INTRODUCTION
Ash is the powdery residue left after the combustion of organic materials, and one of the most common components of sediments at archaeological sites (Weiner Reference Weiner2010). Being the most direct byproduct of combustion, ash is an important indicator of past human activities involving the use of fire (Weiner Reference Weiner2010; Wadley et al. Reference Wadley, Sievers, Bamford, Goldberg, Berna and Miller2011). Therefore, the identification of combustion features is crucial in understanding human evolution, behavior, and practices (Wrangham Reference Wrangham2009; Roebroeks and Villa Reference Roebroeks and Villa2011; Gowlett and Wrangham Reference Gowlett and Wrangham2013). The identification is achieved by studying the microscopic archaeological record using different analytical methods that characterize the mineral and organic components present in ash (Weiner Reference Weiner2010; Berna et al. Reference Berna, Goldberg, Kolska Horwitz, Brink, Holt, Bamford and Chazan2012; Shahack-Gross et al. Reference Shahack-Gross, Berna, Karkanas, Lemorini, Gopher and Barkai2014; Walker et al. Reference Walker, Anesin, Angelucci, Avilés-Fernández, Berna, Buitrago-López, Fernández-Jalvo, Haber-Uriarte, López-Jiménez, López-Martínez, Martín-Lerma, Ortega-Rodrigáñez, Polo-Camacho, Rhodes, Richter, Rodríguez-Estrella, Schwenniger and Skinner2016). Often, these components are organized in distinct layers of sediment, which are the result of single or multiple firing episodes, and thus represent well-defined, short-lived events within a stratigraphic sequence that are suitable for radiocarbon (14C) dating (e.g. Adler et al. Reference Adler, Bar-Yosef, Belfer-Cohen, Tushabramishvili, Boaretto, Mercier, Valladas and Rink2008; Kuhn et al. Reference Kuhn, Stiner, Güleç, Özer, Yılmaz, Baykara, Açıkkol, Goldberg, Molina, Ünay and Suata-Alpaslan2009; Rebollo et al. Reference Rebollo, Weiner, Brock, Meignen, Goldberg, Belfer-Cohen, Bar-Yosef and Boaretto2011; Regev et al. Reference Regev, Finkelstein, Adams and Boaretto2014; Asscher et al. Reference Asscher, Lehmann, Rosen, Weiner and Boaretto2015a, Reference Asscher, Cabanes, Hitchcock, Maeir, Weiner and Boaretto2015b). Considering the widespread occurrence of ash deposits in archaeological settings across the globe, it appears clear that obtaining accurate ages from these features is of major importance for the establishment of absolute chronologies, and thus for sequencing events along the human career.
To date, 14C measurements of ash have always been performed on the organic fraction, namely charcoal and charred seeds that may survive when the combustion process is incomplete (Taylor and Bar-Yosef Reference Taylor and Bar–Yosef2014). However, charcoal suffers from the “old wood” effect (Bowman Reference Bowman1990), whereas charred seeds might be unrelated to the depositional context, especially if they do not occur in sealed clusters (Toffolo et al. Reference Toffolo, Maeir, Chadwick and Boaretto2012; Asscher et al. Reference Asscher, Lehmann, Rosen, Weiner and Boaretto2015a). Charred materials in general are often not preserved in ash due to post-depositional alterations (Cohen-Ofri et al. Reference Cohen-Ofri, Weiner, Boaretto, Mintz and Weiner2006; Rebollo et al. Reference Rebollo, Cohen-Ofri, Popovitz-Biro, Bar-Yosef, Meignen, Goldberg, Weiner and Boaretto2008). Thus, in many cases only the mineral components are left. Among these, the most abundant is calcium carbonate (CaCO3) in the form of calcite, which is the product of thermal decomposition of biogenic calcium oxalate (C2CaO4·H2O) produced by plants (Frost and Weier Reference Frost and Weier2004; Franceschi and Nakata Reference Franceschi and Nakata2005). This calcite phase contains 14C, which in principle derives from atmospheric CO2 sequestered by plants during photosynthesis, and therefore could be used for accurate dating. However, a previous study pointed out that the isotopic signature of ash calcite is different compared to the plant cellulose one, thus hampering a proper age assessment (Regev et al. Reference Regev, Eckmeier, Mintz, Weiner and Boaretto2011). In addition, CO exchange between calcium oxalate and the atmosphere during burning may alter the initial 14C content (Price et al. Reference Price, Dollimore, Fatemi and Whitehead1980; Regev et al. Reference Regev, Eckmeier, Mintz, Weiner and Boaretto2011). If the firing temperature exceeds 600°C, the calcite derived from calcium oxalate turns into calcium oxide (CaO) (Frost and Weier Reference Frost and Weier2004). This mineral phase is unstable at ambient temperature, and when the firing stops, it turns again into calcite by reacting with humidity and CO2 in the atmosphere (Boynton Reference Boynton1980). This high-temperature calcite bears the 14C signature of the atmosphere at the time it nucleates, and therefore could provide accurate 14C dates. In practice, this is not the case because ash always undergoes diagenetic processes that partially or totally alter its original isotopic composition (Koumouzelis et al. Reference Koumouzelis, Ginter, Kozlowski, Pawlikowski, Bar-Yosef, Albert, Litynska-Zajac, Stworzewicz, Wojtal, Lipecki, Tomek, Bochenski and Pazdur2001; Weiner et al. Reference Weiner, Goldberg and Bar-Yosef2002). Recently, we reported that another polymorph of CaCO3, aragonite, forms together with calcite upon carbonation of CaO in experimental and archaeological high-temperature ash (Toffolo and Boaretto Reference Toffolo and Boaretto2014). This pyrogenic aragonite phase is an ideal material for 14C dating. It occurs in archaeological combustion features, it represents the time of ash formation, and since it is susceptible to post-depositional chemical alterations, its presence indicates pristine conditions of preservation, which might include the original 14C signature. Here we show that by carefully isolating pyrogenic aragonite from a destruction horizon of known age at the site of Tel Megiddo, Israel (Figure 1A), it was possible to measure its 14C content and obtain an accurate age determination.

