Introduction
The West Indian fruit fly, Anastrepha obliqua (Macquart) occurs throughout México, Central America, the Caribbean, and South America down to Southern Brazil. It is the major pest of mangoes (Mangifera indica L.) (Sapindales: Anacardiaceae) and plum fruits (Spondias purpurea L.) (Sapindales: Anacardiaceae) (Aluja et al., Reference Aluja, Guillén, De la Rosa, Cabrera, Celedonio, Liedo and Hendrichs1987a, Reference Aluja, Cabrera, Ríos, Guillén, Celedonio, Hendrichs and Liedob; Aluja et al., Reference Aluja, Celedonio-Hurtado, Liedo, Cabrera, Castillo, Guillén and Ríos1996). In Mexico, the control strategies available against A. obliqua include the Sterile Insect Technique (SIT), employed since 2001 (Artiaga-López et al., Reference Artiaga-López, Hernández, Domínguez-Gordillo, Moreno, Orozco-Dávila and Brian2004; Orozco et al., Reference Orozco, Domínguez, Reyes, Villaseñor, Gutiérrez and Brian2004). A fruit fly mass-rearing colony for SIT application requires the collection of large numbers of wild insects obtained as larvae from infested fruits, which are then allowed to pupate. The emerging adults are then placed in an artificial environment that differs from their natural conditions (Moore et al., Reference Moore, Dell, Calkins, Sing and Moore1985; Ochieng-Odero, Reference Ochieng-Odero1994). This can result in the evolution of reproductive barriers between the mass-reared and wild populations (Rössler, Reference Rössler1975; Meza-Hernández et al., Reference Meza-Hernández, Hernández, Salvador-Figueroa, Cruz-López and Barnes2004; Rull et al., Reference Rull, Brunel and Mendez2005). To avoid this and to improve the sexual competitiveness of mass-reared sterile flies, the colonies are renovated periodically. This can be done by replacing the old colony with wild flies, (Rössler, Reference Rössler1975), by outcrossing wild males with laboratory-adapted females (Shelly, Reference Shelly2001; Rull & Barreda-Landa, Reference Rull and Barreda-Landa2007; Gilchrist & Meats, Reference Gilchrist and Meats2014) and by selection (Quintero-Fong et al., Reference Quintero-Fong, Toledo, Ruiz, Rendón, Orozco-Dávila, Cruz and Liedo2016; Sanchez-Rosario et al., Reference Sanchez-Rosario, Pérez-Staples, Toledo, Valle-Mora and Liedo2017).
In phytophagous insects, the host fruit can be an environmental factor that promotes genetically differentiated populations, and eventually, host-race formation. Singh & Cunningham (Reference Singh and Cunningham1981) and Helden et al. (Reference Helden, Dixon and Carter1994) discussed that aphids often show variations in biological and ecological attributes of their populations as a result of heterogeneous environments and host plants. Some species of fruit flies have shown a high plasticity in life history traits in response to different environments, including the host, explaining in part the wide geographical distribution and host range of some species (Mkiga & Mwatawala, Reference Mkiga and Mwatawala2015; Hafsi et al., Reference Hafsi, Facon, Ravigné, Chiroleu, Quilici, Chermiti and Duyck2016). In A. obliqua, it has been observed that females may prefer a host plant species for oviposition when they have a choice, but if they only have one option, they oviposit in a less preferred host (Aluja & Birke, Reference Aluja and Birke1993). Toledo & Lara (Reference Toledo, Lara, McPheron and Steck1996) found differences in the survival and reproduction between populations of A. obliqua collected from naturally infested M. indica and S pondias mombin. Thus, it can be expected that differentiated populations of A. obliqua can be found in different host species. If this is true, the refreshment or enrichment of mass-reared colonies should consider the extent of variation related to host fruits and localities in order to collect samples from one or several host-related populations with the traits better adapted for SIT purposes (Hernández et al., Reference Hernández, Toledo, Artiaga-López and Flores2009). Our goal in this study was to examine whether life history traits, by this meaning mating propensity and mating competitiveness, larval and adult weight, and demographic parameters of A. obliqua, are variable according to the host plant species.
