Syncope can be defined as a transient loss of consciousness and postural tone due to a transient hypoperfusion of the brain. Reference Thijs, Wieling, Kaufmann and van Dijk1 Vasovagal syncope is the most common cause of syncope during childhood. Reference Lewis and Dhala2 The mechanism of vasovagal syncope is not clearly known. However, autonomic nervous dysfunction, reflex vasodilation, vasomotor dysfunction, and genetic factors are considered as potential mechanisms for vasovagal syncope. Reference Brignole, Moya and de Lange3,Reference Nair, Padder and Kantharia4 Vasovagal syncope does not cause fatal consequences; however, recurrent syncope episodes negatively affect the quality of life. Non-pharmacological treatment approaches including aggressive fluid and salt intake, doing exercise, blood pressure increasing manoeuvres, and avoiding predisposing situations are effective in 70% of patients, but syncope episodes still affect approximately 30% of patients. Reference Van Dijk, Quartieri and Blanc5
It is considered that midodrine hydrochloride, which is a selective alpha-agonist, prevents syncope by increasing peripheral resistance and preventing venous pooling with its constrictive effect on arterioles and veins. Midodrine does not cause systemic hypertension even at effective dose and it does not have direct cardiac effects. Reference Mitro, Trejbal and Rybar6,Reference Thulesius, Gjores and Berlin7
It was proven that midodrine was effective in orthostatic hypotension treatment. Reference Low, Gilden and Freeman8,Reference Jankovic, Gilden and Hiner9 Some studies in adults with vasovagal syncope suggest that midodrine treatment may also be an effective preventive treatment. Reference Brignole, Moya and de Lange3,Reference Mitro, Trejbal and Rybar6 However, in the literature, there is a limited number of studies on administering pharmacological treatment to prevent recurrence in paediatric patients with recurrent vasovagal syncope. In the present study, we aimed to investigate midodrine hydrochloride usage and its efficacy in preventing recurrent vasovagal syncope in children.
Material and methods
Patients with syncope admitted to Pediatrics Department of Ankara Education and Research Hospital between June 2017 and October 2019 were evaluated based on diagnostic algorithms for syncope aetiology (Table 1). A detailed medical history was obtained and physical examination, electrocardiography, posteroanterior chest X-ray, electroencephalography, biochemical and metabolic scanning, and cranial magnetic resonance imaging were performed.
Table 1. Algorithm for the evaluation of syncope and proposed diagnostic workup
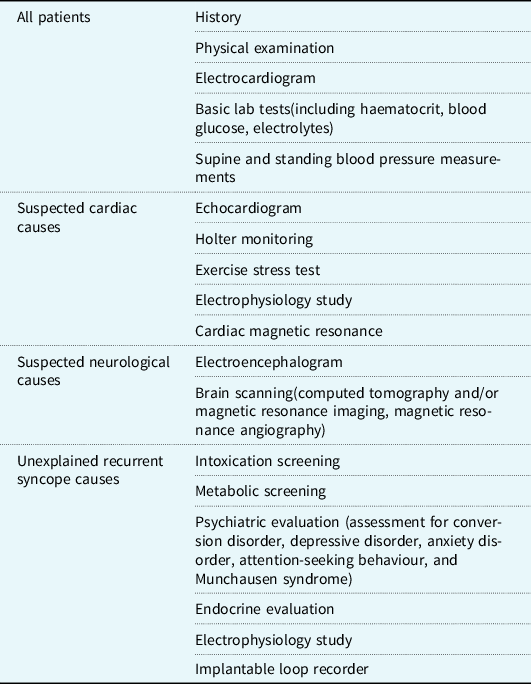
Patients were excluded if they had other causes of syncope. Patients using any medication to prevent vasovagal syncope recurrence were also excluded. Following the examinations, patients who had at least three syncopal episodes in a year were diagnosed with vasovagal syncope. Of those patients, 24 patients who received non-pharmacological treatments but experienced presyncope-syncope episodes three times or more during at least 6 months of follow-up and later started midodrine treatment were retrospectively examined.
Descriptive statistics were obtained for all study variables. Results were expressed in mean ± standard deviation and categorical variables were summarised as frequencies and percentages. All data were analysed using SPSS Statistics version 22.0 for Windows software (IBM Corp., Armonk, New York, United States). A p value of <0.05 was considered statistically significant. Comparative statistical analysis could not be done due to the small size of the study group and the absence of a control group.