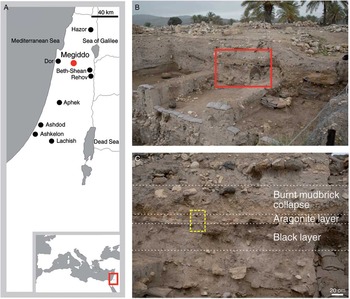
Figure 1 Site location and archaeological context. A: map showing the location of Tel Megiddo and other major Iron Age sites in southern Levant. B: view of the NW trench section of Area K. The rectangle marks the sampling locality. C: view of the portion of the destruction horizon highlighted in B, showing the stratigraphic sequence belonging to Level K-4. The dashed rectangle marks the position of the micromorphology block and the sample dated with 14C.
MATERIALS AND METHODS
Archaeological Context and Sampling
Tel Megiddo, located in northern Israel (Figure 1A), is one of the most important archaeological sites in the Levant, featuring an uninterrupted stratigraphic sequence from the Neolithic to the Persian period (Finkelstein et al. Reference Finkelstein, Ussishkin and Halpern2000, Reference Finkelstein, Ussishkin and Halpern2006, Reference Finkelstein, Ussishkin and Cline2013). A recent 14C dating program, based on clusters of charred olive pits collected from secure depositional contexts, established a high-resolution chronological sequence spanning the period from the Middle Bronze Age III through to the Iron Age IIA (Toffolo et al. Reference Toffolo, Arie, Martin, Boaretto and Finkelstein2014). Within this period, three destruction events characterized by large amounts of in-situ crushed ceramics and burnt debris provided important pegs for both the relative and absolute chronologies. One of these destruction events marks the end of the Iron Age I material culture, which represents the collapse of the late Canaanite city-state (Finkelstein et al. Reference Finkelstein, Ussishkin and Halpern2006). This destruction event has been unearthed in different excavation areas and dated to the range 2920–2760 BP (69.59–70.93 pMC) (Toffolo et al. Reference Toffolo, Arie, Martin, Boaretto and Finkelstein2014). During the 2014 excavation season, preliminary on-site analysis carried out by one of us (RSG) revealed the presence of aragonite within Level K-4, which includes the Iron Age I destruction horizon in Area K. In the NW section of the Area K trench, Level K-4 features three layers belonging to the destruction horizon (Figures 1B and 1C). A black layer rich in charred material covered by potsherds underlies a thin white ashy layer rich in aragonite, which in turn is covered by a massive burnt mudbrick collapse. This locality was selected for detailed sampling. Bulk sediment samples were collected from each layer and stored in plastic vials and bags, which provided enough material for all the laboratory analyses. Two charred olive pits found together within the black layer were collected with their sediment matrix and stored in aluminum foil for 14C dating. Chalk from the local bedrock was used as a reference for stable isotopes and thermogravimetric analyses.
Experimental Samples
An experimental ash rich in aragonite was used as a reference for scanning electron microscope (SEM) analysis and 14C dating. This material was obtained by heating Glycymeris insubrica shells to 900°C for 12 hr in air atmosphere using an electric muffle oven. The resulting quicklime (CaO) was quenched to room temperature in ambient air (Toffolo and Boaretto Reference Toffolo and Boaretto2014). At the time of this study, the ash was 3 yr old and composed of a mixture of aragonite (dominant), calcite, and portlandite [Ca(OH)2]. A crystal of pure geogenic aragonite from a Triassic formation in Loma Badá (Spain), purchased from Mineralogical Research Company (San Jose, California, USA), was used as blank for the density separation method and 14C dating.