Materials and methods
Insects
Infested fruits of S. purpurea (L.), S. mombin L., M. indica L. cv. ‘piña’, and M. indica cv. ‘coche’ were collected at two localities of Chiapas México. In Frontera Comalapa, (15°39′29.95″N, 92°8′34.15″W), one fruit of purple mombin (S. purpurea (L.)) was collected and another fruit from mango piña (M. indica L. cv. ‘piña’) was also collected. In Huehuetan (15°39′29.95″N, 92°8′34.15″W), one fruit from mombin (S. mombin L.) was taken and another from mango coche (M. indica cv. ‘coche’) was harvested. The collected fruits were placed in plastic trays (55 × 45 × 12 cm) and kept for 6 days in laboratory conditions (26 ± 1°C, 75 ± 5% R.H., and a photoperiod of 12:12 h L:D) until the larvae reached third instar. The fruits were then opened, and the larvae were extracted and were placed in plastic containers (10 cm wide × 12 cm high × 20 cm long) with coconut fiber (50% dust–50% short fiber: CF) (Coirtech, Colima, Mexico) to promote the pupariation (Aceituno-Medina et al., Reference Aceituno-Medina, Rivera-Ciprian and Hernández2017).
Each wild host-associated population per locality was considered as a treatment in the statistical analyses. A fifth treatment was a random sample of 1000 larvae taken from the Moscafrut mass-rearing facility (SAGARPA-IICA) located in Metapa de Domínguez, Chiapas, Mexico. This colony was initiated with flies from a laboratory colony at the Subtropical Agricultural Research Laboratory (ARS-USDA), in Weslaco, TX, USA. This strain was started with wild flies collected from S. mombin fruits in Veracruz, Mexico (Moreno et al., Reference Moreno, Ortega-Zaleta and Mangan1997). The mass-rearing population was reared on an artificial diet (Artiaga-López et al., Reference Artiaga-López, Hernández, Domínguez-Gordillo, Moreno, Orozco-Dávila and Brian2004) for at least 113 generations with enrichment introductions of wild material in 2002 and 2011 (Orozco-Dávila et al., Reference Orozco-Dávila, Artiaga-López, Hernández, Domínguez and Hernández2014, Reference Orozco-Dávila, Quintero, Hernández, Solís, Artiaga, Hernández, Ortega and Montoya2017).
Mating competitiveness and propensity
The sexual competitiveness was defined as the number of matings of mass-reared males and wild males from the different fruit host with wild females in field cage conditions. The sexual mating propensity measures the mating index, defined as how ‘eager’ the males and females from the same population are to mate (Calkins & Parker, Reference Calkins, Parker, Dyck, Hendrichs and Robinson2005). Accordingly, mating propensity in this work was determined using females and males from the same fruit host in field cages.
Males and females were released into field cages with open smaller cages (24.5 × 13 × 12 cm) containing flies held according to each host-related and mass-reared population. All the mating observations were done in field cages (3 m in diameter × 2 m in height) covered with amber mesh (ten threads per linear cm). An orange tree (Citrus sinensis L. Osbeck) (Sapindales: Rutaceae), approximately 2 m in height was centrally placed to provide environmental conditions close to field conditions. The mating propensity test was carried out in five independent field cages: (1) 30 S. mombin males + 30 S. mombin wild females, (2) 30 S. purpurea males + 30 S. purpurea wild females, (3) 30 mango cv. ‘piña’ males + 30 mango cv. ‘piña’ wild females, (4) 30 mango cv. ‘coche’ males + 30 mango cv. ‘coche’ wild females, and (5) 30 mass-reared males + 30 wild females (control). The mating competitiveness test was carried out in four independent field cages: (1) 30 S. mombin males + 30 mass-reared males + 30 S. mombin wild females, (2) 30 S. purpurea males + 30 mass-reared males + 30 S. purpurea wild females, (3) 30 mango cv. ‘piña’ males + 30 mass-reared males + 30 mango cv. ‘piña’ wild females, and (4) 30 mango cv. ‘coche’ males + 30 mass-reared males + 30 mango cv. ‘coche’ wild females. We ensured that all treatments were performed in all cages. The males were released at 6:00 h, and 15 min later (6:15 h), the females of the same treatment were released. When a mating was formed, it was collected with an entomological vial. We did 14 trials for S. mombin, eight for S. purpurea, eight for mango piña, eight for mango coche, and 14 for the mass-reared strain. The number of replicates depended on the level of infestation of the host fruit.
