Introduction
Iceland is an oceanic plateau that sits atop the Mid-Atlantic Ridge (MAR) and the impinging Icelandic mantle plume (Sigmundsson, Reference Sigmundsson2006; Thordarson and Larsen, Reference Thordarson and Larsen2007). Because of Iceland's cold sub-polar oceanic climate, it receives high rates of precipitation that, in combination with active volcanism and crustal extension, promote deep-seated meteoric-hydrothermal systems (Muehlenbachs et al., Reference Muehlenbachs, Anderson and Sigvaldason1974; Eiler, Reference Eiler, Valley and Cole2001). Hydrothermally altered crust on Iceland is generally characterized by δ18O values that are lower than those of the mantle (Muehlenbachs et al., Reference Muehlenbachs, Anderson and Sigvaldason1974; Gautason and Muehlenbachs, Reference Gautason and Muehlenbachs1998; Bindeman et al., Reference Bindeman, Gurenko, Carley, Miller, Martin and Sigmarsson2012), and thus crustal assimilation by ascending magmas creates a wide range of sub-mantle δ18O values in Icelandic igneous rocks (e.g. O'Nions and Grönvold, Reference O'Nions and Grönvold1973; Óskarsson et al., Reference Óskarsson, Sigvaldason and Steinthórsson1982; Reference Óskarsson, Steinthórsson and Sigvaldason1985; Hattori and Muehlenbachs, Reference Hattori and Muehlenbachs1982; Björnsson, Reference Björnsson1985; MacDonald et al., Reference MacDonald, Sparks, Sigurdsson, Mattey, McGarvie and Smith1987; Eiler, Reference Eiler, Valley and Cole2001; Bindeman, Reference Bindeman2008; Bindeman et al., Reference Bindeman, Gurenko, Carley, Miller, Martin and Sigmarsson2012; Geiger et al., Reference Geiger, Mattsson, Deegan, Troll, Burchardt, Gudmundsson, Tryggvason, Krumbholz and Harris2016). In this context, δ18O values above +7‰ for Icelandic igneous rocks are extremely rare, and include a devitrified and hydrated pitchstone from Öræfajökull and hydrated acidic xenoliths from Hekla volcano that show δ18O values of around +10‰ (e.g. Muehlenbachs et al., Reference Muehlenbachs, Anderson and Sigvaldason1974; Condomines et al., Reference Condomines, Grönvold, Hooker, Muehlenbachs, O'Nions, Óskarsson and Oxburgh1983; Hemond et al., Reference Hemond, Arndt, Lichtenstein, Hofmann, Óskarsson and Steinthorsson1993; Prestvik et al., Reference Prestvik, Goldberg, Karlsson and Grönvold2001). Igneous rocks with δ18O values exceeding the range that can be produced by closed-system fractionation from a mantle-derived magma (+5.7 to ~7‰; Valley et al., Reference Valley, Lackey, Cavosie, Clechenko, Spicuzza, Basei, Bindeman, Ferreira, Sial, King, Peck, Sinha and Wei2005; Bindeman, Reference Bindeman2008) are thought typically to have assimilated material altered at low temperature or undergone isotope exchange at low temperature themselves (e.g. Bindeman, Reference Bindeman2008; Donoghue et al., Reference Donoghue, Troll, Harris, O'Halloran, Walter and Pérez Torrado2008; Reference Donoghue, Troll and Harris2010; Deegan et al., Reference Deegan, Troll, Barker, Harris, Chadwick, Carracedo and Delcamp2012). Whereas S-type granites are generally characterized by δ18O values in excess of +10‰, because their sedimentary protoliths underwent near-surface low-temperature oxygen exchange (e.g. Savin and Epstein, Reference Savin and Epstein1970; O'Neil et al., Reference O'Neil, Shaw and Flood1977), low-temperature alteration processes on Iceland are less likely to produce high δ18O values because Icelandic meteoric waters are 18O-depleted (–7.7 to –15‰, Árnason, Reference Árnason1976; Hattori and Muehlenbachs, Reference Hattori and Muehlenbachs1982; Rozanski et al., Reference Rozanski, Araguás-Araguás, Gonfiantini, Swart, Lohmann, McKenzie and Savin1993). Moreover, the rate of chemical weathering on Iceland is relatively low because of the generally low mean annual air temperatures, although it should be noted that a 5 to 10°C warmer climate in the Neogene compared to today would have allowed for slightly increased weathering rates (Óskarsson et al., Reference Óskarsson, Riishuus and Arnalds2012). However, the frequent absence of soils within formerly buried flood lavas in the study region support the assumption of rapid burial and consequently low surface-weathering rates.
In this paper, we present whole-rock oxygen isotope data for 12 broadly rhyolitic rock samples from the Borgarfjörður Eystri region, Northeast Iceland, of which a sub-group of intra-caldera rhyolites display unusually high whole-rock δ18O values. For several crucial samples we also analysed oxygen isotopes in feldspar and pyroxene mineral phases, to offer possible explanations for the anomalously high δ18O values detected.
Geological setting
Dyrfjöll, Breiðavik, Kækjuskörð and Herfell are the eroded remnants of a cluster of Neogene central volcanoes around Borgarfjörður Eystri in Northeast Iceland (Berg et al., Reference Berg, Troll, Burchardt, Riishuus, Krumbholz and Gústafsson2014). These volcanoes produced voluminous rhyolite lavas and ignimbrite deposits around 12 Ma, which together make up ≥20% of the surface rock exposures in the area (relative to the 10–12% silicic outcrop typical for Iceland, Gústafsson et al., Reference Gústafsson, Lapp, Thomas and Lapp1989; Burchardt et al., Reference Burchardt, Tanner, Troll, Krumbholz and Gústafsson2011; Martin et al., Reference Martin, Paquette, Bosse, Ruffet, Tiepolo and Sigmarsson2011; Óskarsson and Riishuus, Reference Óskarsson and Riishuus2013). During this extremely violent eruptive phase, the Njarðvik, Dyrfjöll, Breiðavik and Herfell collapse calderas formed and voluminous intra- and extra-caldera ignimbrite sheets and rhyolitic lavas were deposited (Fig. 1, Gústafsson et al., Reference Gústafsson, Lapp, Thomas and Lapp1989; Burchardt et al., Reference Burchardt, Tanner, Troll, Krumbholz and Gústafsson2011). At Dyrfjöll and Breiðavik calderas, the caldera infill is overlain by olivine basaltic hyaloclastites and remnants of pillow lavas that provide evidence for the existence of caldera lakes following rhyolite eruption and caldera collapse (Figs 1 and 2, see Gústafsson, Reference Gústafsson1992). The hyaloclastites are, in turn, overlain by regional flood basalts. The thickness of the caldera-bounded hyaloclastite deposits suggests that the intra-caldera lakes were of substantial size and may have reached ≤350 m depth (Gústafsson, Reference Gústafsson1992). These Neogene caldera lakes were probably similar to modern Icelandic caldera lakes, such as Öskjuvatn at Askja volcano that formed after the 1875 explosive eruption (Sigurdsson and Sparks, Reference Sigurdsson and Sparks1978). In contrast, the Herfell caldera appears to have been completely filled with a thick succession of ignimbrites that can be traced for several kilometres beyond the caldera. Hyaloclastites are absent at Herfell and the ignimbrite infill is overlain directly by olivine basalt lavas belonging to the regional flood basalt suite (cf. Óskarsson and Riishuus, Reference Óskarsson and Riishuus2013). Hyaloclastites are also absent in the Njarðvik caldera, implying that Herfell and Njarðvik did not host extensive caldera lakes (cf. Walker, Reference Walker1975; Lipman, Reference Lipman1984; Gústafsson, Reference Gústafsson1992). The present-day elevation of the Dyrfjöll caldera is ~600 m above sea level, but glacial erosion is estimated to have removed nearly two kilometres of former overburden (Gustafsson, Reference Gústafsson1992). At the time of formation ~12–14 Ma, magmatism in the area is thought to have occurred on the shoulder of the Neogene volcanic rift (Gustafsson et al., Reference Gústafsson, Lapp, Thomas and Lapp1989; Óskarsson and Riishuus, Reference Óskarsson and Riishuus2013), making it probable that the Dyrfjöll volcano reached up to 2000 m in elevation. Moreover, as hyaloclastites only occur in some of the calderas in the region investigated, it is probable that the Neogene sea level did not reach this elevation, consistent with subaerial emplacement structures observed in the adjacent and capping flood-basalt flows (Óskarsson and Riishuus, Reference Óskarsson and Riishuus2013).
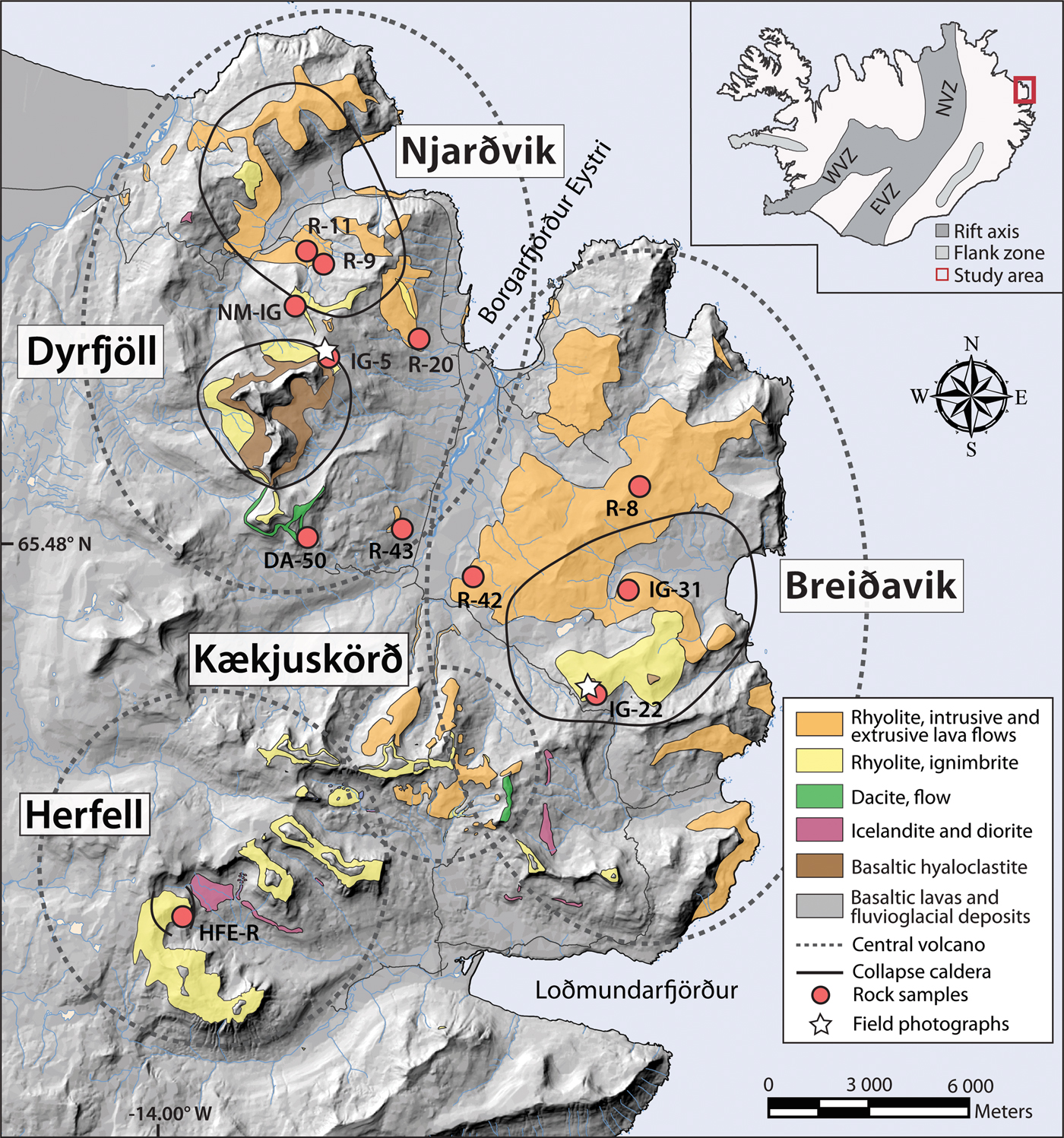
Fig. 1. Geological map of the Borgarfjörður Eystri region in Northeast Iceland, created using the Landmælingar Íslands IS 50 V database together with geological rock units compiled from Gústafsson et al. (Reference Gústafsson, Lapp, Thomas and Lapp1989) and Gústafsson (Reference Gústafsson1992), as well as more recent field observations from the region (Berg et al., Reference Berg, Troll, Burchardt, Riishuus, Krumbholz and Gústafsson2014). Inset shows field area with a red box. Silicic rocks are shown in orange, yellow and green; intermediate rocks in pink; and hyaloclastite deposits in brown. Grey background shading represents basaltic lavas and fluvio-glacial deposits. Sample locations are marked with red circles and approximate caldera margins are indicated with black solid lines. Stippled lines indicate the approximate outline of the individual volcanic centres in the area (Gústafsson et al., Reference Gústafsson, Lapp, Thomas and Lapp1989; Gústafsson, Reference Gústafsson1992; Burchardt et al., Reference Burchardt, Tanner, Troll, Krumbholz and Gústafsson2011; Berg et al., Reference Berg, Troll, Burchardt, Riishuus, Krumbholz and Gústafsson2014). White stars represent the locations of field photographs in Fig. 2.
