbacher17 (Fig. 4c).

Fig. 4. Summary of cannabinoid-induced changes within the placenta. (a) Following THC exposure, labyrinth zone expansion occurs within the placenta, which contributes to increased placental weight. Maternal vasculopathy is also observed in post-THC use. (b) Chorionic villi changes include reduced fetal capillary area, trophoblast, and decidual dysfunction leading to impaired implantation during the early stages of fetal development. (c) Trophoblasts undergo several phenotypic changes in post-THC exposure. These include increased endogenous EC synthesis downstream from cannabinoid induced CB1/CB2 stimulation. Here, NAPE-PLD activity is increased with simultaneous reductions in FAAH enzyme expression. This leads to AEA synthesis and accumulation of ECs within the placenta, which negatively affects placenta viability and fetal development. THC also results in reduced GC expression that, in turn, reduces GLUT1 expression. This results in lower glucose transport across to the fetal surface contributing to IUGR.
The increased availability of illicit synthetic cannabis products, and their rapidly changing chemical composition means that there are limited data on their associated harms in humans.Reference Darke, Duflou, Farrell, Peacock and Lappin61,Reference Tait, Caldicott, Mountain, Hill and Lenton62 In vitro studies involving synthetic cannabinoid, for example, impair the cellular communication between trophoblasts and maternal decidua, in turn, potentially affecting placental implantation.Reference Neradugomma, Drafton, Mor and Mao63 Additionally, phytocannabinoids signal through CB1 and CB2 to induce trophoblast mitochondrial and endoplasmic reticulum stress pathways culminating in the generation of reactive oxygen species (ROS) and an overall reduction in trophoblast viability.Reference Lojpur, Easton, Raez-Villanueva, Laviolette, Holloway and Hardy64–Reference Fonseca, Fernandes and Almada66 Using the BeWo trophoblast tissue culture model, Almada and colleaguesReference Almada, Alves and Fonseca67 observed mitochondrial stress, ROS, and caspase nine activation following synthetic cannabinoid exposure. This pathogenic pathway is dependent on CB1 and CB2 receptor signaling. Accordingly, cannabis use during pregnancy may have significant adverse effects on offspring health.Reference Grant, Petroff, Isoherranen, Stella and Burbacher17,Reference Metz and Stickrath36 The major placental changes seen with cannabis use are summarized in Fig. 4a–c.
Antenatal cannabis use and subsequent child and adolescent outcomes
Despite the rising number of observational studies describing inconsistent, but predominantly adverse, short-term neonatal outcomes in cannabis-exposed fetuses, there remains a paucity of longitudinal studies examining longer term associations of fetal cannabis exposure on subsequent behavioral, academic, and health outcomes during childhood, adolescence, and adulthood. In a systematic review, a possible relationship between gestational cannabis use, and mood disorders, and ADHD, but not psychosis in offspring was described.Reference Roncero, Valriberas-Herrero, Mezzatesta-Gava, Villegas, Aguilar and Grau-López59 Gestational cannabis use has been associated with the severity of depressive symptoms and academic deficits in childrenReference Gray, Day, Leech and Richardson68,Reference Goldschmidt, Richardson, Cornelius and Day69 and subsequently increased rates of cannabis use during adolescence.Reference Day, Goldschmidt and Thomas70 The pediatric literature is relatively sparse in expanding the knowledge on the impact of maternal cannabis consumption on children. Perinatal studies suggest a possible significant mild reduction in birth weight.Reference Leemaqz, Dekker and McCowan16,Reference Grant, Petroff, Isoherranen, Stella and Burbacher17 However, Fergusson and colleaguesReference Fergusson, Horwood and Northstone20 did not find significant effects on neonatal head size using postnatal MRI brain scans, nor any significant adjusted effects on length and head circumference of the newborn. Similarly, Gunn et al. Reference Gunn, Rosales and Center42 did not identify a significant difference in the usual birth anthropometric measurements including head circumference. A review of neuroimaging in children following cannabis exposure highlighted changes to the frontal cortex and basal ganglia of the fetal brain, as well as cortical gray matter and parenchymal volume loss in children aged 10–14 years with intrauterine cannabis exposure.Reference Derauf, Kekatpure, Neyzi, Lester and Kosofsky71 While an association between early life cannabis exposure and subsequent neurodevelopmental impairments may exist, inability to exclude confounding effects of other substances limits the interpretation of any conclusions. There, thus, remains a paucity of studies examining associations between perinatal cannabis consumption and brain growth and development, behavior, and cognition. Warshak et al.,Reference Warshak, Regan, Moore, Magner, Kritzer and Van Hook72 Conner et al. Reference Conner, Bedell, Lipsey, Macones, Cahill and Tuuli73 and Varner et al. Reference Varner, Silver and Rowland Hogue74 described an increase in small babies, neonatal intensive care unit admission, and stillbirth rate, though tobacco smoking and other substance use may be cofactors in some cases. Preterm birth had a cannabis dose–frequency relationship.Reference Conner, Bedell, Lipsey, Macones, Cahill and Tuuli73 Some studies suggest a negative effect on language, memory, executive function, and attention, but do also acknowledge the impact of multiple substance misuse concurrently in some pregnant women.Reference Crean, Crane and Mason75,Reference Cohen and Weinstein76 Pediatricians may not necessarily enquire about cannabis specifically in child development clinics. Furthermore, other environmental and lifestyle factors may partly contribute to any neurobehavioral observations.