Observations and captures of mating pairs were conducted from 6:00 to 12:00 h, which is the time of major activity for A. obliqua (Aluja & Birke, Reference Aluja and Birke1993). To synchronize sexual maturity, wild flies were 16 days old and mass-reared flies were 11 days old (Meza-Hernández et al., Reference Meza-Hernández, Hernández, Salvador-Figueroa, Cruz-López and Barnes2004). Males and females that had mated were chilled for 10 min at 4°C and weighed using an analytical balance (Sartorius TE214S 201 g × 0.1 mg, Sartorius AG, Goettingen, Germany).
Demographic parameters
To estimate the survival of immature stages, we used 36 experimental units (EU) per population or treatment. Each EU consisted of ten eggs that were artificially inoculated in fruit or placed on an artificial diet and kept at 27°C and 70% R.H. Every day for 12 days, the EU were observed to record the proportion of eggs, first, second, and third larval instars. After 12 days, a daily sample of three fruits per treatment was dissected to determine the number of eggs and larvae at first, second, and third instar stages. The identification of each instar was made according to Elson-Harris (Reference Elson-Harris1988) as follows: first instar has a brown mouth hook, the second instar has a brown color in the apical part and black in the posterior part of the hook, and the third instar has a completely black hook.
The adult survival and fecundity was determined using cohorts of ten pairs of each treatment that were placed in 27 dm3 glass cages. Four replicates were done for each treatment (40 couples in total for each population). Adults were fed ad libitum with a mixture of sucrose and enzymatic yeast hydrolysate (3:1) (MP Biomedicals, Santa Ana, CA, USA) (Message & Zucoloto, Reference Message and Zucoloto1989). Water was provided in a 500 ml plastic container with a strip of filter paper. Files were kept at 26 ± 1°C, 75% R.H., and a 14:10 (L:D) h photoperiod. White 75 W fluorescent tubes placed 60 cm above the cages provided light, and the light intensity was ~3500 lux inside the cages. Dead flies by sex were recorded daily.
An oviposition and egg-collecting device was placed 1 day after the first mating was observed, usually when flies were 6 days old, and was replaced every 24 h. The oviposition devices were 4 cm diameter spheres made of furcellaran (Boller, Reference Boller1968). These were made by dissolving 22.7 g of furcellaran (Burtonite 44C powder, Tic Gums Inc. Belcamp, MD, USA) in 1 liter of boiling water. Then, 1.6 ml of green food coloring (McCormick, Mexico, S.A. de C.V.) and 5.6 g of guava flavor powder (Frisco, Kraft Foods de Mexico, S.A. de C.V.) were added. The mixture was poured into spherical plastic templates (4 cm diameter), and the solidified spheres were wrapped with Parafilm membrane (American National Can™, Neenah, WI, USA). Every day for 130 days, a single oviposition device was hung from the top of each cage. After removal, the spheres were unwrapped, cut into thin slices, and dissolved in water (25–27°C) with a bubbling system (aquarium air pump, airflow of 3 liters min−1, 4.5 p.s.i., 110 V, 50/60 Hz). The water was decanted, and the eggs from each daily collection were counted. Then, they were placed over pieces of black cloth placed over a moist sponge in a Petri dish (150 by 25 mm). The eggs were incubated for 5 days at 28 ± 1°C, and egg hatching was estimated by counting the number of unhatched eggs and neonate larvae under a stereomicroscope. Daily records of mortality and fecundity were used to construct life tables and determine demographic parameters.
Data analysis
Data of male weight (mg), female longevity (days), age at first oviposition (days), oviposition period (days), postoviposition period (days), and demographic parameters were estimated for each treatment in each cohort. We use analysis of covariance to determine the effect of host fruit (fixed factor) on mating competitiveness and propensity, and the weight of the males was used as a covariable. We assumed that mango varieties and plant species provide different environments for A. obliqua; therefore, we declared five populations or treatments for the analyses of variance on the demographic parameters. After the means were compared using the Tukey's HSD test, additional demographic parameters were analyzed by orthogonal contrast grouping by host and locality, excluding the mass-reared group. Data on female longevity, age at first oviposition, oviposition period, and post-oviposition period were adjusted to normality and homoscedasticity by log-transformation. Analyses were carried out with the statistical program JMP version 5.0.1. Statistical Discovery Software (SAS Institute, 2003) and R Statistical Software (R Development Core Team, 2014).