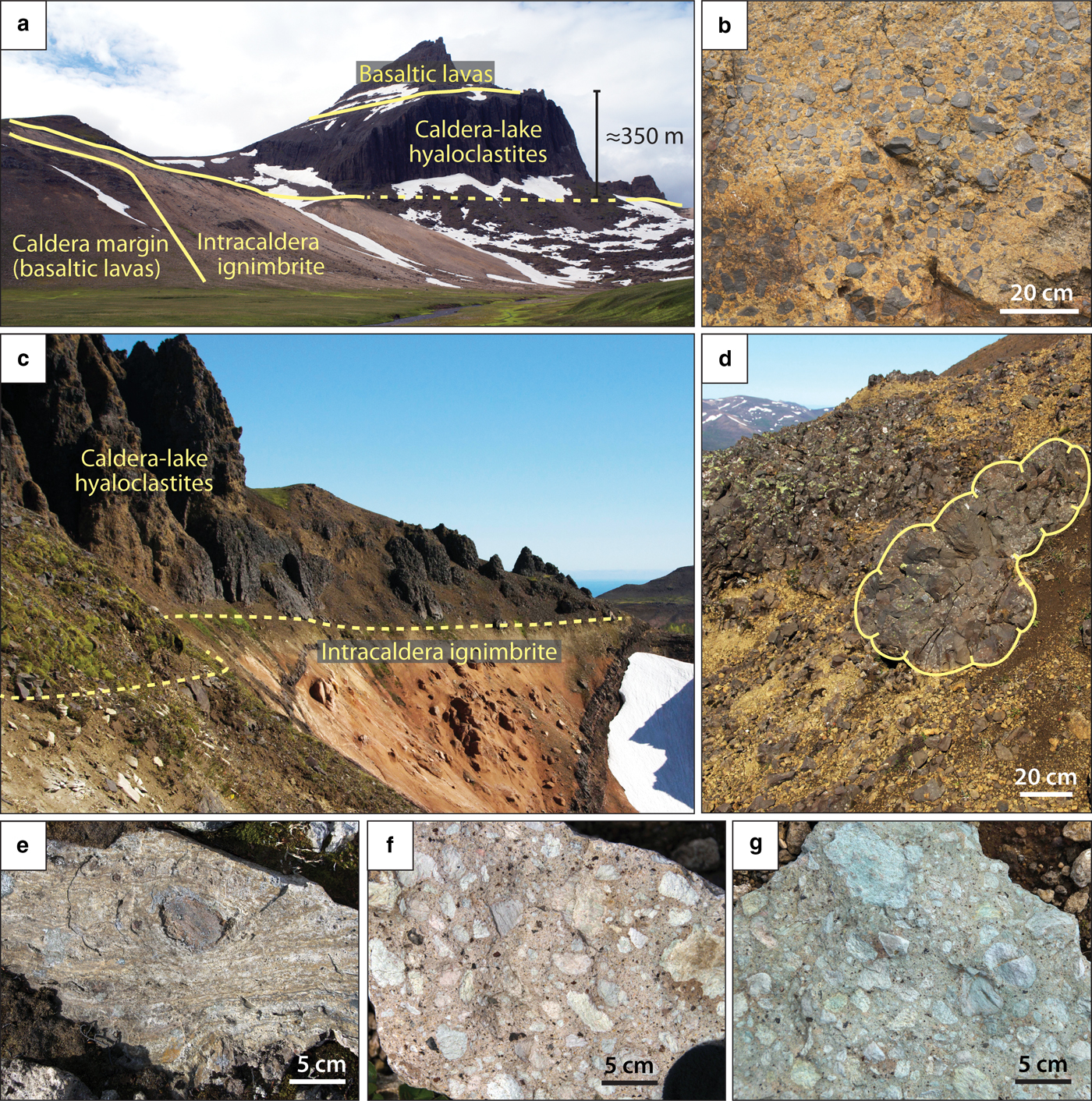
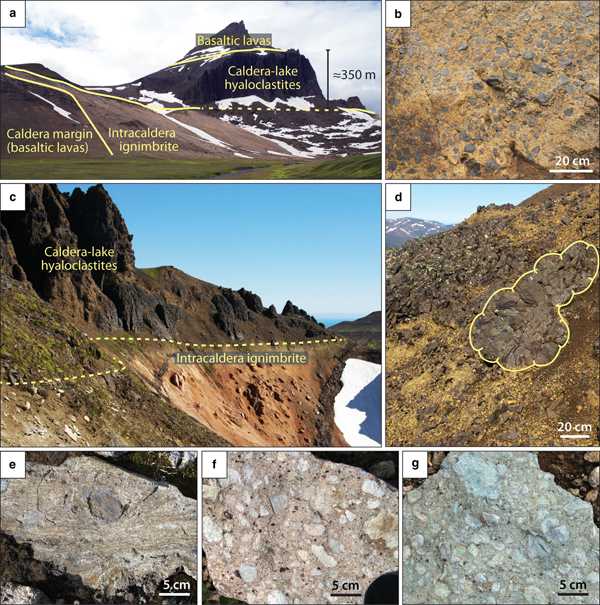
Fig. 2. Field photographs of (a) Urdalsvarp valley at the margin of Dyrfjöll caldera, showing 350 m thick hyaloclastite deposits that formed in a caldera lake setting (photo location marked with a white star in Fig. 1); (b) close-up of basaltic hyaloclastite breccia deposits; (c) the marginal intra-caldera Hvítserkur ignimbrite of the Breiðavik caldera overlain by hyaloclastite deposits; (d) these hyaloclastites comprise common basaltic pillow structures. (e–g) Representative hand specimens of intra-caldera rhyolitic ignimbrite samples from Herfell (e) and Dyrfjöll (f–g), showing welded to lithic-rich rhyolite samples with intense yellow, pink and green alteration colours.
We sampled intra-caldera rocks from the various volcanic centres in the region (n = 6), including four rhyolitic ignimbrites, one rhyolite intrusion and one rhyolite lava (see Fig. 1 and Tables 1 and 2). The Dyrfjöll ignimbrite was sampled at Urdalsvarp and represents a marginal caldera infill (IC-URD-IG-5). Two ignimbrite units were sampled within the Breiðavik caldera: one from Hvítserkur mountain that lies close to the inferred margin of the Breiðavik caldera (IC-HE-IG-22); and another that crops out close to a silicic dome within the central part of Breiðavik caldera (IC-HFE-IG-31). The Herfell ignimbrite was sampled inside the Herfell caldera, but close to the caldera's boundary (IC-HFE-R-1); and a rhyolite intrusion and a lava flow were sampled inside the Njarðvik caldera (IC-HV-R-9 and IC-HV-RD-11).
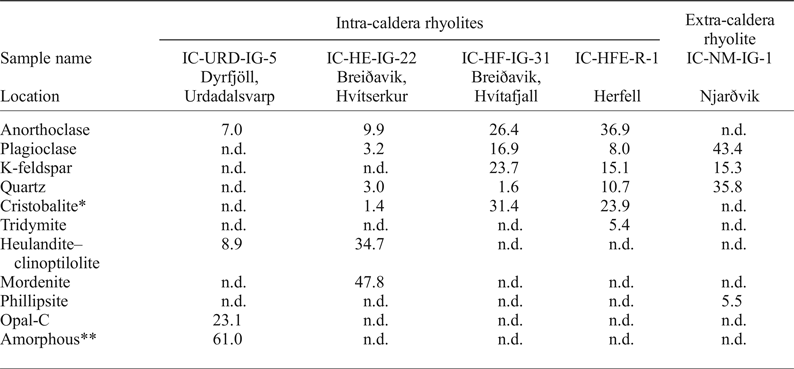
Table 1. Mineral abundances (wt.%) from X-ray diffraction analysis of ignimbrites from Borgarfjörður Eystri, Northeast Iceland.
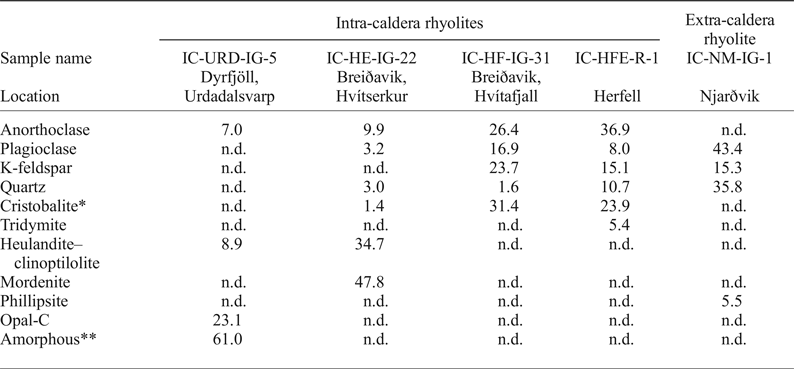
n.d. = not detected; *cristobalite may include opal-C; **amorphous refers to X-ray amorphous material.
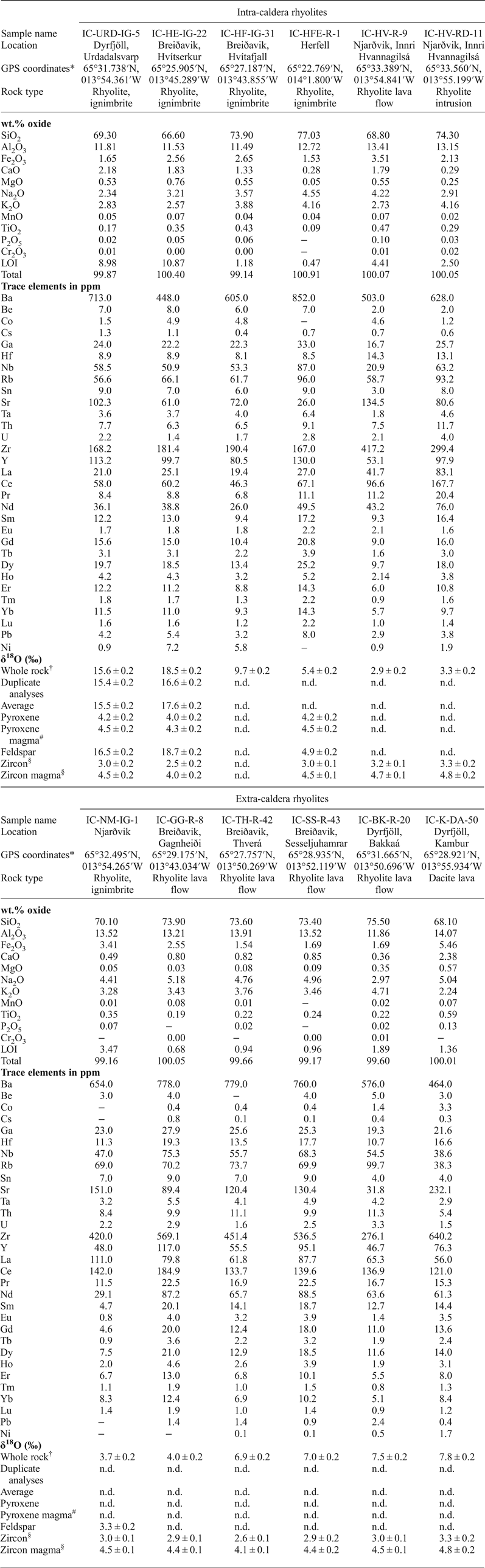
Table 2. Sample description of rhyolites from Borgarfjörður Eystri, Northeast Iceland, including major and trace elements, and oxygen isotope values.