Fourier Transform Infrared Spectrometry (FTIR)
A few milligrams of sediment were homogenized and powdered in an agate mortar and pestle. About 0.1 mg was left in the mortar and mixed with approximately 0.5 mg of KBr (FTIR grade, Sigma-Aldrich) and pressed into a 7-mm pellet using a hydraulic press (Specac) or a hand press (PIKE Technologies). Infrared spectra were obtained at 4 cm–1 resolution in 32 scans within the 4000–400 cm–1 spectral range using a Thermo Scientific Nicolet 380 spectrometer (for archaeological sediments and density separation fractions analyzed at the Weizmann Institute of Science) and an Agilent Technologies Cary 660 spectrometer (for fractions obtained from density separation analyzed at Eberhard-Karls-Universität Tübingen). Phase identification was performed using OMNIC v. 9, standard literature (Farmer Reference Farmer1974; van der Marel and Beutelspacher Reference van der Marel and Beutelspacher1976), and the reference collection of FTIR spectra of standard materials provided by the Kimmel Center for Archaeological Science, Weizmann Institute of Science (http://www.weizmann.ac.il/kimmel-arch/infrared-spectra-library). The crystallinity index, or splitting factor, of hydroxylapatite was calculated using an established method (Weiner and Bar-Yosef Reference Weiner and Bar-Yosef1990).
Phytolith Analysis
Phytolith assemblages were obtained using the rapid extraction method (Katz et al. Reference Katz, Cabanes, Weiner, Maeir, Boaretto and Shahack-Gross2010). The identification of different morphotypes was carried out following the standard literature and a reference collection of archaeological and modern samples (Twiss et al. Reference Twiss, Suess and Smith1969; Mulholland and Rapp Reference Mulholland and Rapp1992; Madella et al. Reference Madella, Alexandre and Ball2005; Albert et al. Reference Albert, Ruíz and Sans2016). Phytoliths were studied using a Zeiss Axio Scope AX10 petrographic microscope at 200×and 400×magnifications.
Archaeological Micromorphology
An intact block of sediment was carved from the NW section of Area K, air-dried for several weeks and finally oven-dried at 40°C for 3 days. The sample was then embedded in a mixture of polyester resin (Elgad, Israel) and acetone (ratio 7:3), with the addition of 10 mL of MEKP catalyst per 1 L of mixture. Once solid, the block was cured in an oven at 50°C for three days and then sliced with a rock saw to obtain 55×75 mm chips, which were shipped to Arizona Quality Thin Sections (Tucson, Arizona, USA) for thin section preparation. All the thin sections were polished to a thickness of 30 µm. Micromorphological analyses were carried out using a Nikon Eclipse 50iPOL petrographic microscope at different magnifications (20×, 100×, 200×, 400×), following the standard literature (Courty et al. Reference Courty, Goldberg and Macphail1989; Stoops et al. Reference Stoops, Marcelino and Mees2010).
X-Ray Diffraction (XRD)
Bulk and sieved (<50 µm) ash samples were analyzed using a D8 Discover GADDS microdiffractometer (µ-XRD2) equipped with a cobalt-sealed tube running at 30kV/30mA, a HOPG primary monochromator, a 500 µm monocapillary optic with a 300 µm exit pinhole, and a large 2D detector (VÅNTEC-500) covering 40°2θ and χ. Samples were prepared on a low background silicon single-crystal wafer, used as sample holder. Phase identification was performed using EVA v. 10 and DIFFRAC Plus Release 2004, and a PDF database from the International Centre for Diffraction Data (ICDD). Crystalline phases were quantified using the Rietveld software package Siroquant v. 3.0.
Raman Micro-Spectrometry (µ-Raman)
Sieved ash samples (<50 µm) were analyzed using a Renishaw inVia Raman microscope. Analyses were carried out using a 532 nm laser with 1800 lines/mm grating at 50% power. Each measurement consists of five accumulations over 5 s at 50×magnification. Phase identification was performed using reference materials of the Angewandte Mineralogie Department at Eberhard-Karls- Universität Tübingen (validated by XRD), and the RRUFF database (http://rruff.info).
Scanning Electron Microscopy (SEM)
Sediment samples were placed on carbon tape and coated with a Au-Pd sputter coater (Edwards S150). Samples were examined with a high-resolution Zeiss Leo Supra 55VP field emission scanning electron microscope. Analyses were performed between 2 and 5 kV at various working distances using a secondary electron detector.
Stable Isotopes Analysis
The carbon and oxygen isotope compositions of chalk and aragonite-rich ash were determined using a GasBench II device connected online to a Finnigan MAT 252 mass spectrometer. Isotope ratios were calibrated using NBS18 (δ13C=–5.00‰, δ18O=–22.96‰, relative to VPDB) and NBS19 (δ13C=1.95‰, δ18O=–2.20‰, relative to VPDB). External reproducibility is better than±0.1‰ for δ13C and±0.1‰ for δ18O measurements. External reproducibility for carbonate concentration is better than±10%.
