INTRODUCTION
The ciliated protozoan, Cryptocaryon irritans, is a parasite that causes ‘white spot disease’ in seawater fish, resulting in weakening or death (Colorni and Burgess, Reference Colorni and Burgess1997). Infection of commercial fish species by C. irritans has threatened the Japan fishing industry. However, the chemotherapeutic agents available to treat fish for C. irritans infection are limited because of human consumption. Immunoprophylaxis is thought to be a safer alternative.
A 32 kDa polypeptide on the surface of the parasite (Cryptocaryon irritans surface antigen [CISA]-32) has been identified as an agglutination/immobilization antigen of C. irritans and is a potential vaccine candidate (Hatanaka et al. Reference Hatanaka, Umeda, Yamashita and Hirazawa2007). So far, several serotypes of Ichthyophthirius multifiliis, the parasite that causes white spot disease in freshwater fish, have been identified by antibody-specific immobilization (Dickerson et al. Reference Dickerson, Clark and Findly1993; Dickerson and Clark, Reference Dickerson and Clark1996). In addition, immunization of the channel catfish Ictalurus punctatus with I. multifiliis immobilization antigens (i-antigens) elicits nearly serotype-specific protection against lethal challenges (Wang et al. Reference Wang, Clark, Noe and Dickerson2002; Swennes et al. Reference Swennes, Findly and Dickerson2007). C. irritans isolates have been reported around the world and have been genetically characterized using ribosomal DNA (rDNA) sequences (Diggles and Adlard, Reference Diggles and Adlard1997; Yambot et al. Reference Yambot, Song and Sung2003; Sun et al. Reference Sun, Zhu, Xie, Wu, Li, Lin and Song2006). They are thought to show various serotypes and express various agglutination/immobilization antigens as in the case of I. multifiliis.
In the present study, we found an agglutination/immobilization antigen on the surface of C. irritans theronts that identifies a novel agglutination/immobilization serotype. Fish antiserum immunized with this novel theront serotype agglutinated/immobilized this serotype in vitro. By immunological analysis, a 37 kDa surface protein (CISA-37) was detected with the fish antiserum. Analysis of the gene encoding this surface protein showed that it contains a potential N-terminal signal peptide and a potential C-terminal glycosylphosphatidylinositol (GPI) anchor site. CISA-37 shares 45% similarity with CISA-32, a putative agglutination/immobilization antigen of another serotype.
MATERIALS AND METHODS
Fish maintenance
Tiger puffer fish, Takifugu rubripes (50·8–91·3 g), hatched in our laboratory were maintained in a 20 000 litre polycarbonate tank. The tank was supplied with seawater kept at 25±1°C at a flow rate of 1·2 l/min; the seawater was sand-filtered and UV irradiated. Tiger puffer fish were fed a commercial 3 mm pellet diet (Nippon Suisan Kaisha, Ltd) twice a day for a total of 3% body weight daily. Laboratory seawater conditions were as follows: salinity, 34 ppt; pH 8·1; chemical oxygen demand, 1·0 mg/l.
Parasite maintenance
The C. irritans population was maintained using the red sea bream, Pagrus major, as a host in a 100 litre polycarbonate tank containing seawater kept at 25±1°C (Hirazawa et al. Reference Hirazawa, Oshima, Hara, Mitsuboshi and Hata2001). Glass slides were placed on the bottom of the aquarium for 15 h to collect adhering C. irritans cysts. Theronts were obtained from the cysts cultured for 5–8 days in a 300 ml plastic beaker containing seawater at 25°C (Yoshinaga and Dickerson, Reference Yoshinaga and Dickerson1994). Theronts released from collected cysts within 12 h were used for experiments. Seawater containing theronts was passed through a cell strainer (2·7 cm in diameter, 5 μm mesh opening) to trap the parasites, which were then transferred to a 1·5 ml microcentrifuge tube and centrifuged at 2000 g for 5 min at 4°C to remove the remaining seawater. The pelleted theronts were stored at −80°C.