Population demographic parameters were estimated from the daily proportion of surviving flies at age x (l x), and daily female offspring per female (m x), following methods described by Birch (Reference Birch1948), Hamilton (Reference Hamilton1966), and Carey (Reference Carey1993).
Results
Mating competitiveness and propensity
There were no significant differences in the average number of matings during the mating propensity test between wild males from mango cv. piña, mango cv. coche, S. mombin, S. purpurea, and mass-reared males (F = 0.95; df = 4, 47; P = 0.4440). The same trend was observed when the data were grouped by host and locality, and when male weight was considered as a covariable (F = 0.11; df = 1, 23; P = 0.738) (fig. 1).

Fig. 1. Matings and weight by host associated population (barrs), for male (- - -) and female (―) during the propensity mating test host-related population fruit of Anastrepha obliqua and mass reared strain.
In the data for the average number of matings in the competitiveness test, the pairwise comparisons only show significant differences between males from mango cv. ‘coche’ vs. mass-reared males (t = 2.31; df = 1,20; P = 0.0317), although in all cases, the largest number of matings corresponded to the wild males (fig. 2).

Fig. 2. Matings during the mating propensity test of Anastrepha obliqua from two host fruits in two different regions and a mass reared strain. [■] Wild male. [□] Mass reared male.
Demographic parameters
Females collected from S. purpurea showed the longest longevity, whereas females from the mass-reared strain showed the shortest (F = 8.90; df = 4, 135; P < 0.0001). Age at first oviposition was shorter for mass-reared females than for wild females recovered from fruits (F = 34.63; df = 4, 135; P < 0.0001). The longest oviposition period was observed in females from S. purpurea, compared with females from mango fruits and the mass-reared group (F = 3.57; df = 4, 135; P = 0.009). Post-oviposition period was shortest for mass-reared females (F = 4.69; df = 4.135; P = 0.0014) (table 1) compared with wild females.
Table 1. Mean (SE) longevity, age at first oviposition, oviposition, and post-oviposition period of Anastrepha obliqua females emerging from two different fruit host species collected in two localities and from a mass-rearing colony.

Lower case letters that differ within columns indicate a significant difference between the means of the five populations.
Orthogonal contrasts indicate that significant differences could be attributed to host fruit (including the mass-reared strain as a fifth level) only in the age at first oviposition. The locality significantly affected the longevity, oviposition period, birth rate, intrinsic rate of increase, finite rate of population increase, mean generation time, and doubling time (tables 2 and 3).
Table 2. Analysis by orthogonal contrast of the effect of the host fruit species and locality on the demographic traits of Anastrepha obliqua.

Table 3. Means + SE of demographic parameters of Anastrepha obliqua females emerging from two different host fruit species collected in two localities and from a mass-reared colony. Four cohorts of ten couples each one.

Lower case letters that differ within rows indicate a significant difference between the means of the five populations.
Survival curves were similar for immature stages across treatments, and small differences were observed in female adults (fig. 3). The mass-reared females showed a survival rate less than other treatments after they are 60 days old. The flies from S. purpurea showed a sudden decrease in survival between 30 and 60 days old, then this trend leveled off compared with other host fruits. The second longest lifespan was observed in females from this host (fig. 3).