Density Separation
This method exploits the specific gravity of minerals. It is known that minerals with different specific gravity can be separated with a centrifuge by using a heavy liquid with a density in between the specific gravity of the selected minerals. In our case, aragonite is heavier (2.93 g/mL) than most minerals found in ash, and especially calcite (2.71 g/mL), and therefore it can be separated by density. We developed a tailored procedure based on a method for shell carbonates (Douka et al. Reference Douka, Hedges and Higham2010). We used sodium polytungstate (SPT) – Na6(H2W12O40) – as heavy liquid, which was purchased from TC-Tungsten Compounds GmbH (Germany) in a purified form poor in C and N isotopes (less than 100 ppm of carbon). High-density SPT has pH 3, which favors the dissolution of carbonates. Therefore, SPT was buffered with an aqueous solution at pH 8, obtained by mixing 93.2% of 1 M Na2HPO4 with 6.8% of 1 M NaH2PO4. The resulting solution has pH 7. Given the large variability in specific gravity of pyrogenic aragonite crystals (2.40–2.93 g/mL), we empirically established that 2.75 g/mL is the best density to reach highest efficiency in separating aragonite from lighter components. The bulk aragonite-rich sediment was dry sieved and 100 mg of the fraction smaller than 50 µm were placed in a 15-mL plastic tube. This powder was mixed with 300 µL of buffer solution and vortexed for a few seconds. After, 4 mL of SPT (ρ=2.86 g/mL) were added and the solution was vortexed for a few seconds, resulting in a density ρ=2.75 g/mL due to mixing of SPT with the buffer. The solution was placed in an ultrasonic bath for 10 min, vortexed for a few seconds, and then centrifuged for 20 min at 4000 rpm. The light fraction floating on SPT, which includes light minerals and organic components, was removed with a pipette. However, due to the high viscosity of SPT, it was not possible to remove completely the supernatant. Therefore, in order to preserve intact the heavy fraction, the tube was frozen with liquid N2, and the tip trimmed using a hose cutter. After melting at ambient conditions, the solution was transferred to a 2 mL centrifuge tube and rinsed 3 times in deionized water using a centrifuge for 3 min at 5000 rpm. The efficiency of the procedure for this particular sample is ca. 30%. Finally, 1 mL of 0.1 M NaOH were added for 5 min to remove humic acids. The solution was clear, and therefore the sample was rinsed 3 times in deionized water using a centrifuge for 3 min at 5000 rpm, and air dried. Light and heavy fractions were analyzed with FTIR and XRD to check their mineralogy. The same procedure was used with the experimental aragonite-rich ash and geogenic aragonite. SPT at higher density (ρ=2.90 g/mL) was used on the heavy fraction obtained from the first round of separation to extract hydroxylapatite, which is heavier than aragonite (3.10 g/mL).
Thermogravimetric Analysis (TGA)
Samples were placed in an alumina crucible and combusted in air atmosphere from room temperature to 1000°C, at a heating rate of 20°C per min, using a SDT Q600 V8.3 Build 101 thermal analyzer.
14C Dating
For the conventional carbonate dissolution method, 200 mg of the purified fraction were dissolved in phosphoric acid for 16 hr to evolve CO2. For the step combustion dissolution, 200 mg of the purified fraction were placed in a quartz ampule under vacuum, and combusted using an electric oven to 550, 600, 700, and 800°C to evolve CO2. Combustion steps lasted 1 hr, and for each step the evolved CO2 was collected as a separate sample. The CO2 released during heating up to 500°C was discarded to avoid possible contribution from residual organics. The different CO2 fractions from acid dissolution and step combustion were converted into graphite for accelerator mass spectrometry (AMS) following Yizhaq et al. (Reference Yizhaq, Mintz, Cohen, Khalaily, Weiner and Boaretto2005). Two charred olive pits recovered from the black layer were treated with a general ABA procedure and the resulting product was combusted to evolve CO2, which was converted to graphite for AMS using the same method (Yizhaq et al. Reference Yizhaq, Mintz, Cohen, Khalaily, Weiner and Boaretto2005). AMS measurements were performed at the D-REAMS laboratory, Weizmann Institute of Science. A marble sample from Ness Ziona (Israel) was used as background. As no CO2 was emitted at 550 and 600°C, the CO2 collected at 700°C was used as background for the 550, 600, and 700°C ash samples. The 800°C fraction was used for the corresponding 800°C samples. Marble dissolved in phosphoric acid was used as background for the non-combusted ash samples. In case of small sample size (below 0.5 mg), oxalic acid II samples of similar size were used for normalization. Small and large background samples were measured, and linear extrapolation was used in order to estimate the best background for each sample size.
RESULTS
Formation Processes of the Destruction Horizon
FTIR analysis of the white ashy sediment revealed the dominant presence of aragonite, calcite, and quartz (SiO2), together with minor amounts of hydroxylapatite [Ca5(PO4)3(OH)], presumably from bone, and burnt clay minerals (Figure 2). The occurrence of the latter suggests that the sediment was exposed to temperatures above 600°C (Forget et al. Reference Forget, Regev, Friesem and Shahack-Gross2015). This is in agreement with the phytolith assemblage of this layer, characterized by cereal inflorescence and leaf/stem morphotypes, many of which are melted due to heating at high temperature (Figure 3). The crystalline phases were analyzed using XRD, which confirmed that most of the bulk sediment is composed of aragonite and calcite, whereas quartz and hydroxylapatite occur in minor amounts. The fraction smaller than 50 µm contains mostly aragonite and a lesser amount of calcite compared to the bulk sediment (Figure 2 and Table 1).