Fish immunization
To monitor immunoreactivities over time, tiger puffer fish were immunized with a 1:1 (v/v) emulsion of Freund's Complete Adjuvant (FCA) and sonicated theronts in phosphate-buffered saline (PBS) (tiger puffer: n=6), theront integral membrane proteins (tiger puffer: n=6), or bovine serum albumin (BSA) as a negative control (tiger puffer: n=6). The dose of injected antigen per fish body weight was 0·5 mg/kg for sonicated theronts and BSA, and 0·05 mg/kg for theront integral membrane proteins. Each tiger puffer was immunized by an intraperitoneal injection of the appropriate emulsion. A booster injection identical to the primary inoculation was given 2 weeks later. All fish in the experiments were bled 4 weeks after the booster injection to obtain small serum samples. Blood was drawn from the caudal vein, and sera were stored at −80°C.
Agglutination/immobilization assays
The assays were performed according to the method of Clark et al. (Reference Clark, Dickerson and Findly1988). Filtered and UV-treated seawater (99 μl) containing approximately 100 parasites were added to each well of a 96-well tissue-culture plate. An aliquot of fish serum (1 μl) was added to each well. The plates were incubated for up to 1 h at 25°C. Agglutination was monitored with a microscope (BX50; Olympus Optical Co.) attached to a CCD camera (FD-120 M; Flovel Co., Ltd).
Extraction of membrane proteins
Integral membrane proteins of C. irritans were extracted by phase separation in Triton X-114 (Sigma-Aldrich) solution, as described previously (Hatanaka et al. Reference Hatanaka, Umeda, Yamashita and Hirazawa2007). Frozen parasites (~200 μg; number of theronts=~500 000) were thawed and resuspended in 100 μl of ice-cold 10 mm Tris-HCl (pH 7·5) in a 1·5 ml microcentrifuge tube. An equal volume of ice-cold extraction buffer (10 mm Tris-HCl, pH 7·5, 300 mm NaCl, 2% [v/v] Triton X-114) was added, and the parasites were incubated on ice for 1 h. The cytoskeletal components were removed by centrifugation at 100 000 g for 1 h at 4°C. The clear supernatant was overlaid on a 6% (w/v) sucrose cushion containing 10 mm Tris-HCl (pH 7·5), 150 mm NaCl and 0·06% (v/v) Triton X-114, and the tube was incubated at 32°C for 5 min. The detergent phase was then separated by centrifugation at 300 g for 3 min at room temperature. The upper aqueous phase was collected and Triton X-114 was added to 0·5% (v/v). After incubation on ice, the mixture was again overlaid on the sucrose cushion, and the tube was incubated at 32°C for 5 min for condensation and was centrifuged again to separate the detergent phase. Integral membrane proteins contained in the detergent phase were precipitated by adding 9 volumes of ice-cold acetone. After incubation for 1 h on ice, the precipitate was pelleted by centrifugation at 16 000 g for 20 min at 4°C. After acetone removal, the pellet was vacuum-dried, resuspended in 100 μl of 10 mM Tris-HCl (pH 7·5) and stored at −80°C.
SDS-PAGE, immunoblotting and N-terminal amino acid sequencing
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) using a discontinuous buffer system was performed on 4–20% gradient gels (Daiichi Pure Chemicals) (Laemmli, Reference Laemmli1970) that were stained by Coomassie brilliant blue R-250 (CBB R-250). Molecular masses were estimated by comparing the migration of the protein of interest with that of commercial molecular weight size standards (Bio-Rad Laboratories).
The protein bands were electroblotted onto Pall Fluoro Trans W Membranes (Nippon Genetics Co., Ltd) using a Trans-Blot SD Semi-Dry Transfer Cell (Bio-Rad Laboratories) at 2 mA/cm2 for 1 h. Filters were blocked by incubation for 1 h at room temperature with Block Ace (Dainippon Pharmaceutical Co., Ltd) containing 0·1% (v/v) Tween 20. Filters were then incubated for 1 h with a 1: 100 dilution of fish serum. After washing the membrane 3 times in 10 mm Tris-HCl (pH 7·5), 150 mm NaCl and 0·1% (v/v) Tween 20, bound fish antibodies were detected with a 1:1000 dilution of rabbit IgG anti-tiger puffer-IgM for 1 h, followed by a 1:25 000 dilution of alkaline phosphatase–conjugated goat anti-rabbit IgG for 1 h. Filters were then developed in nitroblue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl phosphate (BCIP) substrate (Roche Diagnostics). All incubations were performed at room temperature.