Results
Of the 24 patients included in the study, 18 patients were female (75%) (female/male ratio 3/1). The mean age of the patients at the inclusion in the study was 15.12 ± 0.89 years. The mean age of first application to the clinic was 14.2 ± 0.83 years. The age at which the first syncope was observed was 12.4 ± 2.1 years; the mean frequency of syncope episodes was 5.75 ± 2.67 years. The mean duration of symptoms was 18 ± 9.7 months. Mean follow-up period was 21.75 ± 9.9 months. No patients were lost to follow-up (Table 2).
Table 2. Characteristics of the patients with recurrent syncope episodes

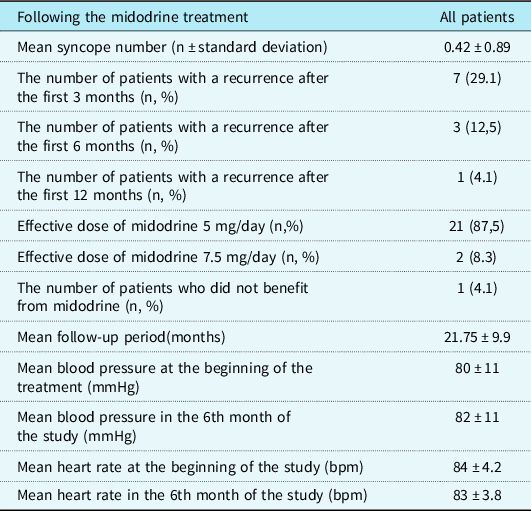
After the treatment was started, the mean number of syncope episodes was 0.42 ± 0.89. In the first 6 months of the follow-up, syncope episodes did not recur in 17 patients while it recurred in 7 patients (29.1%). In the first 3 months of the treatment, syncope episodes recurred in four of seven patients, but the episodes did not recur in the following months. In two of the remaining three patients, the episodes improved with an increase in the treatment dose (7.5 mg/day), but the episodes continued at a decreased rate in one patient.
In total, 21 of the patients received treatment of midodrine at a dose level of 5 mg/day (2.5 mg twice daily) while 3 of them received a 7.5 mg/day (2.5 mg three times daily) treatment. Six patients were still on the treatment with an average treatment period of 19.1 ± 4.4 months and 18 patients discontinued the treatment. The mean treatment period of the patients who discontinued the treatment was 13 ± 1.45 months.
Only two patients had a mild headache at the beginning of the treatment. No side effects were observed in other patients. No statistically significant change was observed in the mean blood pressure and heart rate which were measured prior to the initiation of the midodrine treatment and in the 6th month of the treatment (p = 0.79, p = 0.92; respectively) (Table 3).
Table 3. Charateristics of patients following the midodrine treatment
Discussion
Vasovagal syncope is common both in childhood and adulthood, and it is usually considered as benign symptom. The pathophysiological mechanism is not clear; however, a sudden sympathetic suppression resulting in vasodilation, hypotension, and/or bradycardia is considered to be the cause. The diagnosis is based entirely on clinical signs and symptoms. Reference Driscoll, Jacobsen, Porter and Wollan10–Reference Wieling, Jardine and de Lange12
The starting treatment of vasovagal syncope is providing adequate information about the factors triggering syncope, patient’s awareness of prodromal symptoms, and avoiding prolonged standing, rapid change of position, hot environments, and dehydration. Moreover, non-pharmacological treatment methods such as increased fluid and salt intake, physical exercise, counter pressure manoeuvres are administered. Reference Wang, Li, Liao, Tian, Huang and Dong13 However, syncope episodes continue in many patients despite the treatment. Furthermore, additional treatment approaches are required when frequent recurrences impair the quality of life and the risk of traumatic injury increases. In studies conducted with adults in this patient group, it has been suggested that treatment methods such as atenolol, paroxetine, midodrine, disopyramide, and permanent cardiac pacing are partially beneficial in reducing and preventing vasovagal syncope recurrences. Reference Mahanonda, Bhuripanjo and Kangkate14–Reference Connolly, Sheldon and Roberts17 There are limited numbers of studies on pharmacological treatment in refractory vasovagal syncope in childhood. Salim et al Reference Salim and Di Sessa18 compared fludrocortisone and sodium intake with placebo group, and it was suggested that the placebo group had better results in terms of symptom recurrence (symptoms recurred in 10 of 18 children receiving fludrocortisone and sodium and in 5 of 14 children receiving placebo). In a prospective, randomised study, Reference Zhang, Jin, Wang, Chen, Tang and Du19 a group receiving metoprolol was compared with a group receiving conventional treatment which includes increasing water and salt intake, not standing too long without moving, and avoiding stressful situations which previously caused syncope. During 22 ± 10 months of follow-up, it was identified that the recurrence of vasovagal syncope was similar in children and adolescents receiving metoprolol to the patients receiving conventional treatment (43 versus. 29%, respectively, p = 0.695).