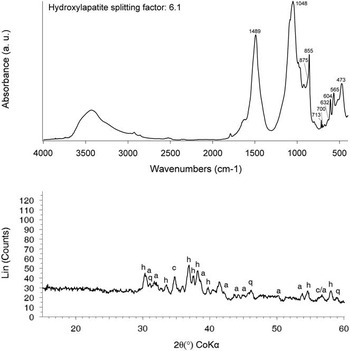
Figure 2 Mineralogical analyses. Top: FTIR spectrum of the white ashy layer (a. u.: arbitrary units). The absorption bands at 1494, 1083, 855, 713, and 700 cm–1 belong to aragonite. Calcite is represented by the absorption bands at 1420 (shoulder), 875 and 713 cm–1. The latter indicates that calcite is enriched in magnesium. Other major components are quartz (1083 shared with aragonite, 797 and 468 cm–1) and hydroxylapatite (604 and 565 cm–1). Note the absence of absorption bands of clay minerals in the 3700–3600 cm–1 region, which is caused by exposure to high temperature. Bottom: diffractograms of the bulk aragonite-rich sediment and the fraction smaller than 50 µm (a. u.: arbitrary units). The main components are quartz (q), aragonite (a), calcite (c), and hydroxylapatite (h). Note that the amount of calcite decreases in the smaller fraction.

Figure 3 Photomicrographs in plane polarized light of opaline phytoliths extracted from the white (aragonite-rich) layer. A: dendritic phytolith from the inflorescence of cereals. B: multicell composed of dendritic phytoliths, from the inflorescence of cereals. C: multicell composed of echinate long cell phytoliths from the inflorescence of cereals (note the irregular appearance caused by partial melting at high temperature). D: melted phytolith characterized by irregular shape and bubbles produced by the escape of water during exposure to high temperature.
Table 1 Quantitative XRD analysis of the white layer rich in aragonite (in wt% of the crystalline phases). Note that these results do not include disordered phases such as burnt clay minerals, opal from phytoliths and highly irregular hydroxylapatite crystals.

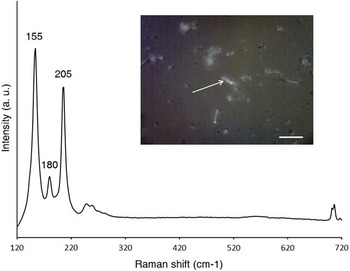
Mineral and organic components were observed within their original depositional context using micromorphology (Figure 4A). Thin sections showed a diffuse boundary between the white aragonite-rich layer and the black layer, and similar microstructure, indicating that they could be two portions of the same depositional unit (Figure 4B). The only major difference is color, which is black in the lower part due to the presence of charred components from incomplete combustion of plant material, and white in the upper part as a result of near-surface, oxidizing conditions that led to high-temperature burning. In the aragonite-rich portion (white layer), the mineral components are organized in intact, horizontally layered stringers of CaCO3 aggregates showing the characteristic texture of high-temperature ash and lime plaster (Figure 4C). These ash aggregates also exhibit the structure of plant tissues, which is represented by phytoliths. Such degree of preservation points to absence of trampling and quick burial. Ash aggregates are surrounded by a large number of needle-shaped CaCO3 crystals smaller than 50 µm (Figure 4D). These crystals were analyzed with µ-Raman spectrometry, confirming their identification as pure aragonite (Figure 5). Scanning electron microscope (SEM) images of the aragonite needles revealed that most of the crystals range between 1 and 10 µm in length and show intact facets, indicating good overall preservation (Figure 6A). Similar aragonite needles were observed in experimental ash and therefore we conclude that the aragonite phase in the white layer is pyrogenic (Figure 6B). All of these lines of evidence suggest that plant material, a mixture of chaff and straw, was laid on the surface of an open area—a practice observed elsewhere at the site (Regev et al. Reference Regev, Cabanes, Homsher, Kleiman, Weiner, Finkelstein and Shahack-Gross2015). This vegetal cover was burnt to high temperature during the destruction event. Plant calcium oxalate and geogenic calcite from local sediments transformed into CaO, which turned into aragonite upon carbonation as soon as the combustion temperature dropped below 100°C, towards the end of the conflagration. The ash layer was then buried by mudbrick debris, which favored the preservation of aragonite crystals. This finds support in the absence of sparitic CaCO3 crystals in thin section, suggesting that ash was not affected by dissolution and reprecipitation processes, and thus it is well preserved. This observation is in disagreement with the δ13C and δ18O values of the carbonate fraction, which fall outside the range of high-temperature ash as shown in Table 2 (Shahack-Gross and Ayalon Reference Shahack-Gross and Ayalon2013 and references therein). Considering that the site is located on top of a chalk hill, we conclude that the stable isotopes results are affected by the presence of geogenic calcite. This phase is devoid of 14C and therefore it is a contaminant for 14C dating (Figure 4C). Similarly, hydroxylapatite from bone mineral might be rich in geogenic carbonate groups due to diagenetic processes. Thus, aragonite must be separated from these phases in order to obtain accurate age determinations.