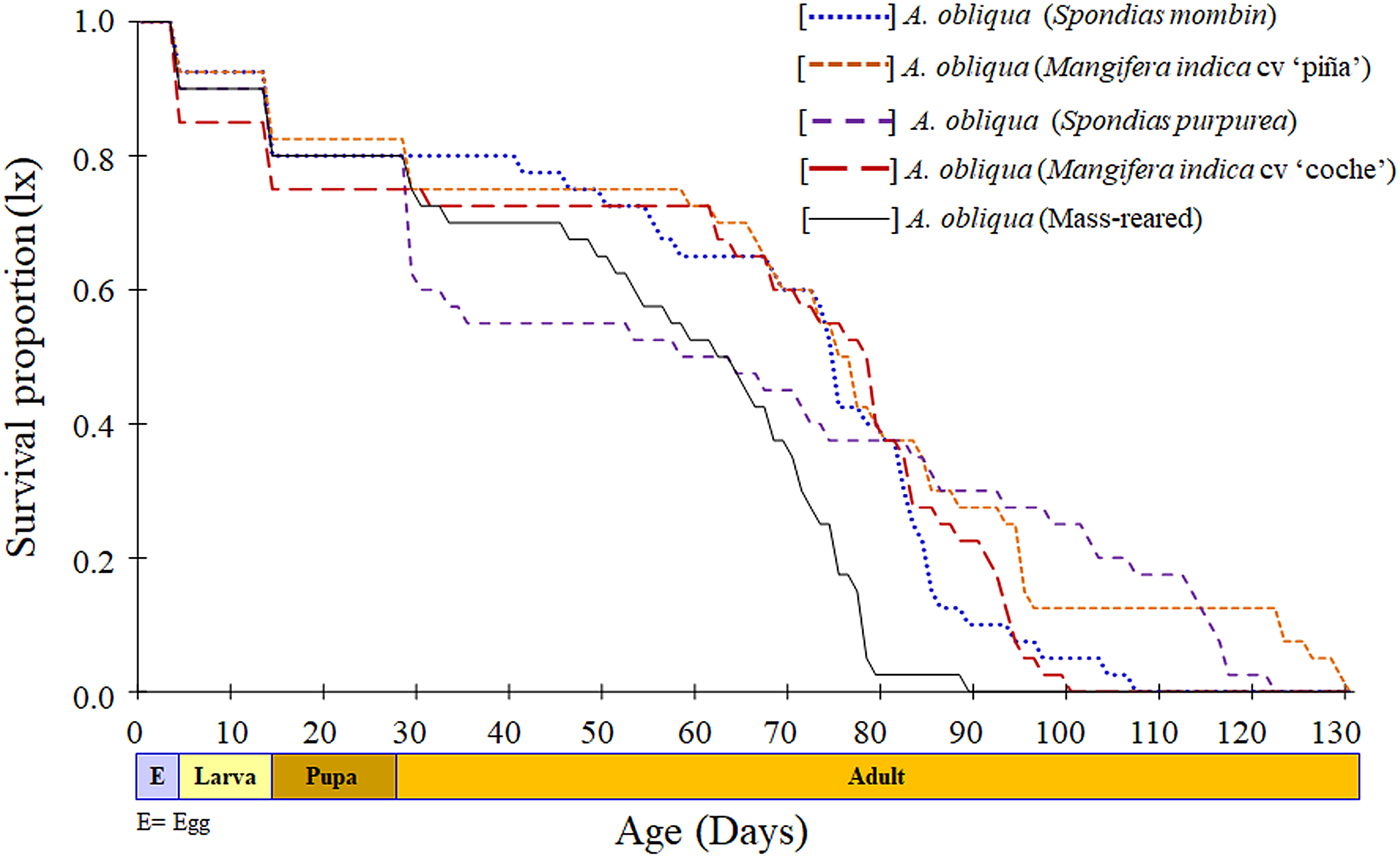
Fig. 3. Survival of Anastrepha obliqua females collected from two host fruits, from two localities, and a mass-reared strain.
The mass-reared females started and finished oviposition of eggs earlier than females collected from wild fruits (fig. 4).