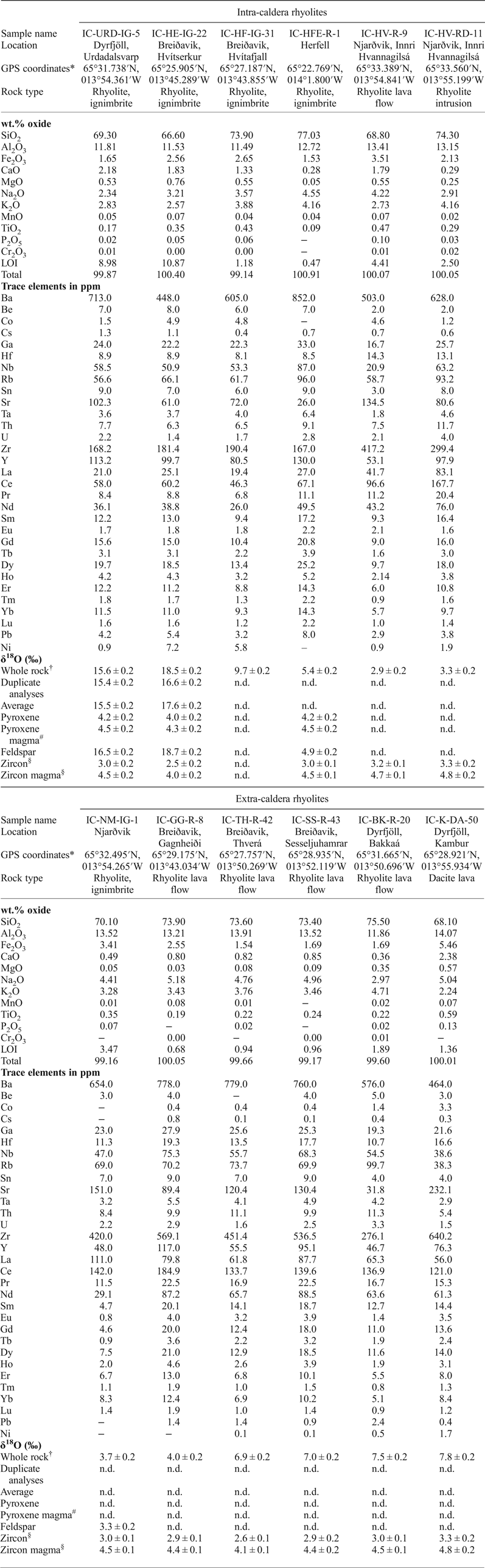
‘–' indicates analysis below detection limit; n.d. = not determined; LOI = loss on ignition. *Reported as: degrees, decimal minutes. †Values reported relative to SMOW (‰). #Calculated using conversion factor from Harris et al. (Reference Harris, Pronost, Ashwal and Cawthorn2005). §See Berg (Reference Berg2016).
For comparison, we also sampled six representative extra-caldera rhyolites from the region, including one rhyolitic ignimbrite, one dacitic lava flow and four rhyolitic lava flows. Although the Njarðvik ignimbrite from Náttmálafjall (IC-NM-IG-1) was sampled close to the outer margin of the caldera, it is distal to its source here. The remaining lava flow samples were collected at a distance of ~1500 to 4000 m to the Njarðvik, Dyrfjöll and Breiðavik calderas (see Fig. 1).
Methods
All rock samples were washed and cut to remove weathered surfaces, and thin sections were subsequently prepared for petrographic observations. Samples were processed further by jaw crushing and milling of hand-picked rock fragments in an agate ball mill at Uppsala University. A portion of each pulverised sample was analysed by X-ray diffraction (XRD) with Rietveld refinement at Activation Laboratories Ltd., Canada. The powdered sample was spiked with known quantities of corundum to permit accurate determination of the mineral proportions present before loading the sample into a sample holder prior to analysis. Following quantification of the crystalline components, the remaining sample content up to 100% was considered to be amorphous material. A Panalytical X'Pert Pro diffractometer equipped with a Cu X-ray source and an X'celerator detector was used for the XRD analyses. The operating conditions were as follows; voltage: 40 kV; current: 40 mA; range: 5–70°2θ; step size: 0.017°2θ; time per step: 50.165 s; divergence slit: fixed; angle: 0.5°. Crystalline mineral phases were identified using the PDF-4 Minerals ICDD database (powder diffraction files from the International Centre for Diffraction Data, http://www.icdd.com/).
Major-element oxides were determined from rock powders by X-Ray Fluorescence (XRF) at ACME Analytical Laboratories Ltd, Vancouver, Canada. Loss on ignition (LOI, i.e. volatile content determined through loss on ignition) was measured by the weight difference after ignition of sample splits at 1000°C. Trace and rare-earth elements were measured by inductively coupled plasma mass spectrometry (ICP-MS) after preparation by multi-acid digestion (HNO3–HClO4–HF) at ACME Analytical Laboratories Ltd, Vancouver, Canada. Sample duplicates have reproducibilities of <0.10 wt.% for major elements and <10 ppm for trace elements. Accuracy was assessed using internal reference materials with reproducibilities of <0.10 wt.% for major elements and <5 ppm for trace elements.
We measured oxygen isotope ratios of 12 whole-rock samples and additional hand-picked mineral separates from four distinct rhyolite ignimbrites from Borgarfjörður Eystri, using both conventional and laser fluorination methods at University of Cape Town, South Africa. All isotope ratios were measured off-line using a Thermo DeltaXP mass spectrometer and all values are reported in standard δ-notation, where δ = (R sample/R standard – 1) × 1000 and R = 18O/16O.
The oxygen isotope composition of 14 whole-rock powders (including two duplicate samples) were analysed using a conventional silicate extraction line (described by Harris and Ashwal, Reference Harris and Ashwal2002). Approximately 10 mg of powdered sample was dried in an oven at 50°C and degassed under vacuum at 200°C. Silicates were reacted with ClF3 (Borthwick and Harmon, Reference Borthwick and Harmon1982) and the liberated O2 was converted to CO2 using a hot platinized carbon rod (Vennemann and Smith, Reference Vennemann and Smith1990; Harris and Ashwal, Reference Harris and Ashwal2002; Fagereng et al., Reference Fagereng, Harris, La Grange and Stevens2008). Further details of the extraction methods of oxygen from silicates can be found in Vennemann and Smith (Reference Vennemann and Smith1990) and Fagereng et al. (Reference Fagereng, Harris, La Grange and Stevens2008). Samples were run on the conventional line together with duplicate samples of the internal quartz standard MQ (δ18O = 10.1‰) that was used to calibrate the raw data to the SMOW scale (Standard Mean Ocean Water; e.g. Sharp, Reference Sharp2007). During the course of this study, analyses of MQ gave a 2σ variation of 0.16‰. The O-isotope ratios of mineral separates were analysed by laser fluorination using purified O2 gas. The internal standard MON GT (δ18O = 5.38‰, Harris and Vogeli, Reference Harris and Vogeli2010) was used to calibrate the raw data to the SMOW scale. The long-term average difference in δ18O values of duplicates of MON GT analysed during this study was 0.13‰, and corresponds to a 2σ value of 0.16‰.
Results
The rhyolites in the study region can be classified into three textural groupings: (1) unwelded to welded and partly glassy ignimbrite deposits; (2) vitrophyric lava flows; and (3) microcrystalline lava flows to slightly coarser sheet intrusions (Gústafsson Reference Gústafsson1992; Berg et al., Reference Berg, Troll, Burchardt, Riishuus, Krumbholz and Gústafsson2014). The welded ignimbrites (1) display fiamme and eutaxitic textures, whereas unwelded ignimbrites contain abundant well-preserved pumice fragments and undeformed-to-cuspate glass shards. The ignimbrites investigated have phenocryst assemblages comprising primarily of plagioclase (up to 500 µm in size) and quartz (≤200 µm), minor clinopyroxene, altered biotite and small ‘iddingsitized’ olivine (<50 µm). The ignimbrites also contain a diverse assortment of lithic clasts, including gabbroic plutonic fragments, basaltic enclaves, as well as felsic xenoliths (see Fig. 3). Overall the matrix is partly glassy, however devitrified glass shards, perlitic cracks and spherulitic textures are ubiquitous especially within the intra-caldera rock suite (cf. Ross and Smith, Reference Ross and Smith1961; Lofgren, Reference Lofgren1971a,b). Vitrophyric rhyolite lavas in region (2) are porphyritic (5–15 area%) with a phenocryst assemblage consisting mainly of plagioclase feldspar, quartz, clinopyroxene and minor olivine (≤1 mm in size) set in a dominantly glassy and laminated groundmass. Glomerocrysts of plagioclase and clinopyroxene can be observed in the vitrophyric rhyolites and dacites (see Berg, Reference Berg2016 for further details). The microcrystalline to coarsely-crystalline rhyolite flows and intrusions (3) are porphyritic (5–10 area%), with a phenocryst assemblage consisting mainly of plagioclase feldspar (≤1 mm), altered clinopyroxene (≤1 mm), as well as minor quartz (≤1 mm) and pyrite (≤200 µm). The groundmass is composed of quartz, alkali-feldspar, minor Fe-Ti oxides and biotite.
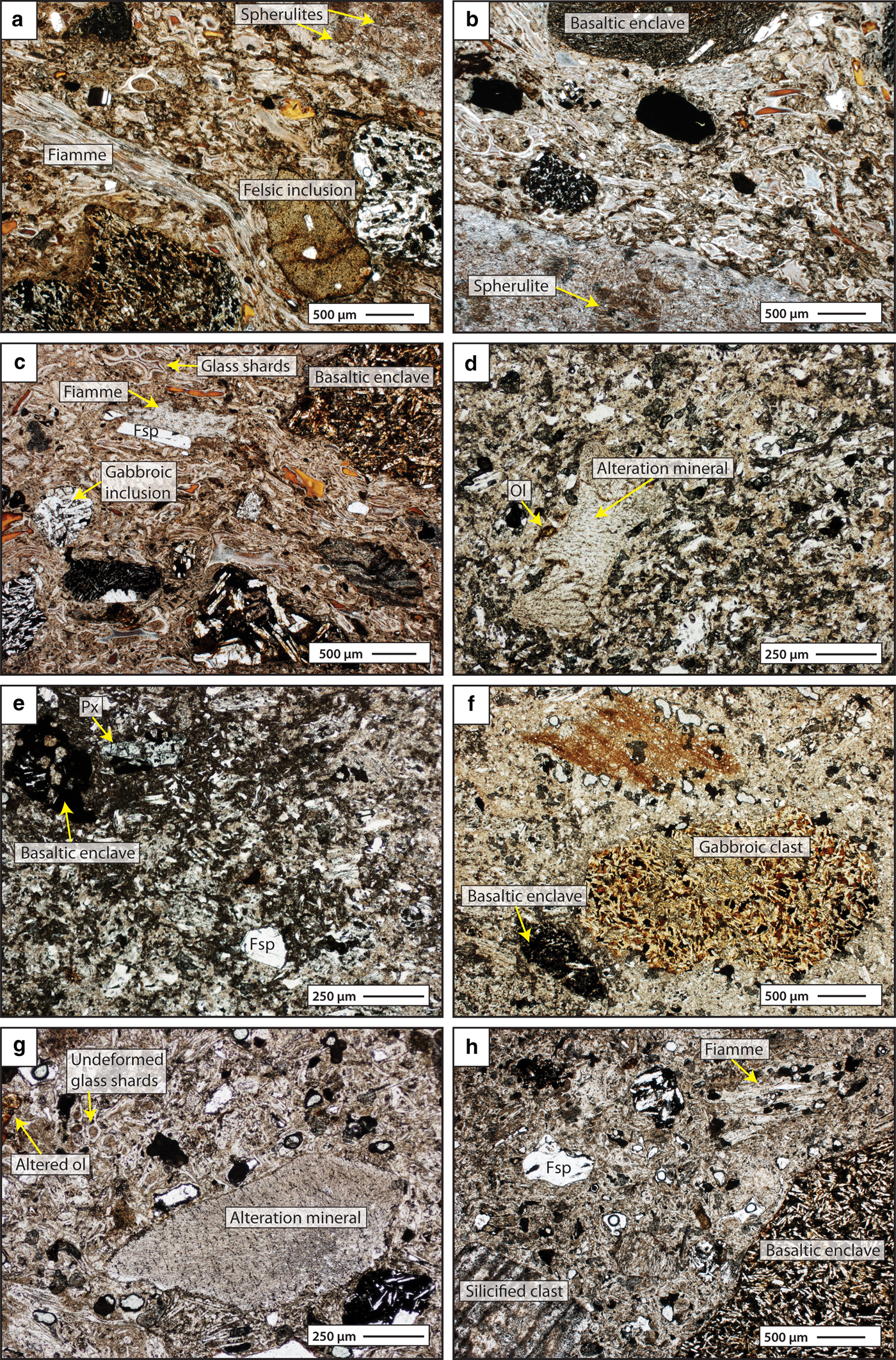
Fig. 3. Representative thin-section micrographs of intra-caldera rhyolites from Borgarfjörður Eystri. (a–c) Sample IC-HFE-IG-31 shows a slightly welded groundmass texture with dispersed feldspar crystals, as well as a variety of inclusions. (d–f) Sample IC-URD-IG-5 is hypocrystalline and poorly welded, with frequent secondary mineralization domains as well as basaltic and gabbroic inclusions. The basaltic enclaves sometimes show relict crenulated contacts to the host groundmass. Sample IC-HE-IG-22 (g–h) is hypocrystalline with secondary, sericite, zeolite and silica mineralization common throughout. All micrographs are shown in plane-polarised light (fsp = feldspar, px = pyroxene, ol = olivine).