Figure 4 Micromorphology of sediments. A: photo of the micromorphology block before removal. A thin section was obtained from the area highlighted by the dashed rectangle (scale bar: 5 cm). B: scan of the thin section prepared from the portion of the block highlighted in (A), showing the microstratigraphy of the sample (scale bar: 1 cm). 1: burnt mudbrick collapse; 2: white (aragonite-rich) layer; 3: black layer. Note the diffuse boundary between the white and black layers. C: photomicrograph of the white layer showing the horizontal stringers of ash (PPL; scale bar: 1 mm). Note the presence of a large fragment of chalk in between stringers. D: photomicrograph of the white layer showing needle-shaped crystals of CaCO3 (XPL; scale bar: 100 µm).

Figure 5 Raman spectrum of a representative needle-shaped crystal from the white (aragonite-rich) layer (indicated by an arrow in the inset), showing Raman bands of aragonite at 155, 180, and 205 cm–1 (scale bar: 20 µm; a. u.: arbitrary units).

Figure 6 SEM images of archaeological and experimental samples. A: cluster of needle-shaped aragonite crystals from the white (aragonite-rich) layer (scale bar: 1 µm). Note that small, thin crystals are surrounded by larger ones with well-developed facets. B: experimental ash rich in clusters of needle-shaped crystals of pyrogenic aragonite (scale bar: 1 µm).
Table 2 Carbon and oxygen stable isotopes values of archaeological and control samples. Values for high-temperature carbonates are based on published data (Shahack-Gross and Ayalon Reference Shahack-Gross and Ayalon2013 and references therein).

Density Separation and 14C Dating
Aragonite needles were extracted from the ash fraction smaller than 50 µm through density separation of minerals with different specific gravity, using sodium polytungstate (SPT) as heavy liquid. By centrifuging the sample in buffered SPT at density ρ=2.75 g/mL and pH 7, most of the aragonite is concentrated in the heavy fraction, together with heavy minerals and heat-altered hydroxylapatite. FTIR and XRD show also the possible presence of a small amount of calcite, although the relative infrared absorptions and X-ray reflections are weak (Figure 7). After further separating hydroxylapatite from aragonite at 2.90 g/mL density, we ascertained with FTIR that the former is not carbonated due to exposure to fire, and therefore it cannot be a contaminant (Figure 8). We then proceeded to dissolution of the purified samples in phosphoric acid and graphitization of CO2 following Yizhaq et al. (Reference Yizhaq, Mintz, Cohen, Khalaily, Weiner and Boaretto2005). The 14C content of fractions with different specific gravity was measured by AMS. In addition, we measured two charred olive pits found together within the black layer. Measurements were evaluated against a published reference dataset of age determinations based on clusters of charred olive pits (Toffolo et al. Reference Toffolo, Arie, Martin, Boaretto and Finkelstein2014). Results are presented in Table 3.

Figure 7 Mineralogical analyses on purified fractions. Top: FTIR spectrum of the heavy fraction separated using SPT at 2.75 g/mL density (a. u.: arbitrary units). Dominant phases are pyrogenic aragonite (absorption bands at 1489, 855, 713, and 700 cm–1) and hydroxylapatite (1048, 632, 604, and 565 cm–1). The high splitting factor (6.1) and the presence of the 632 cm–1 absorption band indicate that hydroxylapatite was exposed to high temperature. Minor phases are quartz (473 cm–1) and possibly calcite (shoulder at 875 cm–1). Bottom: diffractogram of the heavy fraction separated using SPT at 2.75 g/mL density. Note the presence of aragonite (a), hydroxylapatite (h), quartz (q), and possibly calcite (c).

Figure 8 FTIR spectrum of the heavy fraction separated using SPT at 2.90 g/mL density (a. u.: arbitrary units). The dominant phase is hydroxylapatite. Note the absence of the carbonate ν3 absorption band in the 1490–1420 cm–1 region, indicating exposure to high temperature.
Table 3 14C dating results, showing laboratory number, preparation method, material, size, pMC, and 14C age. The last row lists published samples used as reference (Toffolo et al. Reference Toffolo, Arie, Martin, Boaretto and Finkelstein2014).