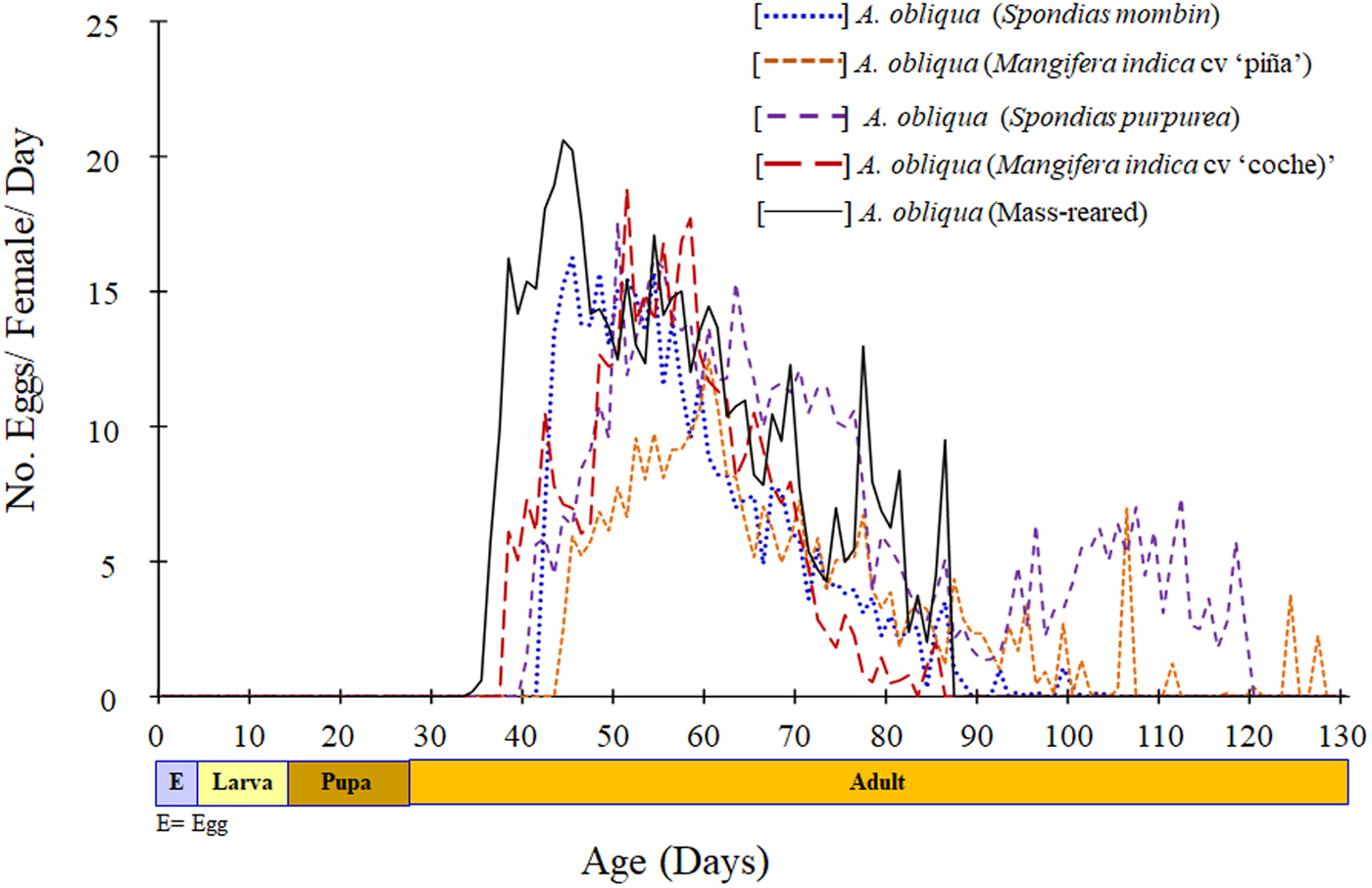
Fig. 4. Fecundity of Anastrepha obliqua females collected from two host fruits, from two localities, and a mass-reared strain.
Gross fecundity and the net reproductive rate (R o) did not show significant differences across host-related populations (F = 1.87; df = 4, 15; P = 0.168). The mass-reared strain showed the highest birth rate (F = 7.36; df = 4, 15; P = 0.002) and the highest intrinsic rate of increase (r m) (F = 8.65; df = 4, 15; P = 0.001). This resulted in the greatest finite rate of population increase (λ) (F = 8.72; df = 4, 15; P = 0.001) and the shortest mean generation (F = 8.65; df = 4, 15; P = 0.004) and doubling times (F = 6.48; df = 4, 15; P = 0.0031). Death rates were not significantly different between groups (F = 2.02; df = 1, 15; P = 0.142) (table 3).