In general, the intra-caldera rhyolites appear to be more affected by secondary mineralization and mineral replacements relative to the extra-caldera suite. In hand specimen, all of the intra-caldera rhyolites show alteration colours that range from white to yellow, pink and green. Moreover, a range of alteration features and alteration minerals can be observed, including chlorite, zeolite, recrystallized quartz aggregates, mineral veining and secondary spherulites. Groundmass plagioclase and clinopyroxene are commonly replaced by sericite and chlorite, respectively, and microcrystalline quartz is also widespread in the groundmass. This secondary alteration appears to be most intense in ignimbrite samples IC-URD-IG-5 and IC-HE-IG-22, which were taken from the margins of the Dyrfjöll and Breiðavik calderas.
Five whole-rock samples including four intra-caldera rhyolites and one extra-caldera sample were selected for XRD analyses with Rietveld refinement and the results are presented in Table 1. The extra-caldera sample shows a fairly typical rhyolitic mineral assemblage of dominantly plagioclase, quartz and potassium feldspar with minor zeolite (phillipsite). In contrast, the intra-caldera samples are highly variable in terms of their mineral content. Sample IC-URD-IG-5, for instance, is extremely silicified, containing 61% amorphous material and 23% opal-C (opal consisting of disordered α-cristoballite). Two more samples, IC-HFE-R-1 and IC-HF-IG-31, contain high-temperature silica phases, consisting of 24% cristoballite (+5% tridymite) and 31% cristoballite, respectively. Finally, sample IC-HE-IG-22 is distinct from the other intra-caldera samples owing to the fact that up to 83% of the sample mass can be accounted for by the zeolite minerals heulandite and mordenite.
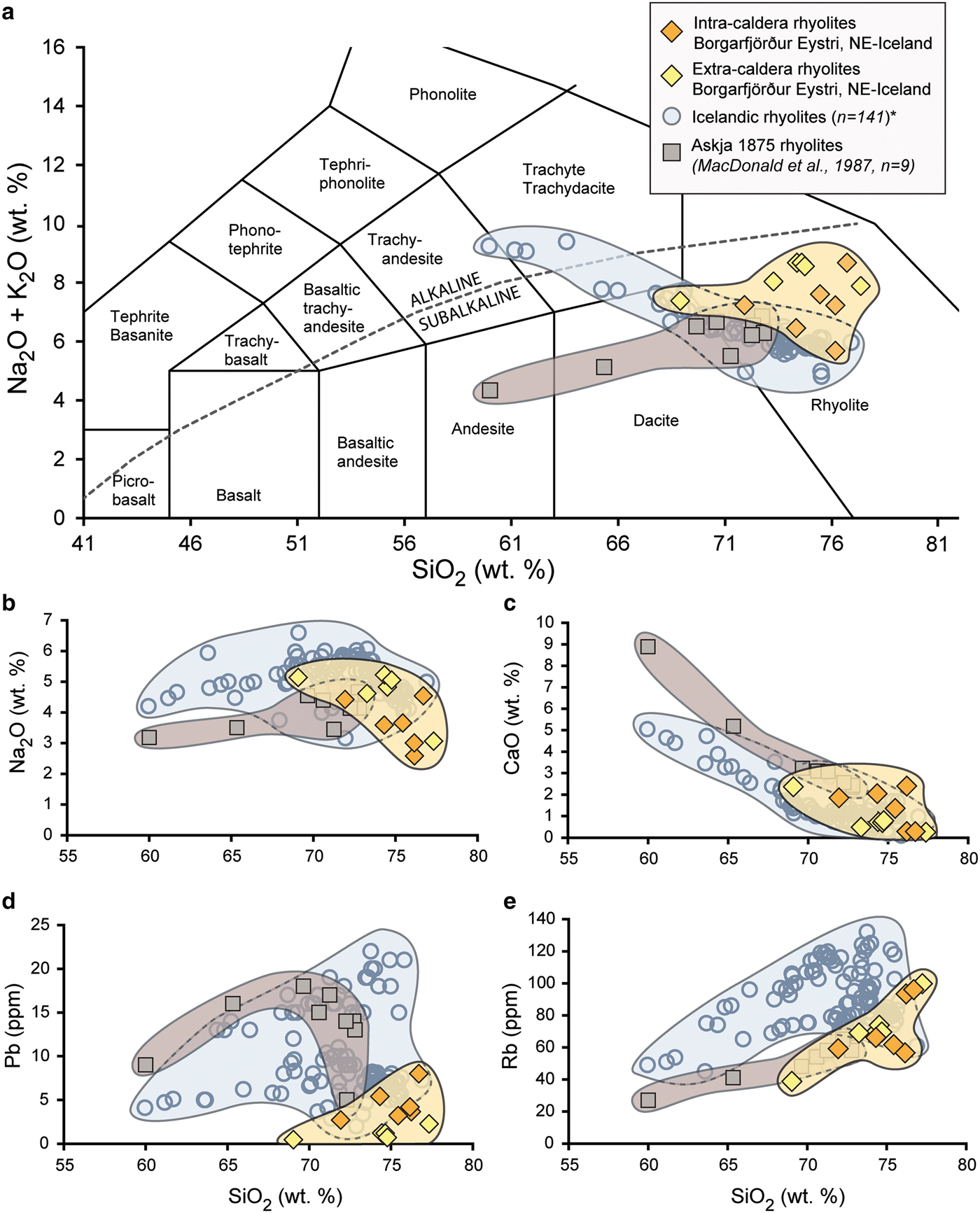
When plotted on a TAS diagram, the intra- and extra-caldera samples analysed range from subalkaline high-silica dacite to rhyolite with SiO2 contents between 69 and 77 wt.% (on a volatile-free basis, see Fig. 4a, Le Maitre et al., Reference Le Maitre, Bateman, Dudek, Keller, Lameyre Le Bas, Sabine, Schmid, Sorensen, Streckeisen, Woolley and Zanettin1989). By employing Harker plots for selected fluid-mobile major and trace elements (Na2O, CaO, Rb, Pb, Fig. 4b–e), we can see that the extra-caldera rhyolites overlap frequently with the documented data range for evolved rocks in Iceland, which define linear or curvilinear trends towards rhyolite compositions and which typically reflect magma differentiation processes (Wood, Reference Wood1978; Gunnarsson et al., Reference Gunnarsson, Marsh and Taylor1998; Troll and Schmincke, Reference Troll and Schmincke2002). The intra-caldera rhyolites, in contrast are scattered relative to the unaltered Icelandic rhyolites with respect to their major- and trace-element compositions (c.f. Donoghue et al., Reference Donoghue, Troll, Harris, O'Halloran, Walter and Pérez Torrado2008; Reference Donoghue, Troll and Harris2010).
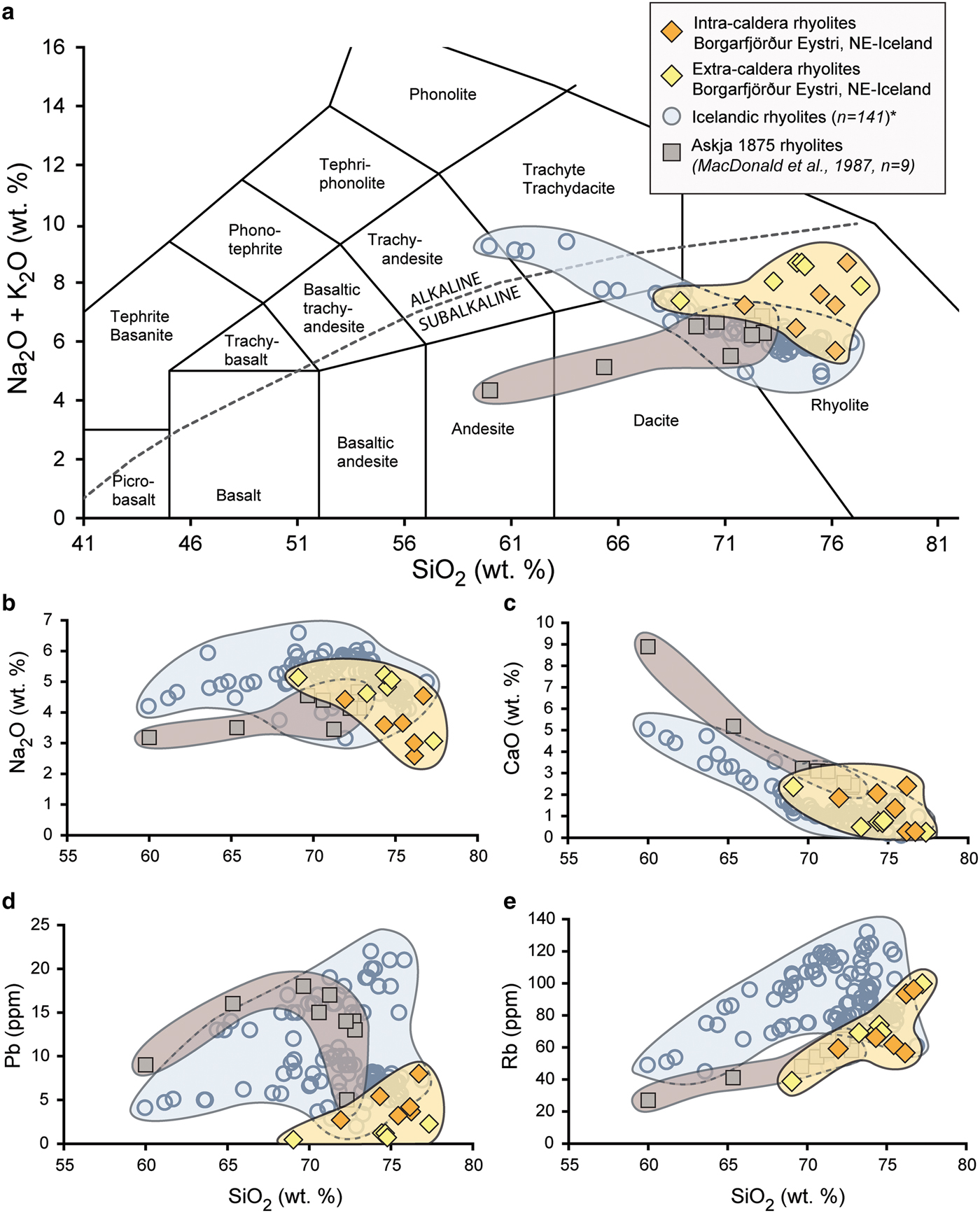
Fig. 4. Major- and trace-element plots of intra- and extra-caldera rhyolites from Borgarfjörður Eystri are shown in orange and light yellow, respectively, while unaltered Icelandic rhyolites are shown in blue (*data compiled from GEOROC database, http://georoc.mpch-mainz.gwdg.de/georoc/). The rhyolites from the Askja 1875 eruption are singled out and are shown here in brown (MacDonald et al., Reference MacDonald, Sparks, Sigurdsson, Mattey, McGarvie and Smith1987). (a) On a Total Alkali vs. silica diagram (TAS, Le Maitre et al., Reference Le Maitre, Bateman, Dudek, Keller, Lameyre Le Bas, Sabine, Schmid, Sorensen, Streckeisen, Woolley and Zanettin1989) the samples from Borgarfjörður Eystri classify as subalkaline dacite to rhyolite and define a group that shows considerable spread relative to the comparative literature data from Iceland. (b–e) Plots of selected fluid-mobile major and trace elements (Na2O, CaO, Pb and Rb) vs. SiO2 for Borgarfjörður Eystri dacite to rhyolite samples relative to Icelandic rhyolites. Note, Askja rhyolite samples are singled out again as an example of a cogenetic suite. The documented Icelandic dacite to rhyolite compositions define broad curvilinear trends on all plots, representing dominantly magmatic differentiation processes, whereas the altered Borgarfjörður Eystri rhyolites are more widely scattered, particularly the intra-caldera samples, which records the loss and gain of fluid-mobile elements during secondary hydrothermal overprinting as well as zeolite and secondary silica formation.