The robustness of the density separation method is supported by the measurement on experimental ash, which excludes contribution of dead carbon from SPT, and the measurement on 14C-dead geogenic aragonite, which shows no contamination from modern carbon due to sample preparation and the NaOH step. The bulk ash appears to be much older than the fraction smaller than 50 µm, likely because of the presence of coarse-grained calcite from weathered chalk. Both samples do not match the age of olive pits. The fraction lighter than 2.65 g/mL is largely outside the expected pMC range, as a result of the occurrence of geogenic calcite from local sediments. A similar situation characterizes the fraction lighter than 2.75 g/mL, although the presence of a larger amount of aragonite accounts for the higher pMC. The fractions heavier than 2.75 g/mL, where most of the aragonite is located, exhibit the closest pMC values compared to the reference samples, thus establishing a direct correlation between specific gravity and 14C age. These values are ca. 100 yr BP older than expected. This discrepancy might be caused by incomplete removal of calcite during SPT treatment, as hinted by FTIR and XRD. In fact, a contamination value as low as 1% could account for the observed age shift (Bowman Reference Bowman1990). Therefore, instead of dissolving the purified fraction in phosphoric acid, the sample was combusted under vacuum. It is known that the decomposition temperature of CaCO3 is related to the degree of atomic order of the crystal, with well-ordered crystals decomposing at higher temperature (Cuif et al. Reference Cuif, Dauphin, Berthet and Jegoudez2004; Stalport et al. Reference Stalport, Coll, Szopa, Person, Navarro-Gonzalez, Cabane, Ausset and Vaulay2007; Lindroos et al. Reference Lindroos, Regev, Oinonen, Ringbom and Heinemeier2012). This implies that pyrogenic aragonite, a disordered type of CaCO3, releases CO2 at lower temperature compared to geogenic calcite. This property was observed in the sample under study using TGA, which showed that the Tel Megiddo ash starts decomposing at 500°C, whereas local chalk releases CO2 only above 600°C (Figure 9). By heating the sample at different temperatures, a clear trend emerges from the pMC values (Figure 10). The CO2 evolved at 550°C, which belongs to the aragonite phase, matches the pMC values of charred olive pits and thus falls within the expected range of the destruction horizon, whereas the CO2 evolved at higher temperature shows progressively lower pMC values, due to the contribution of geogenic calcite. A similar pattern was observed for the fraction lighter than 2.75 g/mL, which contains a small, yet significant amount of aragonite.

Figure 9 TGA plots of bedrock chalk from Tel Megiddo (A) and the aragonite-rich layer (B). Note that the latter starts decomposing at lower temperature compared to chalk.

Figure 10 pMC values for the archaeological samples treated with acid dissolution and step combustion (sample numbers are listed in Table 3). The shaded bar indicates the expected pMC range of the destruction horizon based on measurements carried out on charred seeds from the black layer and from other locations at Tel Megiddo (Toffolo et al. Reference Toffolo, Arie, Martin, Boaretto and Finkelstein2014). Note that the only samples that fall within this range are those produced by combusting purified aragonite-rich ash at 550°C.
DISCUSSION
The 14C dating results demonstrate that pyrogenic aragonite is a closed carbon system that records the 14C signature of the atmosphere in the moment it nucleates, shortly after a firing event. Even though aragonite is thermodynamically unstable at ambient temperatures and pressures, meaning that it will eventually recrystallize into calcite with possible isotopic exchange (Lippmann Reference Lippmann1973), this condition largely depends on environmental factors such as presence of water and low pH. In fact, aragonite can survive intact for a long time, as in the case of the nautiloid shells from the Buckhorn asphalt quarry, dated to ca. 300 million yr ago (Seuss et al. Reference Seuss, Titshack, Seifert, Neubauer and Nützel2012), and the crystals embedded in chondrite meteorites from 4.6 billion yr ago (Lee and Lindgren Reference Lee and Lindgren2015). In both instances, the absence of water left aragonite essentially pristine.
Similar settings must have determined the preservation of aragonite at Tel Megiddo, where it provided accurate age determinations as seen from pMC values. Tel Megiddo is located within the Mediterranean climate zone and receives an amount of precipitations comparable to southern and western Europe (e.g. Mehta and Yang Reference Mehta and Yang2008). Considering that the aragonite layer is located about two meters below the topsoil (Figure 1B), we infer that the thick mudbrick collapse has favored the preservation of aragonite from rainwater seeping through the section. The large amount of clay in this layer is especially important, as more porous sandy sediments might lead to in-depth penetration of rainwater and movement of groundwater. Therefore, we conclude that aragonite could occur also in temperate zones elsewhere around the world, provided that it is not close to the topsoil and embedded in sandy sediments at open-air sites.