Integral membrane proteins (~100 μg) were further subjected to reversed-phase chromatography on a μRPC C2/C18 SC 2·1/10 column (GE Healthcare Biosciences) with the SMART System (GE Healthcare Biosciences) using a linear gradient of 0–100% acetonitrile containing 0·1% trifluoroacetic acid at a flow rate of 0·1 ml/min. Eluted proteins were monitored by absorbance at 280 nm. The ~37 kDa protein was then subjected to automated N-terminal amino acid sequencing on a PPSQ-21 protein sequencer (Shimadzu Biotech).
cDNA cloning of CISA- 37
Total RNA (0·5 μg) was extracted from theronts (number of theronts=~1 000 000) using the RNeasy Mini kit (Qiagen). First-strand cDNA was synthesized using a 5′/3′ rapid amplification of cDNA ends (RACE) kit (Roche Diagnostics) for 3′-RACE. The degenerate primer 5′-ACNGCNGTNGCNGAYTGGAC-3′ was synthesized based on the N-terminal amino acid sequence. The first cDNA was amplified by the polymerase chain reaction (PCR) in a 50 μl reaction containing 25 μl of HotStarTaq Master Mix (Qiagen), 10 pm degenerate primer and anchor primer, the specific 3′-end of synthesized cDNA for 3′-RACE, and 1 μl of cDNA template. PCR amplification was as follows: initial denaturation at 94°C for 15 min, then 30 cycles of 94°C for 30 sec, 55°C for 1 min, and 72°C for 2·5 min, followed by a final elongation at 72°C for 10 min. Amplified products were subcloned into the pT7blue plasmid (Novagen) and used to transform JM109 competent cells (Takara Bio, Inc.). For 5′-RACE, the first-strand cDNA was synthesized using the 5′-Full RACE Core Set (Takara Bio, Inc.) with a 5′-phosphorylated primer (5′-TTGAATGAGCAGTACCTGCCC-3). The first-strand cDNA was amplified by PCR in a 50 μl reaction containing 25 μl of HotStarTaq Master Mix, 10 pm primer sets (5′-AAGCTGAACAGG AAGCTTTAACTAC-3′ and 5′-AAATTAGGTTCTGAAATTAAAATTACACC-3′), and 1 μl of cDNA template. PCR amplification was as follows: an initial denaturation at 94°C for 15 min, then 30 cycles of 94°C for 30 sec, 58°C for 1 min, and 72°C for 2 min, followed by a final elongation at 72°C for 10 min. The nucleotide sequences obtained by 3′- and 5′-RACE over the coding regions of CISA-37 were confirmed by PCR in a 50 μl reaction containing 10×PCR buffer (Stratagene), 200 μM dNTPs, 0·5 U Pfu DNA polymerase, 1 μl cDNA, and 10 pm specific primer sets: 5′-AAAATAATGAGTAAATTTATAATTGC-3′ and 5′-CAATAAAATAGGAATTTTATGCAAAC-3′. PCR amplification was as follows: an initial denaturation at 94°C for 15 min, then 30 cycles of 94°C for 30 sec, 58°C for 1 min, and 72°C for 2 min, followed by a final elongation at 72°C for 10 min. Amplified products were subcloned into the pHSG299 plasmid (Takara Bio Inc.) and used to transform JM109 competent cells (Takara Bio Inc.). At least 5 clones were sequenced on a 3100 DNA sequencer (Applied Biosystems).
RT-PCR
Reverse transcription (RT) was carried out using total RNA (0·5 μg) extracted from theronts of each serotype, as described above, using the ProSTAR First-Strand RT-PCR kit (Stratagene). RT products were amplified by 30 cycles of PCR using primers 5′-GATTTCATCTTTAGCTGTAATGACATCAGC-3′ and 5′-ATCAAAATATGATCTTAATTAGC-3′ to amplify the CISA-32 gene, and 5′-AAAATAATGAGTAAATTTATAATTGC-3′ and 5′-CAATAAAATAGGAATTTTATGCAAAC-3′ to amplify the CISA-37 gene. PCR amplification was as follows: an initial denaturation at 94°C for 15 min, then 30 cycles of 94°C for 30 sec, 58°C for 30 sec, and 72°C for 2 min, followed by a final elongation at 72°C for 10 min.