Discussion
The host fruit and locality affected the sexual competitiveness, propensity, larval and adult weights, and the demographic parameters of A. obliqua. According to the demographic parameters, the population of S. mombin showed the highest reproduction rate starting at an early age. Even in the propensity test, it had the highest number of matings. However, males with greater sexual competitiveness and longevity corresponded to those collected from S. purpurea.
We found significant differences in the competitiveness between mango cv. coche vs. mass-reared only, but, in all other comparisons, the wild males were selected at least twice as often as mass-reared males by the wild females. Although the mating propensity test indicated that there are no significant differences in the mating behavior of flies between host fruits or locality, the sexual mating propensity indicated that 43% of the mass-reared males mated while the competitiveness test indicated that only 10% of the mass-reared males mated. The sexual mating propensity is a qualitative measure, indicating that 30.40% of the males and females collected from the different fruit hosts mated under field cage conditions. These results suggest that mass-reared males had a low level of competence for mating, which could be a result of the artificial methods used to maximize mass production, compromising their sexual competence. Research with other tephritid fruit fly species has shown some degree of reproductive isolation between mass-reared and wild flies (McInnis et al., Reference McInnis, Lance and Jackson1996; Miyatake & Shimizu, Reference Miyatake and Shimizu1999). However, the factors and traits of reproductive isolation selection are not yet known. A periodic renewal of mass-reared colonies is highly recommended, but it is necessary to first check for mating compatibility and competitiveness of the introduced strain with the target populations, as was done for A. ludens in Orozco-Dávila et al. (Reference Orozco-Dávila, Hernández, Meza and Domínguez2007).
Our data indicated that the weight of males did not modify the host fruit effect on the competitiveness of A. obliqua. Weight has been frequently suggested as an indicator of vigor or fitness for fruit flies (Liedo et al., Reference Liedo, Salgado, Oropeza and Toledo2007), but studies show different results. Our findings are in accordance with Rhagoletis cerasi (L.) (Diptera: Tephritidae) (Jaastad, Reference Jaastad1998). Heavier males of Ceratitis capitata Wiedemann (Diptera: Tephritidae) and Anastrepha suspensa Wiedemann (Diptera: Tephritidae) display greater sexual calling activity as well as greater efficiency in marking and defending territory when forming leks, indicating that male weight is a key factor selected by females (Burk & Webb, Reference Burk and Webb1983; Churchill-Stanland et al., Reference Churchill-Stanland, Stanland, Wong, Tanaka, McInnis and Dowell1986).
Generally, there were differences in the demographic parameters between the mass-reared strain and strains from other host fruits. Carey (Reference Carey1984) and Krainacker et al. (Reference Krainacker, Carey and Vargas1987, Reference Krainacker, Carey and Vargas1989) found that host species affected the demographic parameters of C. capitata, even in varieties where there can be differences. According to Papadopoulos & Katsoyannos (Reference Papadopoulos, Katsoyannos and Brian2004), C. capitata showed differences in the duration of the larval stage, mortality rate, pupal period, longevity, and fecundity when they were developed in three different varieties of apples (Malus domestica Borkh) (Rosales: Rosaceae), namely, Golden Delicious, Granny Smith, and Red Delicious. Sugayama et al. (Reference Sugayama, Kovaleski, Liedo and Malavasi1998) reported the same findings for A. fraterculus, concluding that flies developed in guava fruits (Psidium guajava L.) (Myrtales: Myrtaceae) had higher survival and reproduction rates than apple cultivars Golden Delicious, Gala, and Fuji. In turn, Bactrocera zonata (Saunders) (Diptera: Tephritidae) showed a higher pupal recovery rate with guava fruit than with banana (Musa paradisiaca L.) (Zingiberales: Musaceae) (Rauf et al., Reference Rauf, Ahmad, Rashdi, Ismail and Khan2013). In the case of A. obliqua, Celedonio-Hurtado et al. (Reference Celedonio-Hurtado, Liedo, Aluja and Guillén1988) found differences in the immature developmental time and survival when flies were reared on M. indica, S. mombin, and artificial diets. Thus, the host fruit affects demographic parameters, but probably the extent of this depends on experimental micro-environmental factors that cannot be identified in all treatments.