Cannabis exposure and birth abnormalities
Early human and animal studies suggested that cannabis use could cause genetic damage and chromosomal abnormalities. However, more recent systematic reviews and a position statement have not found an association between maternal cannabis use and birth abnormalities.40,Reference Gunn, Rosales and Center42,Reference Conner, Bedell, Lipsey, Macones, Cahill and Tuuli73 The relationship is considered more likely due to tobacco use or other confounding factors. Nevertheless, questions are still raised from studies that use less extensive controls. An analysis of a state birth defect register found that for people who reported cannabis use, 19 of the 54 assessed conditions were significantly increased among those who used cannabis.Reference Forrester and Merz77 A recent spatial–temporal analysis of increasing cannabis use and the incidence of gastroschisis also postulated a causal linkReference Reece and Hulse78 although this is in contrast to a previous study of recreational drug use as assessed by hair analysis and fetal anomalies, which showed no significant association after adjusting for confounding variables.Reference David, Holloway and Thomasson79 While these observations raise questions regarding rising rates of congenital anomalies in parallel with the timing of legalization of marijuana in parts of the USA, causality has not been established.Reference Siega-Riz, Keim-Malpass, Lyons and Alhusen80
Cannabis use and breastfeeding
THC is transferred into breast milk at low concentrations, with infants ingesting a mean of 2.5% of the maternal doseReference Baker, Datta and Rewers-Felkins81 and a concentration of 9.5 ng/ml.Reference Siega-Riz, Keim-Malpass, Lyons and Alhusen80 THC can be detected in breast milk up to 6 days after last maternal use.Reference Bertrand, Hanan, Honerkamp-Smith, Best and Chambers82 Studies looking at the long-term outcomes of offspring after cannabis use during breastfeeding are limited.Reference Tennes, Avitable and Blackard83,Reference Astley and Little84 Breastfeeding from chronic THC users has been reported to be associated with delayed motor development at 1 year of age.Reference Astley and Little84 However, it is difficult to isolate the impact of marijuana intake via breast milk from in utero exposure. The use of other illicit substances by these mothers remains a confounding factor, and the heterogeneity of the small sample sizes limits the interpretation of the results.Reference Mourh and Rowe37 Due to concerns regarding the rebound of cannabis use rates in the postpartum period compared with use during pregnancy, the World Health Organization (WHO) highlights a focus on psychosocial interventions during pregnancy. The WHO also recommends that mothers with substance use disorders should be encouraged to breastfeed unless the risks clearly outweigh the benefits.85
Cannabis and adiposity, the liver, and gastrointestinal system
Cannabis is associated with adiposity distribution, serum leptin, appetite, and gastrointestinal symptoms. THC is highly lipophilic, rapidly absorbed and stored in adipose tissue from where it passively diffuses back into the bloodstream,m but may still be detectable in fat biopsies after 4 weeks and in urine up to 11 weeks after the last consumption.Reference Gunasekaran, Long, Dawson, Hansen, Richardson, Li, Arnold and McGregor86 Enhanced levels of THC and its metabolites thus become evident on lipolysis after food deprivation. Cannabinoid receptor 1 (CB1)-activating ECs, via CNS effects, regulate appetite and are associated with the development of metabolic syndrome and ameliorated obesity, type II diabetes, fatty liver, and insulin resistance in animal models.Reference Hirsch and Tam87 This appetite-stimulating property of CB1 has resulted in its use for improving food intake in patients with reduced appetite associated with cancer chemotherapy, HIV, or anorexia nervosa.Reference Hirsch and Tam87 CB1 antagonists/inverse agonists, therefore, held appeal for reducing appetite, abdominal adiposity, and cardiometabolic risk factors, however, psychiatric side effects resulted in withdrawal from such use for obesity treatment.Reference Bermudez-Silva, Viveros, McPartland and Rodriguez de Fonseca88 This was the fate of Rimonabant that had demonstrated efficacy for ameliorating obesity, insulin resistance, and hepatic steatosis.Reference Christensen, Kristensen, Bartels, Bliddal and Astrup89 Hepatic stellate cells express CB1 and cannabinoid receptor 2 (CB2) that are up-regulated and associated with liver fibrosis,Reference Julien, Grenard and Teixeira-Clerc90,Reference Dai, Zhang and Ye91 while CB1 has also been associated with hepatic steatosis.Reference Jeong, Osei-Hyiaman and Park92 Any potential association of cannabis consumption with fatty liver may have consequences for the mother and fetus, as maternal gestational fatty liver signifies insulin resistance, increased risk of gestational diabetes mellitus (GDM), and associates with fetal macrosomia independent of maternal GDM.Reference Lee, Kim and Koo93 Cannabis use can result in cannabis hyperemesis syndromeReference Bhatt and Queen94 and has also been linked with irritable bowel syndrome (IBS).Reference Aziz, Palsson, Whitehead, Sperber, Simrén and Törnblom95–Reference Adejumo, Ajayi, Adegbala and Bukong97 Nevertheless, a putative role for exogenous cannabinoid has been proposed for treatment of pain syndromes, including migraine, fibromyalgia, and IBSReference Russo98 as well as a role in gastrointestinal symptoms, such as nausea and vomiting, cannabinoid hyperemesis syndrome, anorexia, weight loss, and chronic abdominal pain.Reference Goyal, Singla, Gupta and May99