DNA extraction, PCR amplification and analysis
Genomic DNA was extracted from pooled theronts using the QIAquick DNA Extraction Kit (QIAGEN) and stored at −80°C until use. Extracted genomic DNA was amplified by PCR in a 50 μl reaction containing 25 μl of HotStarTaq Master Mix (Qiagen), 10 pM specific primers (5′-GTTCCCCTTGAACGAGGAATTC-3′ and 5′-CGCATTTCGCTGCGTTCTTC-3′) (Diggles and Adlard, Reference Diggles and Adlard1997), and 1 μl of genomic DNA template. PCR amplification was as follows: an initial denaturation at 94°C for 15 min, then 30 cycles of 94°C for 30 sec, 58°C for 1 min, and 72°C for 2 min, followed by a final elongation at 72°C for 5 min. Amplified products were subcloned into the pT7Blue plasmid vector (Novagen) and transformed into JM109 competent cells (Takara Bio Inc.). At least 5 clones were sequenced on a 3100 DNA sequencer (Applied Biosystems). The sequences of all samples were edited and aligned by GENETYX-MAC, version 10.1, with slight manual modifications. The nucleotide sequences of the C. irritans 18S and ITS-1 region of the rDNA obtained were compared with other isolate sequences in the GenBank database; DQ270008 (Huidong, China), DQ270009 (Huidong, China), AF490382 (Penghu, Taiwan), AF490383 (Kaoshiung, Taiwan), AY029269 (Israel), AY29271 (Heron Island, Australia), and AY29272 (Moreton Bay, Australia).
RESULTS
Agglutination/immobilization of C. irritans parasites by tiger puffer serum
In the presence of tiger puffer serum immunized with C. irritans theronts or with theront integral membrane proteins, C. irritans parasites ceased to swim and began to form large aggregates that settled to the bottom of the well (Fig. 1). A 1:100 dilution of immunized serum caused agglutination of live parasites, whereas a 1:10 dilution of fish serum immunized with BSA caused little agglutination (data not shown). Antiserum from rabbit or fish immunized with theronts of the previously described serotype G-32 (Hatanaka et al. Reference Hatanaka, Umeda, Yamashita and Hirazawa2007) caused little agglutination (data not shown).

Fig. 1. Agglutination/immobilization of theronts of serotype G37 by tiger puffer antiserum from fish immunized with sonicated theronts (A) or integral membrane proteins of theronts (B). Theronts were incubated for 1 h at room temperature in a 1:100 dilution of immune serum.
Identification of the putative agglutination/immobilization antigen of serotype G37
Theront membranes were extracted through differential centrifugation and phase separation in the nonionic detergent Triton X-114. Fig. 2A shows the protein composition of whole theronts, as resolved by non-reducing SDS-PAGE (lane 1), and of theront integral membranes, as resolved by non-reducing (lane 2) or reducing (lane 3) SDS-PAGE. The whole-theront fraction contained a complex mixture of proteins that included a prominent ~37 kDa protein that was the major component of the integral membrane protein fraction under both non-reducing and reducing conditions and was named CISA-37. To characterize CISA-37 as a potential agglutination/immobilization antigen, immunoblot analysis was carried out using serum from tiger puffer immunized with theronts. CISA-37 was strongly detected by antiserum from immunized tiger puffer fish under non-reducing and reducing conditions (Fig. 2B, lanes 2 and 3).

Fig. 2. SDS-PAGE and immunoblot analyses of theront serotype G37 integral membrane proteins. (A) Intact theronts (10 μg) resolved on 4–20% SDS-polyacrylamide gradient gels under non-reducing (lane 1) conditions. Theront integral membrane proteins (2 μg) resolved on 4–20% SDS-polyacrylamide gradient gels under non-reducing (lane 2), and reducing (lane 3) conditions in the presence of 2-mercaptoethanol and visualized using CBB R-250. The arrowhead indicates the position of the 37 kDa antigen. (B) Immunoblot analysis: 2 μg of theront integral membrane proteins were resolved by non-reducing (lanes 1 and 2) or reducing (lane 3) SDS-PAGE and then immunoblotted using the following antisera: lane 1, serum with BSA as a negative control; lanes 2 and 3, serum from tiger puffer immunized with theronts. Relative molecular masses of standard markers are indicated on the left. The arrowhead indicates the position of the 37 kDa antigen.
Purification of CISA-37 by reverse-phase chromatography
CISA-37 was successfully purified by reverse-phase chromatography for N-terminal amino acid sequencing (Fig. 3). The N-terminal amino acid sequence of CISA-37, as determined by automated sequencing (data not shown), was: NH2-Ala-Trp-Val-Glu-Lys-Thr-Ala-Val-Ala-Asp-Trp-Thr-Gly-Thr-Phe-Ile-Val-Val-Lys-Ala.