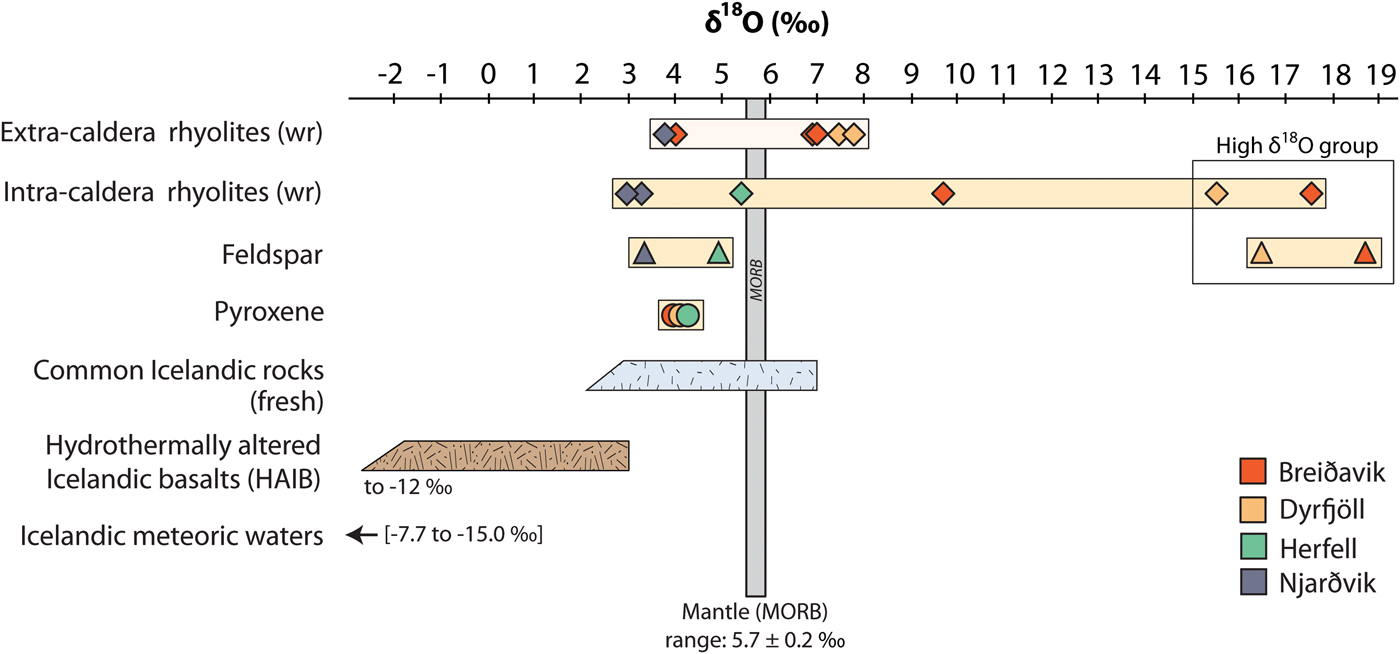
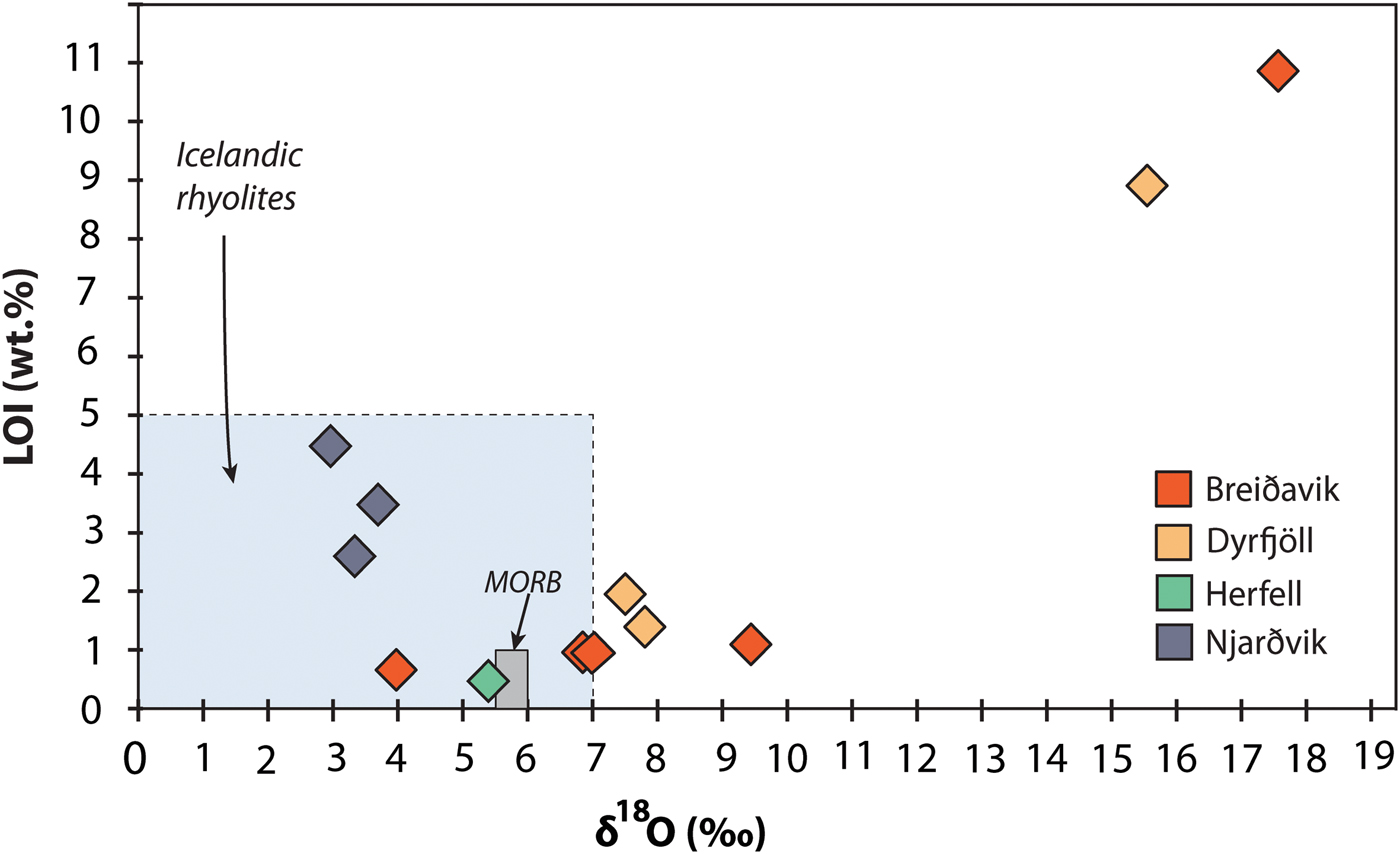
Whole-rock oxygen isotope values of the extra-caldera rhyolites range from +3.7 to +7.8‰ (n = 6), which are similar to the values obtained previously for Icelandic rhyolites (Fig. 5, Condomines et al., Reference Condomines, Grönvold, Hooker, Muehlenbachs, O'Nions, Óskarsson and Oxburgh1983; Sigmarsson et al., Reference Sigmarsson, Condomines and Fourcade1992; Hemond et al., Reference Hemond, Arndt, Lichtenstein, Hofmann, Óskarsson and Steinthorsson1993; Gunnarsson et al., Reference Gunnarsson, Marsh and Taylor1998; Hards et al., Reference Hards, Kempton, Thompson and Greenwood2000; Prestvik et al., Reference Prestvik, Goldberg, Karlsson and Grönvold2001; Macpherson et al., Reference Macpherson, Hilton, Day, Lowry and Grönvold2005, Carley et al., Reference Carley, Miller, Sigmarsson, Coble, Fisher, Hanchar, Schmitt and Economos2017). The intra-caldera samples, in turn, range from +2.9 to +17.6‰ (n = 6), and notably, the Dyrfjöll and Breiðavik (Hvítserkur) rhyolite samples have δ18O values of +15.5 and +17.6‰, respectively (Fig. 5). These δ18O values are higher than any Icelandic igneous rock reported previously and duplicate analyses of these samples were carried out to ensure analytical reproducibility (Table 2). Notably, all the extra-caldera rhyolites in this study show LOI values of <3.5 wt.% and are thus within the range of Icelandic rhyolites in the literature in which the LOI values are known to reach up to 5 wt.% (GEOROC database, http://georoc.mpch-mainz.gwdg.de/georoc/). In contrast, the intra-caldera samples have highly variable LOI values, ranging from 0.5 up to 11.0 wt.% (Table 2). A subgroup of these samples shows exceedingly high LOI values (>9 wt.%, see Fig. 6) and concomitant high degrees of replacement by secondary minerals (Figs 2 and 3).
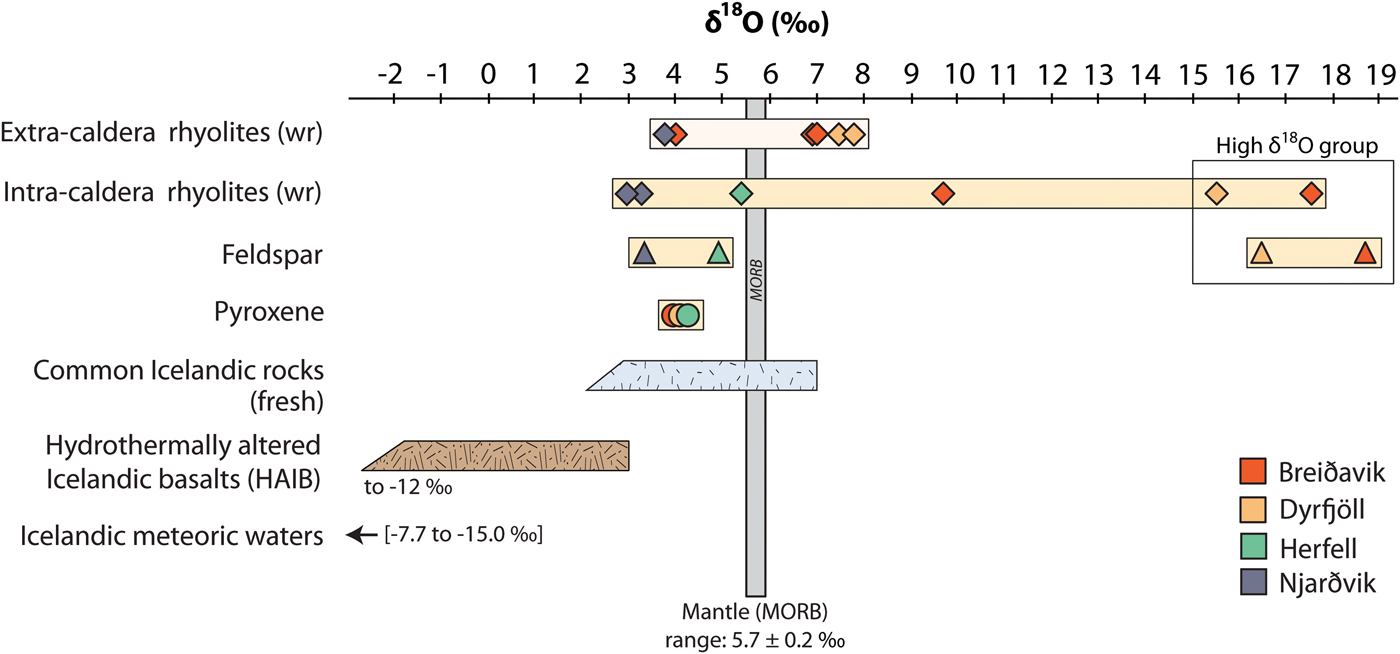
Fig. 5. Oxygen isotope variation in whole-rock and mineral separates of intra- and extra-caldera rhyolites from Borgarfjörður Eystri. Each volcanic system has been assigned a specific colour (see inset). Duplicate analyses were performed for the Dyrfjöll and Hvítserkur ignimbrites of the high δ18O group (see Table 2). Literature δ18O values of fresh Icelandic rocks (after Condomines et al., Reference Condomines, Grönvold, Hooker, Muehlenbachs, O'Nions, Óskarsson and Oxburgh1983; Sigmarsson et al., Reference Sigmarsson, Condomines and Fourcade1992; Hemond et al., Reference Hemond, Arndt, Lichtenstein, Hofmann, Óskarsson and Steinthorsson1993; Gunnarsson et al., Reference Gunnarsson, Marsh and Taylor1998; Hards et al., Reference Hards, Kempton, Thompson and Greenwood2000; Prestvik et al., Reference Prestvik, Goldberg, Karlsson and Grönvold2001; Macpherson et al., Reference Macpherson, Hilton, Day, Lowry and Grönvold2005), and hydrothermally altered Icelandic basalts (HAIB, after Hattori and Muehlenbachs, Reference Hattori and Muehlenbachs1982; Bindeman et al., Reference Bindeman, Gurenko, Carley, Miller, Martin and Sigmarsson2012) are presented in blue and brown, respectively. Literature values of meteoric waters are from Árnason (Reference Árnason1976), Hattori and Muehlenbachs (Reference Hattori and Muehlenbachs1982) and Rozanski et al. (Reference Rozanski, Araguás-Araguás, Gonfiantini, Swart, Lohmann, McKenzie and Savin1993).