Despite the effect of the host on the population parameters of fruit fly species, the ability to exploit different host species seems to be the most important adaptation trait for survival and reproduction in wild condition. Some of the most economically important tephritid pests have the ability to infest large numbers of hosts (Aluja & Mangan, Reference Aluja and Mangan2008). Although A. obliqua is restricted to plant species of the Anacardiaceae family, it still can be considered an oligophagous species that has the ability to switch hosts. This ability probably compensates for the loss in fitness attributed to one particular host.
The shorter time to initiate reproduction in the mass-reared strain is consistent with the previous reports for A. obliqua and other mass-reared tephritid species (Carey & Vargas, Reference Carey and Vargas1985; Foote & Carey, Reference Foote and Carey1987; Vargas & Carey, Reference Vargas and Carey1989; Miyatake & Yamagishi, Reference Miyatake and Yamagishi1992; Miyatake, Reference Miyatake1998; Hernández et al., Reference Hernández, Toledo, Artiaga-López and Flores2009). This shorter time can be explained by the selection of early reproducing individuals during colonization. Sexual maturation in wild females is delayed while longevity increased. This could mean a trade-off between reproduction and longevity exists, because females from mass-rearing showed the shortest lifespan and the earliest age at first oviposition as a result of their high developmental rate to sexual maturation. Fast development is a highly desirable trait for mass-rearing. It results in greater population growth rates and, therefore, more efficient rearing (Liedo & Carey, Reference Liedo and Carey1994). The differences in the parameters of populations, such as the finite rate of population increase, the generation, and doubling times, can be attributed to this characteristic of mass-reared strains.
Our results showed non-significant difference between host fruits, but Aluja & Birke (Reference Aluja and Birke1993) reported that females of A. obliqua preferred mombin fruits over mango fruits for ovipositing when both were available. When only mangos were available, they oviposited in them, indicating that A. obliqua could identify a mombin as a host that provides more nutritional advantages, and this choice affects the life history traits of the males and females of the emerged flies and their fitness (Kaspi et al., Reference Kaspi, Mossinson, Drezner, Kamensky and Yuval2002; Passos-Roriz & Joachim-Bravo, Reference Passos Roriz and Joachim-Bravo2013; Hafsi et al., Reference Hafsi, Facon, Ravigné, Chiroleu, Quilici, Chermiti and Duyck2016). In this sense, our results indicate that for the Malthusian intrinsic rate of population increase, r m, and most of the demographic parameters, which relate reproduction and survival rates, a population from S. mombin would allow artificial colonization in less time, considering that it has a high reproduction rate at an early age. Even in the propensity test, it had the highest number of matings. However, males with greater sexual competitiveness and longevity for colonization corresponded to those collected from S. purpurea. In mass-reared males, the artificial food compared with a fruit host could be providing some additional nutritional advantage, but with the consequence of diminished competitiveness. According to Boggs & Freeman (Reference Boggs and Freeman2005), increasing allocation and bioavailability of larval food resources improves age-specific survival and reproduction as result of the efficient digestion and assimilation of nutrients that, once absorbed, exert a positive effect on growth and life history traits (Carbonell-Capella et al., Reference Carbonell-Capella, Buniowska, Barba, Esteve and Frígola2014). This could explain the differences in survival and reproduction of A. obliqua developed in the different host fruits compared with flies developed in artificial food under mass-rearing conditions.
According to the demographic parameters, the population of S. mombin would allow artificial colonization in less time, considering that it has a high reproduction rate starting at an early age. However, males with greater sexual competitiveness and longevity for colonization corresponded to those collected from S. purpurea. Although a better and safer approach would be to start with a genetically diverse colony, it is recommended to cross individuals of S. mombin and S. purpurea populations, and the resulting hybrid could be used to establish a colony for mass breeding.
Acknowledgements
The authors thank Margarita Najera, Bigail Bravo, Floriberto López Mendez, and Julio Lanza for technical assistance during fruit collection, fecundity and survival tests, and field cage experiments. José Manuel Gutiérrez Ruelas, for the support granted during the development of the project. This work was funded by the Programa Nacional Moscas de la Fruta (DGSV-SENASICA-SAGARPA). E.H. thanks CONACYT for scholarship support (CVU 71688/201421007). This study forms part of E.H.’s doctoral thesis