Fig. 3. Purification of CISA-37 from theront integral membrane protein fractions by reverse-phase chromatography. The integral membrane protein fraction of theronts was applied to a μRPC C2/C18 SC 2·1/10 column (GE Healthcare Biosciences) with a linear gradient of acetonitrile containing 0·1% trifluoroacetic acid at a flow rate of 0·2 ml/min (A). Dashed line shows the gradient of acetonitrile. The peak indicated by the arrowhead was resolved by reducing SDS-PAGE and visualized with CBB R-250 (B).
cDNA cloning of CISA-3 7
CISA-37 cDNA fragments were generated from theront RNA by 3′-RACE using degenerate primers. Subsequently, the 5′ untranslated region of CISA-37 was identified by 5′-RACE using specific primers. The CISA-37 cDNA contained a 993-bp open reading frame encoding 331 amino acids, including a putative 19-amino acid residue signal peptide (Fig. 4A) (GenBank; AB381932). In the gene encoding CISA-37, TAA (nts: 382–384, 388–390, 655–657 and 986–988) and TAG (nts: 376–378 and 448–450) codons were used as glutamine codons, as has been previously observed in the CISA-32 gene (GenBank; AB262047) (Hatanaka et al. Reference Hatanaka, Umeda, Yamashita and Hirazawa2007). A Kyte-Doolittle hydropathy plot of the derived amino acid sequence shows that the C-terminal portion of CISA-37 has a hydrophobic segment characteristically found in proteins containing a C-terminal GPI anchor (Gerber et al. Reference Gerber, Kodukula and Udenfriend1992) (Fig. 4B). The potential omega (ω) site in CISA-37 for propeptide cleavage and GPI attachment – as predicted by the big-PI Predictor program – is S307 (Eisenhaber et al. Reference Eisenhaber, Bork and Eisenhaber1999).

Fig. 4. Sequence analysis of CISA-37. (A) Nucleotide sequence of the CISA-37 cDNA and the derived amino acid sequence. The complete CISA-37 cDNA was determined from cDNA generated from total theront RNA using 3′- and 5′-RACE with degenerate primers. The 20 N-terminal amino acid residues determined by Edman degradation are underlined. The potential N-terminal signal peptide is boxed. TAGs and TAAs in the cDNA sequence, thought to be used as glutamine codons, are underlined. The position of the putative omega (ω) site for propeptide cleavage and GPI attachment is circled (Ser307). (B) Kyte-Doolittle hydropathy plot of the CISA-37 amino acid sequence (window size, 7 residues).
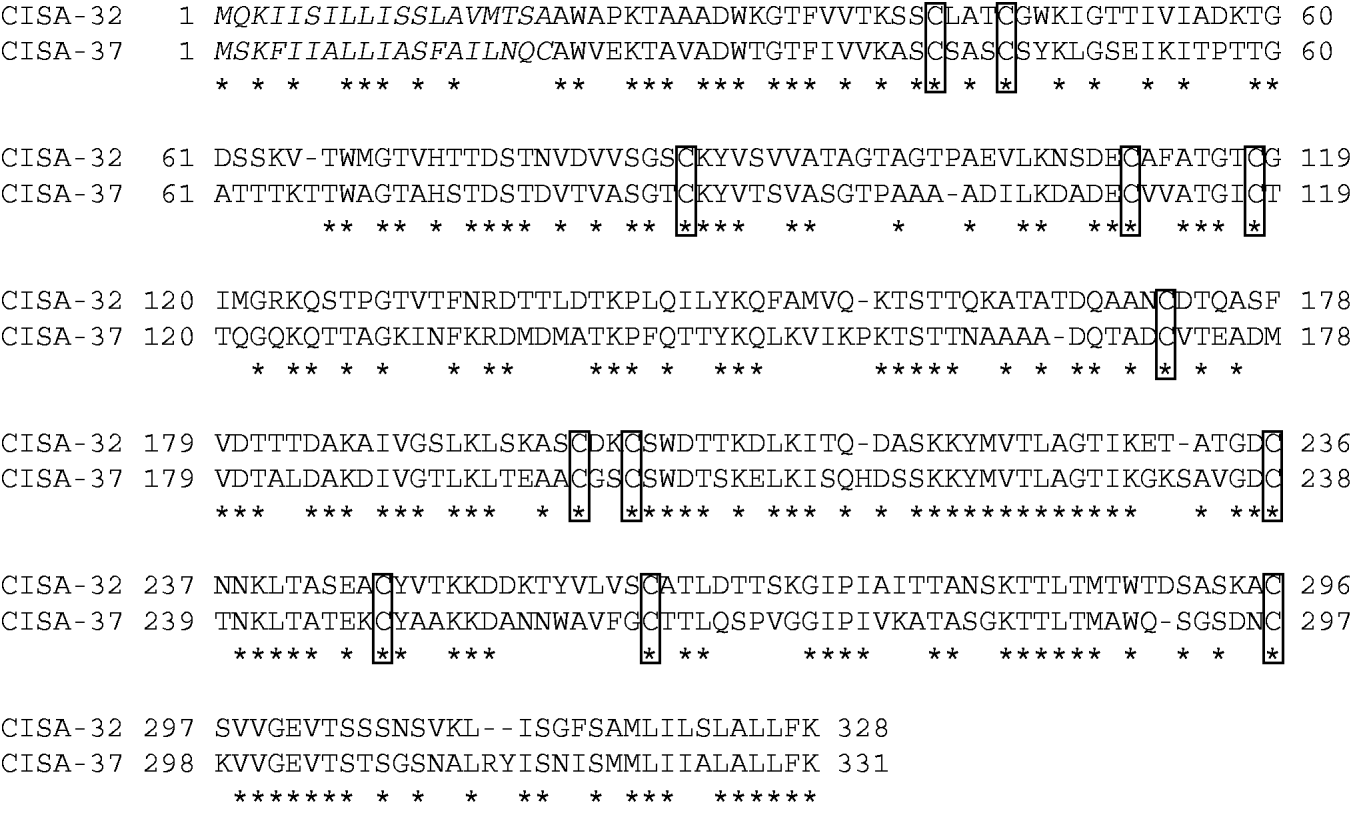
Alignment of deduced CISA amino acid sequences
Alignment of the deduced amino acid sequences of the two serotypes, shown in Fig. 5, indicates a 45% similarity. Twelve cysteine residues of the putative mature peptides were conserved between the CISAs.