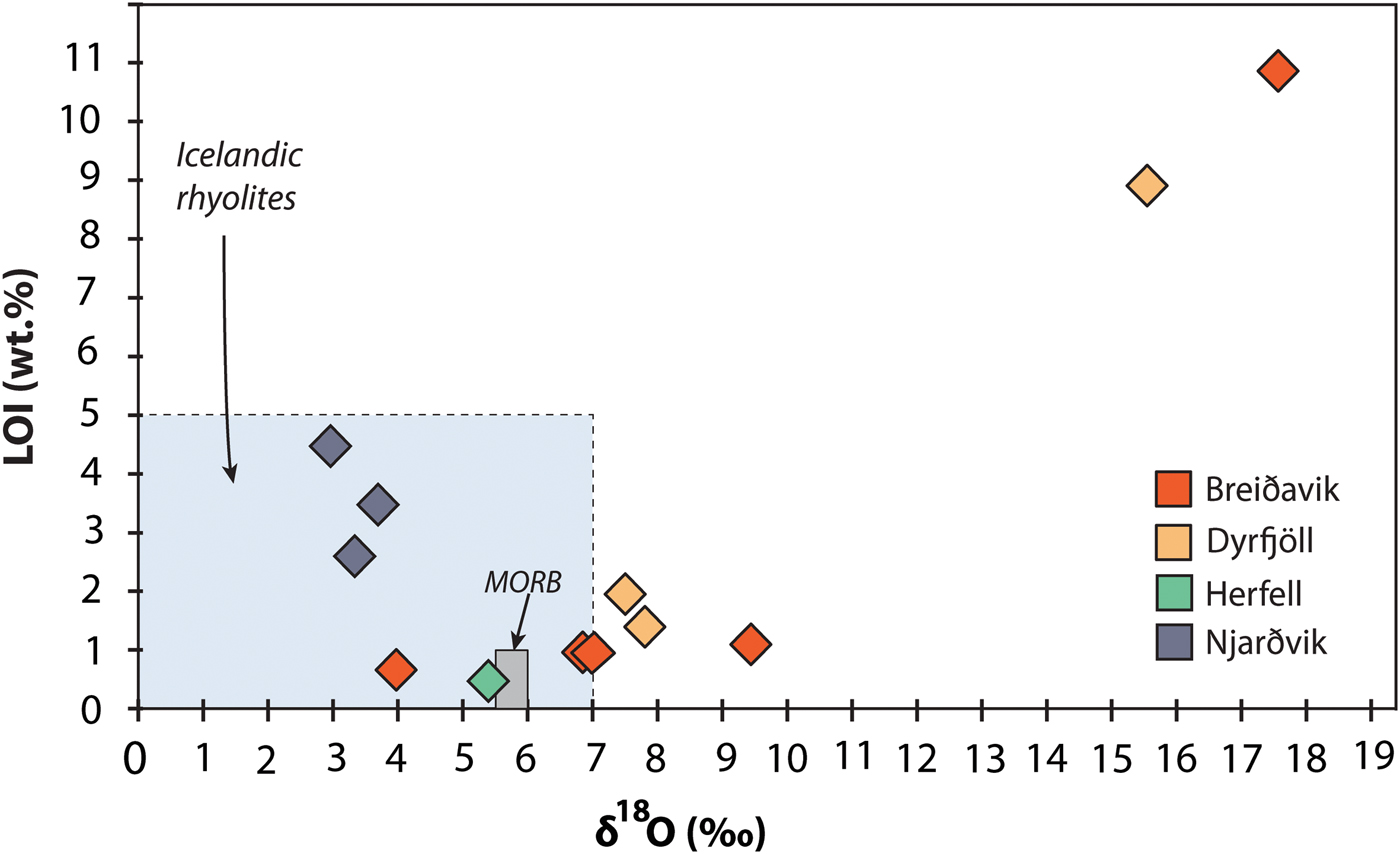
Fig. 6. Whole-rock δ18O variation relative to LOI (loss on ignition) values of the Borgarfjörður Eystri rhyolite samples. Literature values for Icelandic rhyolites are from the GEOROC database, http://georoc.mpch-mainz.gwdg.de/georoc/. The intra-caldera rhyolites span a wide range in δ18O values, which is also reflected in the δ18O composition of feldspar. The high δ18O group notably correlates with elevated LOI values.
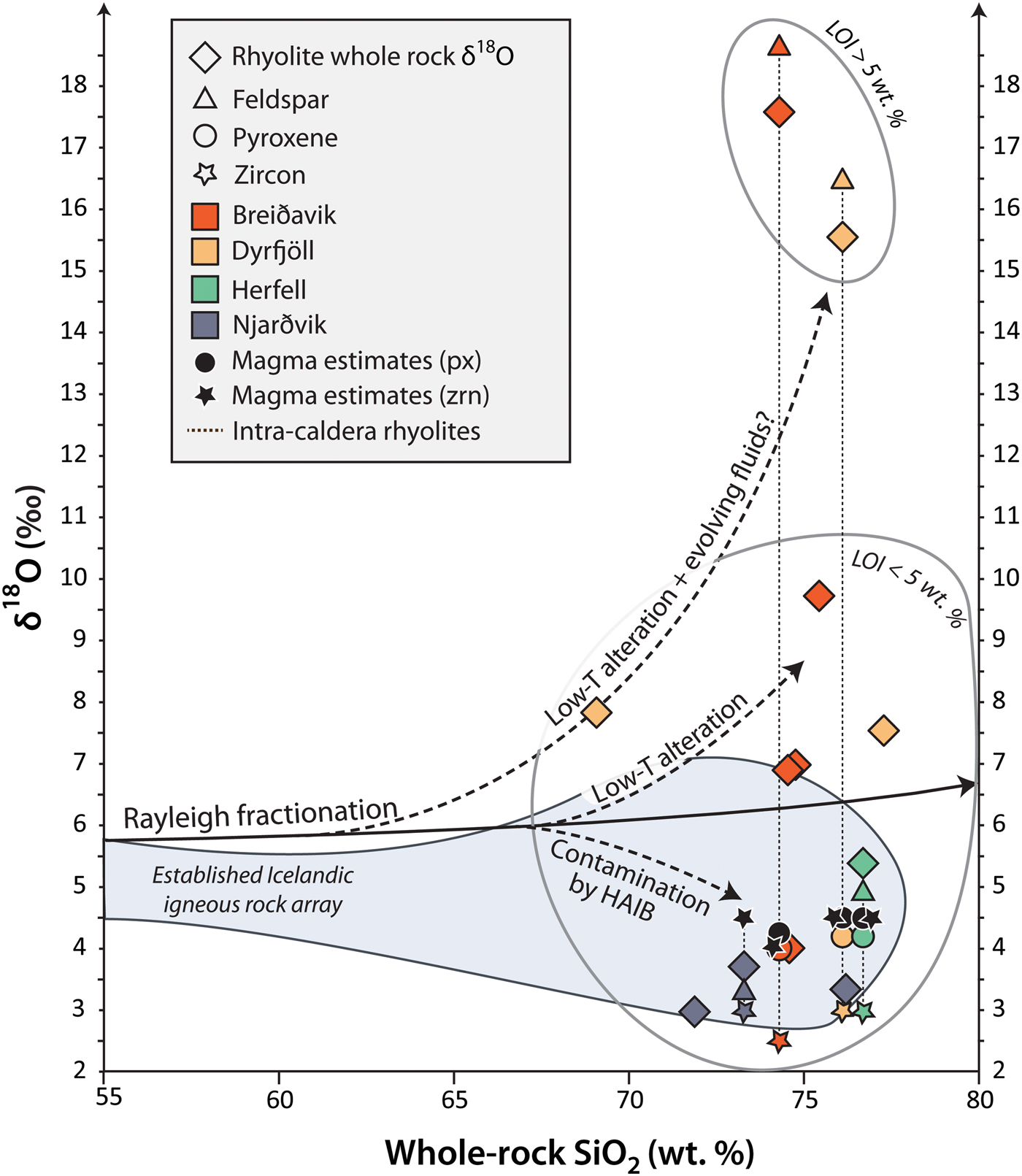
Feldspar separates from the intra-caldera samples show values from +4.9 to +18.7‰ (n = 3), which broadly overlap the respective whole-rock δ18O values. Conversely, the δ18O values of pyroxene separates from the intra-caldera sample suite define a narrow range of +4.0 to +4.2‰ (n = 3, see Fig. 5 and Table 2). Assuming that mineral δ18O values below +5.5‰ reflect magmatic values, we can use the conversion factor from Harris et al. (Reference Harris, Pronost, Ashwal and Cawthorn2005) to estimate δ18O magma values from pyroxene (which is preferable because it shows less variability than feldspar). A range of magma δ18O values from +4.3 to +4.5‰ is derived from pyroxene for the rhyolite parental magmas by this approach, which is consistent with pristine magma δ18O values calculated from zircon from these samples (Berg, Reference Berg2016, Fig. 7 and Table 2). These low δ18O magma values are also in good agreement with the range of primary δ18O magma values reported from other silicic eruptions on Iceland (Bindeman, Reference Bindeman2008; Bindeman et al., Reference Bindeman, Gurenko, Carley, Miller, Martin and Sigmarsson2012; Carley et al., Reference Carley, Miller, Wooden, Padilla, Schmitt, Economos, Bindeman and Jordan2014; Schattel et al., Reference Schattel, Portnyagin, Golowin, Hoernle and Bindeman2014, Geiger et al., Reference Geiger, Mattsson, Deegan, Troll, Burchardt, Gudmundsson, Tryggvason, Krumbholz and Harris2016; Carley et al., Reference Carley, Miller, Sigmarsson, Coble, Fisher, Hanchar, Schmitt and Economos2017).

Fig. 7. Oxygen isotope values vs. SiO2 content. Minerals and whole rocks are plotted with their measured isotopic compositions, and are colour coded for each volcanic centre. Selected magma estimates from relatively robust minerals pyroxene (px) and zircon (zrn) are presented with black solid symbols. Pyroxene δ18O data were converted to magma values following the method outlined in Harris et al. (Reference Harris, Pronost, Ashwal and Cawthorn2005). Zircon data and magma estimates are presented in Berg (Reference Berg2016). The intra- and extra-caldera rhyolites show both positive and negative variation around the Raleigh fractionation trend (e.g. Bindeman, Reference Bindeman2008), which indicates disequilibrium with alteration fluids. Note that feldspar and some whole rocks are more susceptible to alteration than pyroxene, and that high δ18O values correlate with the hydrous content of the rocks (see sample IC-HE-IG-22 and IC-URD-IG-5). HAIB = hydrothermally altered Icelandic basalts. LOI = loss on ignition. Icelandic reference field drawn after Sigmarsson et al. (Reference Sigmarsson, Condomines and Fourcade1992), Hemond et al. (Reference Hemond, Arndt, Lichtenstein, Hofmann, Óskarsson and Steinthorsson1993), Gunnarsson et al. (Reference Gunnarsson, Marsh and Taylor1998), Hards et al. (Reference Hards, Kempton, Thompson and Greenwood2000), Prestvik et al. (Reference Prestvik, Goldberg, Karlsson and Grönvold2001) and Macpherson et al. (Reference Macpherson, Hilton, Day, Lowry and Grönvold2005).
Discussion
The variations in oxygen isotope ratios among the intra- and extra-caldera rhyolites define a sub-group of high δ18O intra-caldera samples that differs vastly from our regional sample suite and from Icelandic rhyolites in general. In the following discussion we use a combination of field observations, petrography and geochemical data to explain the variations in our data set and the processes that could have produced the exceptionally high δ18O values.