Sex differences in the effect of cannabis
Sex differences in the consumption and effects of cannabis have been observed. Males are more likely to initiate cannabis use or develop a cannabis use disorder (CUD) than females, but the trajectory from first use to CUD is higher in females.Reference Fogel, Kelly, Westgate and Lile100,Reference Cooper and Haney101 This may have implications for the antenatal and early postnatal periods. Male cannabis smokers exhibit higher circulating levels of THC and more cardiovascular effects than female cannabis users.Reference Meier, Pardini, Beardslee and Matthews102 By contrast, cannabis use to treat nausea or abdominal pain is more common in females than in males.Reference Hernandez, Paty and Price103 Sex differences in the effects of cannabis may relate in part to the amount and distribution of body fat, with females having more subcutaneous fat that may provide a depot. Men experience increased appetite and caloric intake, while women describe more anorexia after cannabis consumption but in withdrawal, men feel more insomnia and women more nausea, abdominal pain, and anxiety.Reference Fattore and Fratta104,Reference Cuttler, Mischley and Sexton105
There is growing evidence of neurobiological differences involving the EC system and cannabinoid metabolism between male and female cannabis consumers.Reference Craft, Marusich and Wiley106–Reference Pacheco, Childers, Arnold, Casiano and Ward108 Cannabis use may perpetuate functional nausea and vomiting disorders (FNVDs) such as the cannabis hyperemesis syndrome.Reference Aziz, Palsson, Whitehead, Sperber, Simrén and Törnblom95 It is plausible that some women will use cannabis to ameliorate pregnancy-associated nausea or that gestational cannabis use could result in hyperemesis mistakable for hyperemesis gravidarum.Reference Metz and Borgelt23 Rodent models have provided additional insights into the mechanisms of neurobiological sex differences in offspring exposed to cannabinoids in utero. Bara et al. exposed pregnant Wistar rats to synthetic cannabinoid WIN55,212–2 and THC, with the effects studied in the litter pups.Reference Bara, Manduca and Bernabeu107 Male pups demonstrated significantly lower levels of socialization compared with drug and sex controls. Further, increased excitability of prefrontal cortex pyramidal neurons was observed along with long-term suppression of synaptic plasticity within the medial prefrontal cortex.
Conclusions and future directions
“Cannabis” refers to a heterogeneous group of substances of varying properties, potencies, routes of consumption, speed of absorption, and effect. Studies have not consistently or conclusively quantified the level of risk to the fetus or offspring of the pregnant user independent of other substances. Gestational cannabis is not routinely screened for in most countries beyond an antenatal interview. Consequently, there are inadequate data to suggest a fetal cannabis syndrome, cannabis-related neonatal abstinence syndromes, or a relationship with congenital abnormalities related to perinatal cannabis use. Given the current uncertainties regarding potential adverse effects on the fetus, current guidance that cannabis and cannabinoid products be avoided during pregnancy and lactation seem appropriate. However, this should be balanced against potential harms of alcohol, tobacco, and other substances that have more established adverse fetal effects, that may be used as alternative substances during pregnancy. Given the prevalence of cannabis usage during pregnancy relative to other substances, it is time that documentation of perinatal cannabis becomes a more prominent aspect of neonatal/pediatric care beyond concerns of domestic consumption in young people, and the cumulative concerns that result in child protection input. To what degree there could be a cumulative dose-dependent risk independent of, or associated with, other substances remains unclear. Future longitudinal studies with high-quality data regarding the routes of administration and more accurate quantification of gestational consumption of cannabis and other substances throughout pregnancy will improve knowledge about longer term cognitive, broader developmental, and cardiometabolic sequelae of perinatal cannabis exposure. Such data are required to strengthen the evidence for guiding women of reproductive age and clinicians involved in their care about potential intergenerational harms of perinatal cannabis use.
Acknowledgements
None
Disclosures
The authors have no potential conflict of interest to declare that are relevant to the manuscript.
Grant support
None.
Disclosures
The authors have no potential conflict of interest to declare that are relevant to the manuscript.
Author contributions
Oyekoya T Ayonrinde – conception of the review article, drafting, and critical revision of the manuscript.
Oyedeji A Ayonrinde – drafting and critical revision of the manuscript.
Derrick Van Rooyen – drafting and critical revision of the manuscript.
Robert Tait – drafting and critical revision of the manuscript.
Mikaela Dunn – drafting and critical revision of the manuscript.
Shailender Mehta – drafting and critical revision of the manuscript.
Scott White – drafting and critical revision of the manuscript.
Oyekunle K Ayonrinde – drafting and critical revision of the manuscript.