Fig. 5. Alignment of the deduced amino acid sequences of CISA-32 and CISA-37. Asterisks indicate identical residues. Cysteine residues conserved in the mature peptides of both CISAs are boxed.
RT-PCR and immunoblot analyses of the two serotypes
To investigate whether each strain expresses only its specific agglutination/immobilization antigen or also expresses the agglutination/immobilization antigen of the other serotype, RT-PCR with specific primers to CISA-32 and CISA-37 was carried out with RNA from theronts of both serotypes. The product amplified with the CISA-32-specific primers was detected only in serotype G32 theronts (Fig. 6A, lanes 1 and 2) and vice versa (Fig. 6A, lanes 3 and 4). Nested PCR did not detect the amplified CISA-32-specific product in the serotype G37 or vice versa. The PCR products amplified from each theront cDNA had bands of the predicted size (984 bp and 1032 bp for CISA-32 and CISA-37, respectively), and thus were subcloned, sequenced, and confirmed to be the CISA-32 and CISA-37 cDNA sequences. In line with these results, a single ~32 kDa protein in the Triton X-114–soluble fraction of serotype G32 theronts was detected by tiger puffer serum immunized with serotype G32 theronts (Fig. 6B, lane 1) but not by serum from fish immunized with serotype G37 theronts (Fig. 6B, lane 2), and conversely, a single ~37 kDa protein was observed when the Triton X-114–soluble fraction of serotype G37 was blotted with tiger puffer serum immunized with serotype G37 theronts but not with serum from fish immunized with serotype G32 theronts (Fig. 6B, lanes 3 and 4).

Fig. 6. Expression of CISA-32 and CISA-37 by RT-PCR (A) and immunoblot (B) analysis. (A) RT-PCR analysis for expression of CISA-32 (lanes 1 and 3) or CISA-37 (lanes 2 and 4) genes in serotype G32 (lanes 1 and 2) or G37 (lanes 3 and 4) theronts. Total RNA from both theronts was analysed using specific primers to detect the CISA-32 or CISA-37 gene. Amplification products were electrophoresed in a 1·5% agarose gel and stained with ethidium bromide. (B) Immunoblot analysis: 2 μg of theront integral membrane proteins of serotype G32 (lanes 1 and 3) and G37 (lanes 2 and 4) were resolved by non-reducing SDS-PAGE and then immunoblotted using the following antisera: lanes 1 and 2, serum from tiger puffer immunized with serotype G32 theronts; lanes 3 and 4, serum from tiger puffer immunized with serotype G37 theronts. Molecular masses of standard markers are indicated on the left.