In a study where children with recurrent vasovagal syncope received midodrine, midodrine was used as a first line therapy for recurrent syncope episodes. A total of 26 children were followed for 6 months. During the follow-up period, syncope recurrence rate was 22% in the Group 1 (receiving midodrine) and 80% in the Group 2 (receiving only conventional non-pharmacological treatment). It was reported that both effectivity rate observed with the tilt test and the rate of remaining syncope free were significantly higher in the patients receiving midodrine treatment than those receiving conventional treatment (p = 0.03 and p = 0.023, respectively) despite a low dose level of 2.5 mg/day as the starting dose of midodrine treatment was administered to the patients included in the study. Reference Qingyou, Junbao and Chaoshu20 However, this study was conducted on those who were hospitalised for syncope, agreed to take tilt tests, and had “positive” tilt table test results. According to current American Heart Association and European Society of Cardiology guidelines, Reference Brignole, Moya and de Lange3,Reference Shen, Sheldon and Benditt21 tilt tests are no longer required to diagnose simple vasovagal syncope. This new requirement is the result of a high incidence of “false negatives”. The use of tilt table test in the diagnosis of vasovagal syncope should be considered to those patients who have a very typical history of vasovagal syncope, but do not faint during orthostatic testing, and those patients who do not have a vasovagal history, but faint during orthostatic testing and have false positive results. Similarly, there is no point in evaluating the effectiveness of a treatment for the same reasons. We did not use tilt table tests in the present study. Other tests for aetiology were performed and the diagnosis of vasovagal syncope was made through medical history and elimination of other diseases.
Midodrine is a peripheral vasoconstrictor alpha-agonist which increases peripheral vascular resistance and reduces venous pooling. Midodrine, following oral administration, is rapidly metabolised to the active matter desglymidodrine. Its effects on alpha adrenergic receptors on the veins results vasoconstriction. Desglymidodrin passes poorly through the blood–brain barrier, and therefore has little or no effect on the central nervous system. Midodrine has little cardiostimulatory or arrhythmic effect on normal hearts when compared to beta-adrenergic receptors that predominantly mediate cardiac inotropic effects. Reference Ward, Gray and Gilroy15,Reference Sra, Maglio and Biehl23–Reference Kaufmann, Saadia and Voustianiouk26
Properties of midodrine are ideal for improving the response to orthostatic stress as all acute and chronic orthostatic intolerance forms are primarily related to decreased vasoconstriction and/or increased venous pooling. Therefore, it creates a tendency to treat or prevent hypotension with increased blood volume. The administration of midodrine increases systolic and diastolic blood pressure in patients with decreased blood pressure. It was suggested in a series of studies conducted in adults with orthostatic hypotension, paroxysmal orthostatic tachycardia, and vasovagal syncope that midodrine had a beneficial effect. Reference Sra, Maglio and Biehl23–Reference Kaufmann, Saadia and Voustianiouk26
In clinical practice, the first intervention for vasovagal syncope is non-pharmacological methods. Reference Brignole, Moya and de Lange3,Reference Shen, Sheldon and Benditt21,Reference Sanatani, Chau and Fournier22 In the present study, pharmacological treatment was administered only when patients had at least three syncope recurrences after utilising non-pharmacological treatments such as aggressive fluid and salt intake, using counterpressure manoeuvres, and exercise. It was shown that administering midodrine was safe and effective in the treatment of children with vasovagal syncope who did not benefit from traditional non-pharmacological treatment. It prevented syncope recurrence in 87.5% of patients in the first 6 months, and it was successful with a rate of 96% in remaining patients with dose escalation in 1 year follow-up.
Mitro et al reported in their study on 41 adults with recurrent vasovagal syncope episodes and a positive tilt table test result that 38 of 39 patients (97%) with negative repeated tilt table test remained free of syncope recurrence during 19 ± 9 months of follow-up in patients who received midodrine. Effective midodrine dose was 5 mg/day in 25 patients and 10 mg/day in 16 patients. Reference Mitro, Trejbal and Rybar6 In our study, effective midodrine dose was 5 mg/day in 21 patients and 7.5 mg/day in 3 patients. Moreover, similar to the results of the study by Mitro et al, 23 of 24 patients (96%) remained free of syncope recurrence. Sra et al Reference Sra, Maglio and Biehl23 administered midodrine to 11 adult patients diagnosed with vasovagal syncope resistant to other medications. They reported that symptoms completely disappeared with a dose of 7.5 mg/day in five patients (46%). There was significant improvement in four patients, one patient discontinued the treatment due to its side effects, and one patient still had syncope episodes despite midodrine treatment. Ward et al Reference Ward, Gray and Gilroy15 performed a double-blind, placebo-controlled trial in 16 tilt positive patients with frequent syncope episodes. They reported that 14 of 16 patients receiving placebo and only 6 of 16 patients receiving midodrine had positive repeated tilt table test results. They also suggested that midodrine treatment caused a significant increase in the number of symptom-free days.