Magmatic vs. hydrothermal signatures
The δ18O values of igneous rocks typically reflect a combination of their original magmatic values (e.g. preserved in phenocrysts), and superimposed subsolidus changes to magmatic δ18O values (e.g. Taylor, Reference Taylor1968; Cousens et al., Reference Cousens, Spera and Dobson1993; Bindeman, Reference Bindeman2008; Deegan et al., Reference Deegan, Troll, Barker, Harris, Chadwick, Carracedo and Delcamp2012). Mantle-derived basaltic magmas have δ18O values of 5.7 ± 0.2‰ that can increase by up to ~1‰ through closed-system fractional crystallization (Valley et al., Reference Valley, Lackey, Cavosie, Clechenko, Spicuzza, Basei, Bindeman, Ferreira, Sial, King, Peck, Sinha and Wei2005; Bindeman, Reference Bindeman2008). The addition of external (e.g. crustal or fluid) components can then result in magma δ18O values being shifted above or below this range. The δ18O values resulting from open-system magmatic processes can be distinguished from secondary processes through e.g. study of alteration mineralogy (phyllosilicates, silica, zeolites) and water content of a rock (Taylor, Reference Taylor1968; Götze et al., Reference Götze, Tichomirowa, Fuchs, Pilot and Sharp2001; Donoghue et al., Reference Donoghue, Troll and Harris2010; Deegan et al., Reference Deegan, Troll, Barker, Harris, Chadwick, Carracedo and Delcamp2012). In such cases, original magmatic oxygen isotope ratios may be preserved in some mineral phases that are more resistant to secondary isotope exchange, such as quartz or zircon (Valley et al., Reference Valley, Lackey, Cavosie, Clechenko, Spicuzza, Basei, Bindeman, Ferreira, Sial, King, Peck, Sinha and Wei2005; Bindeman, Reference Bindeman2008; Budd et al., Reference Budd, Troll, Deegan, Jolis, Smith, Whitehouse, Harris, Freda, Hilton, Halldórsson and Bindeman2017) and oxygen isotope disequilibrium between co-existing minerals and/or whole-rock can help determine the degree of overprint by post-crystallization processes (e.g. Cousens et al., Reference Cousens, Spera and Dobson1993; Harris et al., Reference Harris, Pronost, Ashwal and Cawthorn2005).
Intra- and extra-caldera rhyolites from Njarðvik and Herfell have LOI values that are within the documented range for Icelandic rhyolites (≤5 wt.%, Figs 6 and 7; Owen et al., Reference Owen, Tuffen and McGarvie2013; GEOROC database), and they have δ18O values similar to reported whole-rock δ18O values of Icelandic rhyolites (Condomines et al., Reference Condomines, Grönvold, Hooker, Muehlenbachs, O'Nions, Óskarsson and Oxburgh1983; Sigmarsson et al., Reference Sigmarsson, Condomines and Fourcade1992; Hemond et al., Reference Hemond, Arndt, Lichtenstein, Hofmann, Óskarsson and Steinthorsson1993; Gunnarsson et al., Reference Gunnarsson, Marsh and Taylor1998; Hards et al., Reference Hards, Kempton, Thompson and Greenwood2000; Prestvik et al., Reference Prestvik, Goldberg, Karlsson and Grönvold2001; Macpherson et al., Reference Macpherson, Hilton, Day, Lowry and Grönvold2005, Carley et al., Reference Carley, Miller, Sigmarsson, Coble, Fisher, Hanchar, Schmitt and Economos2017). The Herfell ignimbrite yields a magma δ18O estimate from pyroxene that overlaps with magma values estimated from magmatic zircon from the same rock unit (Berg, Reference Berg2016) and from detrital zircon in the region (Carley et al., Reference Carley, Miller, Sigmarsson, Coble, Fisher, Hanchar, Schmitt and Economos2017). This particular sample is thus interpreted to reflect a magmatic δ18O value (Figs 1, 5 and 7, Table 2). In contrast, rhyolites from the Breiðavik and Dyrfjöll volcanic centres are more variable in terms of both their oxygen isotope ratios and LOI values (Figs 6 and 7). The extra-caldera samples plot together with common Icelandic rocks, and the extra- and intra-caldera samples that are mildly elevated in δ18O have low LOI, whereas intra-caldera samples with elevated δ18O usually show high LOI as well (see Figs 6 and 7).
The extensively hydrated Dyrfjöll and Breiðavik (Hvítserkur) intra-caldera rhyolites samples (LOI = 9–11 wt.%) have whole-rock and feldspar δ18O values >15‰, i.e. the whole-rocks are ~10‰ higher than the values recorded in their co-existing pyroxene and zircon (Berg, Reference Berg2016). While the pyroxene and zircon values reflect predominantly magmatic values, the high δ18O whole-rock and mineral values are not representative of the original magma, and the elevated δ18O values in Dyrfjöll and Breiðavik (Hvítserkur) intra-caldera rhyolites thus reflect various degrees of secondary alteration processes after deposition. This hypothesis is consistent with the relative paucity of ‘igneous’ minerals and the presence of opal-C and zeolites in the intra-caldera Dyrfjöll and Breiðavik samples, respectively (Table 1). Secondary alteration would also be consistent with the apparent strong susceptibility of rock groundmass and feldspar to hydrous alteration processes as opposed to pyroxene (cf. Taylor, Reference Taylor1968; Gregory et al., Reference Gregory, Criss and Taylor1989; Cousens et al., Reference Cousens, Spera and Dobson1993). Alternatively, it may be argued that late-stage magmatic assimilation affected the groundmass and feldspar compositions only (cf. Duffield and Ruiz, Reference Duffield and Ruiz1998), but this model is unlikely because assimilation processes on Iceland usually shift magma towards lower δ18O values (cf. Bindeman, Reference Bindeman2008; Bindeman et al., Reference Bindeman, Gurenko, Carley, Miller, Martin and Sigmarsson2012; Gurenko et al., Reference Gurenko, Bindeman and Sigurdsson2015). Moreover, the samples with the highest δ18O values also have high LOI values and distinct alteration mineralogy, consisting of high-temperature silica, opal-C and zeolites. This convincingly argues against a crustal assimilation origin of the exceptionally high δ18O values reported in this investigation.
The large oxygen isotope variability in the investigated dacitic-to-rhyolitic whole-rock and mineral pairs from Borgarfjörður Eystri (+2.9 to +17.6‰) must therefore reflect a two-stage evolution for the high δ18O group, that we envisage as follows: (1) Sub-mantle δ18O magma values (<5.7‰) recorded in regional whole rocks, pyroxene and some feldspar crystals reflecting combined fractional crystallization of mantle-derived magmas and a degree of assimilation of hydrothermally altered 18O-depleted crust (cf. MacDonald et al., Reference MacDonald, Sparks, Sigurdsson, Mattey, McGarvie and Smith1987; Valley et al., Reference Valley, Lackey, Cavosie, Clechenko, Spicuzza, Basei, Bindeman, Ferreira, Sial, King, Peck, Sinha and Wei2005; Lackey et al., Reference Lackey, Valley, Chen and Stockli2008; Bindeman et al., Reference Bindeman, Gurenko, Carley, Miller, Martin and Sigmarsson2012: Zierenberg et al., Reference Zierenberg, Schiffman, Barfod, Lesher, Marks, Lowenstern, Mortensen, Pope, Bird, Reed, Friðleifsson and Elders2013; Geiger et al., Reference Geiger, Mattsson, Deegan, Troll, Burchardt, Gudmundsson, Tryggvason, Krumbholz and Harris2016). (2) Variable degrees of post-magmatic, secondary alteration of groundmass and feldspar then occurred in intra-caldera hydrothermal settings, which resulted in the observed hydrothermal alteration mineralogy and the high δ18O whole-rock and feldspar values for these specific samples (cf. Taylor, Reference Taylor1968). Preferential groundmass alteration is not unusual during secondary alteration processes, and has been documented previously for ignimbrite and rift-zone intrusive rocks elsewhere (e.g. Cousens et al., Reference Cousens, Spera and Dobson1993; Deegan et al., Reference Deegan, Troll, Barker, Harris, Chadwick, Carracedo and Delcamp2012), and effectively reflects an incomplete or partial alteration process. Accepting that the high δ18O and LOI values reflect hydrothermal alteration (stage 2), then the single sample from Breiðavik caldera with elevated δ18O but low LOI is probably also part of this group. This sample may have undergone further dehydration, e.g. due to re-heating inside the active Breiðavik caldera (e.g. due to proximity to a small intrusion; Donoghue et al., Reference Donoghue, Troll and Harris2010). The detailed processes that characterize stage 2 are discussed in more detail below.
Origin of high-δ18O values
A number of processes could be responsible for the high-δ18O values recorded in several intra-caldera rhyolites from Borgarfjörður Eystri. Below we will discuss: (1) low-temperature hydration; (2) low-temperature hydrothermal alteration; (3) high-temperature isotope exchange processes; and (4) isotope fractionation in fluids by evaporation.
Low-temperature hydration
Glassy volcanic rocks with high H2O contents are not normally considered compositionally representative of their parent melt, but are commonly products of post-emplacement rehydration. This is the case for explosive eruptive products that lost most of their volatiles during eruption (e.g. Taylor, Reference Taylor1968; Lofgren, Reference Lofgren1971b; Troll and Schmincke, Reference Troll and Schmincke2002; Seligman et al., Reference Seligman, Bindeman, Watkins and Ross2016). Icelandic rhyolites usually have LOI contents up to 5 wt.% (Owen et al., Reference Owen, Tuffen and McGarvie2013; GEOROC database), for example, the Thorsmörk ignimbrite in southern Iceland has a LOI of 3.85 wt.% (Jørgensen, Reference Jørgensen1980), and the Solheimar ignimbrite from Katla has a LOI of 0.70 wt.% (Lacasse et al., Reference Lacasse, Sigurdsson, Carey, Jóhannesson, Thomas and Rogers2007). Sub-solidus hydration processes on Iceland have been shown to dominantly involve meteoric water (Árnason, Reference Árnason1976; Sveinbjörnsdóttír and Johnsen, Reference Sveinbjörnsdóttir and Johnsen1992). Present-day meteoric waters in coastal areas on Iceland show δ18O values from –8 to –11‰ (Árnason, Reference Árnason1976; Hattori and Muehlenbachs, Reference Hattori and Muehlenbachs1982), and the long-term weighted mean δ18O value of precipitation in Reykjavik is –7.7‰ (Rozanski et al., Reference Rozanski, Araguás-Araguás, Gonfiantini, Swart, Lohmann, McKenzie and Savin1993). Meteoric waters in Neogene Iceland were probably marginally less depleted in 18O compared to today, because of a warmer Earth and Iceland's slightly lower latitude at 12–14 Ma (e.g. Hattori and Muehlenbachs, Reference Hattori and Muehlenbachs1982; Sheppard, Reference Sheppard, Valley, Taylor and O'Neil1986). It follows, therefore, that the intra-caldera rhyolites with elevated δ18O values and high water contents (LOI = 9 to 11 wt.%), cannot be derived by simple addition of meteoric water, which would lower rather than raise the δ18O value of the hydrated whole-rock suite.
Low-temperature hydrothermal alteration
The intra-caldera rhyolites with elevated LOI and δ18O values show a variety of alteration colours and secondary zeolite and silica mineralization (Figs 2e–g and 3, Tables 1 and 2). Hydrothermal alteration processes on Iceland usually involve the formation of phyllosilicates at a temperature of ~200°C (i.e. kaolinite, smectite, illite, montmorillonite – Kristmannsdóttir, Reference Kristmannsdóttir1982; Gislason and Eugster, Reference Gislason and Eugster1987) and secondary quartz and/or zeolites at ~150–200°C (Walker, Reference Walker1960; Walker and Carmichael, Reference Walker and Carmichael1962; Franzson et al., Reference Franzson, Thordarson, Björnsson, Gudlaugsson, Richter, Fridleifsson and Thorhallsson2002). Indeed, our quantitative XRD results indicate that our two samples with exceptionally high δ18O values, IC-URD-IG-5 (15.4‰) and IC-HE-IG-22 (16.6‰) contain secondary opal-C and the zeolite-group minerals heulandite–clinoptilolite and mordenite, respectively (see Table 1). Assuming that Neogene Icelandic meteoric water also had a δ18O value of ca. –7.7‰ (Rozanski et al., Reference Rozanski, Araguás-Araguás, Gonfiantini, Swart, Lohmann, McKenzie and Savin1993), clinoptilolite that formed in equilibrium with this water would have a δ18O value of ~+25‰ at ambient temperature, because the clinoptilolite-water fractionation is +32.9‰ at 20°C (Feng et al., Reference Feng, Faiia, WoldeGabriel, Aronson, Poage and Chamberlain1999). However, the clinoptilolite-water fractionation decreases significantly at higher temperatures, and Δclinoptilolite-water is +11.4‰ at 200°C, +17.6°C at 100°C and +25.7‰ at 50°C (Feng et al., Reference Feng, Faiia, WoldeGabriel, Aronson, Poage and Chamberlain1999). Note that fractionation factors for heulandite and mordenite are not available, but it is unlikely that the different zeolites would have significantly different per mil fractionation factors. Similarly, secondary silicification during hydrothermal alteration increases the δ18O value of the rock because of a large silica-water fractionation factor at low-temperature, i.e. Δquartz-water is +11.6‰ at 200°C, +20.7‰ at 100°C and +28.7‰ at 50°C (Matsuhisa et al., Reference Matsuhisa, Goldsmith and Clayton1979; Götze et al., Reference Götze, Tichomirowa, Fuchs, Pilot and Sharp2001). As a consequence, near-surface hot-spring-type alteration at low temperature in Neogene Iceland could have locally produced a zeolite and silica-rich assemblage with high δ18O values. The mineralogy of the altered intra-caldera rhyolites throughout our sample suite justifies the involvement of secondary replacement minerals to cause 18O enrichment. To reach δ18O whole-rock values of +18‰, the intra-caldera rhyolites would be required to have almost fully equilibrated isotopically with the fluid at 50°C because water with a δ18O value of –7.7‰ would be in equilibrium with quartz with a δ18O value of 21.0‰ and zeolite (clinoptilolite) with a δ18O value of 18.0‰. This scenario, however, is at odds with our mineralogical observations (Fig. 3), as some minerals, such as pyroxene, apparently maintained their original magmatic δ18O values. The data in total leads us to surmise that unrealistically low temperatures (<50°C) would be required during alteration to compensate for pyroxene with magmatic δ18O values by producing exceedingly high groundmass δ18O values. Alternatively, shifts in the isotope composition of the alteration fluids that affected the groundmass of the samples need to be considered, in the sense that alteration fluids affecting the groundmass became progressively more positive with ongoing duration of the alteration process (see next section).