Phylogenetic relationships among serotype G-32, G-37, and other C. irritans isolates
A total of 321 bp of rDNA nucleotide sequence were determined for the G32 and G37 serotypes that included 137 bp from the 3′ end of the 18S region, 170 bp from the ITS-1 region, and 14 bp from the 5·8S region (Fig. 7). ITS rDNA sequence of serotype G32 was identical to that of isolates from Pingtung in Taiwan (GenBank: AF490384) and from the USA (GenBank: AY029270). On the other hand, the sequences of serotype G37 were not identical to those of any isolate but showed the highest similarity (93·438% similar) with the Israel (GenBank: AY029269) and Malaysia (GenBank: AF490385) isolates by the FASTA program.

Fig. 7. Alignment of 18S and ITS-1 rDNA sequences of Cryptocaryon irritans strains and Ichthyophthirius multifiliis. Asterisks indicate identical nucleotides in the consensus sequences.
DISCUSSION
In the present study, we demonstrated the existence of a novel agglutination/immobilization serotype of C. irritans. Tiger puffer antiserum raised against proteins isolated from theronts of serotype G37 agglutinated/immobilized parasites of this serotype but rabbit and fish antisera raised against serotype G32 (from our previous study) (Hatanaka et al. Reference Hatanaka, Umeda, Yamashita and Hirazawa2007) did not. In the case of I. multifiliis, the protective immunity of channel catfish (Ictalurus punctatus) elicited by i-antigen is nearly serotype-specific (Wang et al. Reference Wang, Clark, Noe and Dickerson2002; Swennes et al. Reference Swennes, Findly and Dickerson2007). Also, in the case of C. irritans, our results indicate that fish immunity to C. irritans elicited by the agglutination/immobilization antigen is also serotype-specific.
As all the cysteine residues in both CISAs without N-terminal signal peptides were conserved, CISA-37 and CISA-32 likely have similar structures. TAA and TAG codons are found both in the CISA-37 and CISA-32 genes, and these codons are probably used as glutamine codons rather than termination codons as determined from our previous analysis of elongation factor-1α (EF-1α) sequences (Hatanaka et al. Reference Hatanaka, Umeda, Yamashita and Hirazawa2007). The CISA-37 protein is thought to be a GPI-anchoring protein for surface antigens of Tetrahymena and Paramecium spp. (Azzouz et al. Reference Azzouz, Ranck and Capdeville1990; Ko and Thompson, Reference Ko and Thompson1992). However, we were unable to determine the putative function of the 2 CISA proteins in C. irritans from the deduced amino acid sequences. Although the cysteine residues in the mature peptides of both CISAs were conserved and the hydropathy profiles were similar (data not shown), CISA-37 was not detected by immunoblot analysis under reducing conditions, whereas CISA-32 was detected by immunoblot analysis only under non-reducing conditions (Hatanaka et al. Reference Hatanaka, Umeda, Yamashita and Hirazawa2007).
C. irritans seems to express a single agglutination/immobilization antigen on its surface, according to RT-PCR analysis. The surface antigens of Tetrahymena and Paramecium spp. switch to alternative variants in response to changes in environmental conditions such as temperature, but are usually stable under set conditions (Caron and Meyer, Reference Caron and Meyer1989; Smith et al. Reference Smith, Berkowitz, Potoczak, Krause, Raab, Quinn and Doerder1992). During the present study, C. irritans was maintained under constant conditions. Therefore, under different conditions, different agglutination/immobilization antigen(s) may be expressed on the surface of C. irritans parasites, showing different serotype(s).
The relationship between the rDNA nucleotide sequence of C. irritans and its serotype is still unclear. The rDNA nucleotide sequences of serotype G32 were identical to isolates from Pingtung in Taiwan and from the USA. However, the serotypes and primary structures of agglutination/immobilization antigens of different isolates have not been fully investigated. Future work to identify the serotype-rDNA nucleotide sequence relationship will be required for the development of fish vaccines against infection by C. irritans.