Unlike these studies, in a randomised double-blind crossover study Reference Romme, van Dijk and Go-Schon27 (STAND Trial) conducted in the Netherlands on midodrine efficacy in adult patients with recurrent vasovagal syncope episodes resistant to non-pharmacological treatment, a total of 28 patients alternately received placebo and midodrine treatments for 3 months. Unlike previous studies using the head-up tilt test, it was aimed to measure the efficacy by recording recurrent syncope and presyncope episodes in daily life. There was no dose adjustment in patients with recurring episodes, and the patients received a fixed dose of midodrine 5 mg BID. Researchers stated that there was no statistically significant difference between additional midodrine and placebo treatment in terms of syncopal recurrence, and that the efficacy of midodrine treatment was much less than expected in patients who did not respond to non-pharmacological treatment. Therefore, they did not recommend administering midodrine as routine additional treatment in those patients. They explained the different results from previous studies which investigated midodrine efficacy without a control group, decreases in symptom frequency could be expected in patients with vasovagal syncope regardless of treatment, and thus, placebo-controlled studies should be conducted to investigate whether midodrine treatment had therapeutic effect on the expected decrease in the frequency of recurrence following the diagnosis. However, they did not make any dose adjustments in patients with recurring episodes, and the patients received a fixed dose of midodrine 5 mg BID in the study. We suggest that syncope recurrences may be prevented with increasing dose escalation in some of patients and this may affect the results of the study. Moreover, the study was valuable in that it was randomised and double-blind; however, it was conducted in an adult population. There is limited study on vasovagal syncope mechanism in paediatric age, especially in adolescence, and different results may be obtained compared to adulthood.
Midodrine is considered quite safe in terms of side effects. In previous studies, it was reported that supine hypertension, headache, pilomotor reactions, and gastrointestinal symptoms were rare. In the STAND Trial Reference Romme, van Dijk and Go-Schon27 conducted in the Netherlands, when the patients were compared in terms of side effects, those who received placebo reported more side effects than those who received midodrine treatment, which is quite interesting (headache, cold sensations, and nausea in those receiving midodrine; headache and fatigue/loss of energy in those receiving placebo, 57 versus. 48%). Midodrine is actually a “prodrug” converted into desglymidodrine which is an active metabolite with high bioavailability. This offers several advantages. As a prodrug, it enters into the system as a partially active agent and minimises the possibility of gastrointestinal side effects. As midodrine does not pass through the blood–brain barrier, it does not have any stimulant effect on the central nervous system. Therefore, it does not have a potential for abuse. Reference Grubb, Karas, Kosinski and Boehm25,Reference Kaufmann, Saadia and Voustianiouk26 In the present study, there was no side effect other than mild headache in 2/24 patients.
Limitations
There were many major limitations to the present study such as having a relatively small study population and the lack of placebo-control. It is known that clinical severity of vasovagal syncope varies considerably. Although, it is considered that clinical improvement may not dependent the medicine, the patient group did not benefit from non-pharmacological treatment methods and, as a result, were included in the study with frequent presyncope-syncope episodes. As the results of our study suggest, the administration of midodrine was quite effective in adolescents. Although it was thought that the medicine was effective in preventing symptoms throughout the period of use, most of the patients in our study receiving the medicine for a while after the symptoms disappeared and discontinued the treatment when symptoms did not recur. It was observed that syncope episodes did not recur in those patients during the follow-up for a mean of 12 months following the discontinuation of the study medicine.
Conclusion
Midodrine is an effective agent that can be used in children with resistant vasovagal syncope who do not respond to conventional non-pharmacological treatment and also it has minimal side effects. It is suggested that midodrine 5 mg is sufficient in children. However, large-scale, double-blind, placebo-controlled prospective studies will be necessary to better determine the role and efficacy of midodrine in the treatment of recurrent vasovagal syncope in children.
Acknowledgements
None.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Financial support
The author(s) received no financial support for the research, authorship, and/or publication of this article.