High-temperature isotope exchange processes
Fluids in geothermal systems and hot springs are predominantly sourced from local precipitation (Hoefs, Reference Hoefs1973; Árnason, Reference Árnason1976; Donoghue et al., Reference Donoghue, Troll and Harris2010). Circulating 18O-depleted waters in Neogene Iceland (e.g. –7.7‰; Rozanski et al., Reference Rozanski, Araguás-Araguás, Gonfiantini, Swart, Lohmann, McKenzie and Savin1993) would tend to progressively increase their δ18O values along fluid pathways during the course of medium- to high-temperature exchange with the country rock (the ‘oxygen shift’ of Hoefs, Reference Hoefs1973). Such water-rock isotopic exchange is thought to be slow at temperatures <200°C, yet geothermal steam waters at Hekla record an isotopic shift of +7.5‰ and thermal water at the Theistareykir high-temperature field in Northeast Iceland shows an oxygen isotopic shift of up to +6.5‰ (Hoefs, Reference Hoefs1973; Sveinbjörnsdóttír et al., Reference Sveinbjörnsdóttír, Ármannsson, Ólafsson, Óskarsson, Markússon and Magnusdottir2013). Therefore, at the distal end of a hydrothermal circulation cell, geothermal fluids may become significantly enriched in 18O (e.g. Hoefs, Reference Hoefs1973; Donoghue et al., Reference Donoghue, Troll, Harris, O'Halloran, Walter and Pérez Torrado2008). Hydrothermal alteration in a near-surface hot-spring environment (~100 to 200°C) fed by waters that already exchanged oxygen at high temperature with the country rock would then produce alteration minerals (clay, zeolites and silica) considerably enriched in 18O. For example, if the alteration fluid is –1‰ rather than –7.7‰, the alteration minerals would be ~7‰ higher, which would permit crystallization of zeolite and silica with δ18O values ≥+20 and +17‰, respectively, during equilibration at 100°C (cf. Matsuhisa et al., Reference Matsuhisa, Goldsmith and Clayton1979; Feng et al., Reference Feng, Faiia, WoldeGabriel, Aronson, Poage and Chamberlain1999). By considering a lower temperature for this process, a greater zeolite/silica-water fractionation has to be assumed. The wide range of δ18O values recorded in the intra-caldera rhyolites studied could hence be accounted for by epithermal (~100 to 200°C) interaction with evolved fluids that had undergone previous O-isotope shifts by progressive water-rock interaction during hydrothermal circulation.
Evaporation of thermal pools
Another process by which the δ18O composition of fluids can be raised is through Rayleigh-type distillation during evaporation (Craig, Reference Craig, Gordon and Horibe1963). In a subaerial and evaporating hydrothermal system, the residual liquid becomes enriched in 18O (Craig, Reference Craig, Gordon and Horibe1963; Faure, Reference Faure1986). Progressive vapour loss through evaporation from, for example, a hot or even boiling lake would therefore increase the δ18O value of the remaining water gradually and any mineral that eventually precipitates from such a fluid would similarly become enriched in 18O. In fact, when quartz precipitates from an aqueous liquid in an open hydrothermal system undergoing 30% vapour loss and a temperature decline from 300 to 150°C, Rayleigh distillation will increase the δ18O value of the quartz by ~10‰ (cf. Chiba et al., Reference Chiba, Chacko, Clayton and Goldsmith1989; Rhodes and Oreskes, Reference Rhodes and Oreskes1999; Donoghue et al., Reference Donoghue, Troll, Harris, O'Halloran, Walter and Pérez Torrado2008). As a result of evaporation processes, shallow surface brines in modern Iceland usually have δ18O values that are 3‰ higher than the brine water measured in drill holes at these sites (e.g. +2 and –1‰ respectively, Ólafsson and Riley, Reference Ólafsson and Riley1978). It is thus plausible that boiling and evaporation can drive the meteoric-hydrothermal alteration fluids towards further enrichment and promote subsequent high δ18O whole-rock values in the vicinity of crater and caldera lakes.
Model for high-δ18O intra-caldera rhyolites in Borgarfjörður Eystri
Fossil geothermal systems associated with central volcanoes are common in the Quaternary and Neogene formations of Iceland (Arnórsson, Reference Arnórsson1995), and might have also provided favourable conditions for boiling water systems within e.g. the Dyrfjöll and Breiðavik calderas. A ~10‰ increase in the δ18O value of meteoric waters is possible via Rayleigh evaporation in such pools upon hydrothermal heating and associated vapour flux (e.g. Giggenbach and Stewart, Reference Giggenbach and Stewart1982). The high δ18O waters of a caldera lake can then feed back into the hydrothermal system via e.g. caldera faults and fractures (e.g. Walter and Troll, Reference Walter and Troll2001; Troll et al., Reference Troll, Walter and Schmincke2002; Donoghue et al., Reference Donoghue, Troll, Harris, O'Halloran, Walter and Pérez Torrado2008), thus promoting infiltration of the underlying rock succession and permitting progressive fluid-rock interaction. This would lead to progressively 18O-enriched fluids over the lifetime of such a circulating hydrothermal system and would result in elevated δ 18O values in the altered rocks that host the high-level (near surface) part of the system.
In this respect, we note that caldera margins are particularly susceptible to low-temperature hydrothermal alteration because fumarole activity at these localities is usually cooler than in the centre of the caldera (e.g. Taylor, Reference Taylor1968; Walter and Troll, Reference Walter and Troll2001; Donoghue et al., Reference Donoghue, Troll, Harris, O'Halloran, Walter and Pérez Torrado2008). Importantly, the intra-caldera rhyolites with the highest δ18O values (IC-URD-IG-5 and IC-HE-IG-22) are found at the margins of former intra-caldera lakes (Fig. 1), where epithermal (~100 to 200°C) alteration was probably dominant. This is similar to the occurrence of altered, marginal intra-caldera tuffs in other settings, such as those at Fuente de Los Azulejos on Gran Canaria. There, low-temperature hydrothermal caldera margin rocks show intense weathering colours from green to pink and a mineral assemblage of zeolites, clays, sheet silicates and secondary silica with δ18O compositions of up to +18‰ (Donoghue et al., Reference Donoghue, Troll, Harris, O'Halloran, Walter and Pérez Torrado2008). Although at lower latitude than Iceland, the Fuente de Los Azulejos caldera margin rocks are also enriched in 18O beyond the equilibrium fractionation of the phases involved when local meteoric water is taken as the dominant water source. Similarly, magma-rock interaction involving circulating hydrothermal fluids has been suggested to have played a significant role in the formation of the Los Azulejos rocks by producing 18O enriched hydrothermal fluids. This leads us to suggest that δ18O enriched hydrothermal fluids may be widespread in intra-caldera and caldera-margin settings.
With this model for the high δ18O sample group, the variation among the intra-caldera rhyolites is explained by the presence of former caldera lake water that fed fumarole activity, thus enabling localized formation of exceptionally 18O enriched hydrothermal fluids that are now bound in a range of hydrous replacement minerals. The lower δ18O values in intra-caldera samples from both Njarðvik and Herfell calderas coincide with an absence of hyaloclastites in these two calderas, which hints at the absence of major caldera lakes and suggests a comparatively dry environment at these sites. The H2O-poor, intra-caldera rhyolite with mildly elevated δ18O values from the centre of Breiðavik caldera (δ18O = 9.7‰, IC-HF-IG-31), in turn, was probably affected initially by the enriched 18O hydrothermal waters of the caldera lake, much like the high-δ18O group, but was subsequently dehydrated during later heating, conceivably during a magmatic resurgence episode in the central part of the caldera. A similar situation was reported by e.g. Donoghue et al. (Reference Donoghue, Troll and Harris2010) from Gran Canaria. There, the intra-caldera cone sheet swarm contains individual intrusive sheets that are hydrothermally altered, but some were subsequently dehydrated due to later incoming cone sheet intrusions, thus offering an analogue for the Icelandic sample in question.
Summary and implications
Extra-caldera rhyolites on Iceland record regional low δ18O magmatic signatures and an element of ‘regular’ low-temperature alteration, whereas the δ18O compositions of some of the intra-caldera rhyolites reported here are among the highest δ18O values thus far known for igneous rocks on Iceland. Although at this time we present a relatively small δ18O dataset, our new data are consistent with: (1) secondary alteration textures; (2) geographical position of samples at the margins of former caldera lake settings; (3) whole-rock H2O contents; (4) alteration mineralogy; and (5) scatter in major- and trace-element compositions of altered high-silica rocks versus well defined evolutionary trends of unaltered intermediate and high-silica rock suites on Iceland. It appears that the high-δ18O intra-caldera rhyolites of our study underwent hydrothermal alteration by fluids with higher δ18O values than local meteoric waters on Iceland. Fluid enrichment may have been achieved through progressive wall-rock exchange, or it may have been an effect of surface evaporation in caldera lake settings, or a combination of these processes. Thus, low-temperature meteoric waters were potentially capable of feeding circulating hydrothermal systems with 18O-enriched fluids in the investigated intra-caldera lake setting, proably as a result of fractionation due to interaction with the rocks encountered during the course of fluid circulation. Another factor to achieve exceptionally enriched 18O fluids could be evaporation in hot or boiling intra-caldera lakes. Large oxygen isotopic shifts are plausibly linked to caldera margins because of intense fracturing and rapid heat loss of the system at these sites (e.g. Donoghue et al., Reference Donoghue, Troll, Harris, O'Halloran, Walter and Pérez Torrado2008), which may reflect a more widespread phenomenon in intra-caldera and especially caldera margin settings.
The wider implication of our finding is that high-δ18O rocks may be locally present in the Icelandic crust as they might derive from old hydrothermal systems now buried under extensive lava piles. This implies that significant heterogeneity in δ18O composition may exist locally within the Icelandic crust as spatially restricted domains that vastly exceed δ18O mantle values. This realization could be important in relation to digestion of Icelandic crust by magma as discrete high-δ18O pockets in buried central volcanoes may complicate δ18O magmatic signatures if taken up by ascending melts. Indeed, high δ18O domains in the Icelandic crust may also help to explain the occurrence of occasional high δ18O zircon on Iceland as a result of partial melting of preconditioned, altered crust (cf. Gurenko et al., Reference Gurenko, Bindeman and Sigurdsson2015).
Acknowledgements
We are grateful to L.E. Gústafsson for assistance during fieldwork and S. Roopnarain and F. Rawoot for help in the stable-isotope laboratory. We also thank L. Dallai, D. Chew, A.K. Barker, R. Gertisser, B. Ellis, S. Mollo and E. Kooijman for stimulating discussions on the stable isotope systematics of the region. In addition, J.S. Lackey and I.N. Bindeman are thanked for reviews of the manuscript. Funding from NORDVULK, the Swedish Research Council (VR), the Royal Swedish Academy of Sciences (KVA), and from Uppsala University (UU) are gratefully acknowledged.