Accurate 14C dating of pyrogenic aragonite was accomplished through a careful characterization of the depositional context, including bulk analyses (FTIR, XRD, phytoliths) and descriptions of intact portions of sediment (micromorphology) to reconstruct the formation processes of the destruction horizon, understand the preservation status of sediments, and verify the occurrence of contaminants. In addition, µ-Raman provided the first spectroscopic characterization of single pyrogenic aragonite needles in archaeological sediments. Thus, the link between experimental and archaeological samples suggested in a previous study is now confirmed (Toffolo and Boaretto Reference Toffolo and Boaretto2014). With this approach, we were able to locate and target one specific carbonate compound for 14C dating. Currently, this is not the rule in 14C dating of anthropogenic carbonates such as lime plasters and mortars, as usually a mixture of different carbonate phases provides the CO2 for AMS measurements, thus leading to contradicting results in several cases (Lindroos et al. Reference Lindroos, Heinemeier, Ringbom, Braskén and Sveinbjörndóttir2007; Boaretto and Poduska Reference Boaretto and Poduska2013; Ringbom et al. Reference Ringbom, Lindroos, Heinemeier and Sonck-Koota2014). The target compound was extracted from the bulk sediment using density separation in a heavy liquid, an established method in geochemistry and 14C dating of shell carbonates (Douka et al. Reference Douka, Hedges and Higham2010). We do note, however, that in the case of pyrogenic aragonite the needles are characterized by a high ratio of surface area to volume, and therefore dissolve almost immediately in high-density SPT, which is at pH 3. This would be negligible if the sample was 100% CaCO3 as in shells, but it is a major shortcoming in the case of heterogeneous sediments such as ash. This problem was overcome by adding a buffer based on a mixture of different sodium phosphates that shifts the pH of SPT to 7. AMS measurements on fractions with different densities clearly showed that calcite is a major source of contamination due to its geologic origin as a weathering product of chalk, whereas aragonite-rich fractions exhibit younger 14C ages. The fact that the latter are 100 yr BP off compared to the expected age of the destruction horizon points to incomplete removal of calcite. This calcite phase is presumably attached to heavier aragonite crystals, thus resulting in a “composite” specific gravity that falls within the range of aragonite. For this reason, dissolution in phosphoric acid was replaced with step combustion under vacuum, which should also overcome the contribution of carbonate hydroxylapatite from bones (Stiner et al. Reference Stiner, Kuhn, Weiner and Bar-Yosef1995). A direct correlation between temperature and 14C age emerges from AMS measurements, in both the heavy and light fractions of the ash. This is the result of decomposition of aragonite at 550°C, followed by the decomposition of a progressively larger amount of geogenic calcite at higher temperatures, which “dilutes” the 14C content of aragonite. Considering that most of the carbonates in the purified fraction are aragonite crystals, it seems that part of such crystals decompose above 550°C together with calcite. This might be due to a higher degree of atomic order within the crystal lattice, as in the case of needles larger than 20 µm observed with SEM, which presumably reached their size through a relatively slow and steady growth process.
Our results show that pyrogenic aragonite is a new short-lived material suitable for 14C dating. The occurrence of pyrogenic aragonite has been recently documented in several combustion features and lime plasters found at archaeological sites of different age located in Israel (Toffolo and Boaretto Reference Toffolo and Boaretto2014: Table 1). This limited geographic distribution is due to the fact that pyrogenic aragonite is best identified using FTIR, but this method has been used sparingly in other parts of the world (Weiner Reference Weiner2010). Given the presence of archaeological pyrogenic aragonite in different sedimentary contexts and chronological periods spanning the last 30,000 yr, we believe that its occurrence could be much more widespread than thought. For this reason, the dating method presented here could find extensive applications, regardless of geographic location and chronological period, up to the limit of 14C. Once aragonite has been identified, it is necessary to exclude possible sources of contamination. The main ones are geogenic calcite and diagenetic carbonates, such as carbonate hydroxylapatite and reprecipitated calcite. The possible presence of secondary calcite does not exclude the presence of aragonite. This is because diagenesis does not affect sediments homogeneously, and pockets of pristine aragonite may survive next to areas rich in secondary calcite within the same layer of sediment. This is a common situation in caves (e.g. Karkanas et al. Reference Karkanas, Bar-Yosef, Goldberg and Weiner2000; Weiner et al. Reference Weiner, Goldberg and Bar-Yosef2002). As a general rule of thumb, one should keep in mind that each ash deposit is characterized by a unique mineral assemblage, and therefore SPT density should be adjusted case by case to identify the fraction most suitable for step combustion. This in turn requires a thorough understanding of formation processes. Using this approach, a number of common archaeological features and materials involving the use of fire could be accurately dated, provided that aragonite is preserved: destruction horizons, hearths, cooking installations, lime kilns, furnaces, metal workshops, limestone-tempered pottery, lime plasters, and mortars. These features and artifacts are directly related to short-lived human activities, and thus could significantly contribute to the establishment of accurate 14C chronologies together with organic materials, or in their absence. Furthermore, pyrogenic aragonite could be a viable alternative to the current 14C dating method applied to lime mortars, which showed its limits due to the often altered isotopic composition of calcite and the inability to target one specific carbonate compound (Boaretto and Poduska Reference Boaretto and Poduska2013).
Finally, we wish to note that also high-temperature calcite might preserve the original 14C signature of the atmosphere, similar to pyrogenic aragonite. In the cases where pyrogenic aragonite does not nucleate after fire due to adverse environmental conditions, high-temperature calcite could be used for 14C dating. This calcite fraction is extremely disordered at the atomic level and thus it is lighter than diagenetic or geogenic calcite, which might occur within the same layer of sediment. Therefore, it can be extracted by density separation using the same procedure developed for aragonite. This would extend the application of the method presented herein to several other anthropogenic carbonate materials in which aragonite is not present.
Acknowledgments
This research was funded by the Alexander von Humboldt Foundation through postdoctoral fellowship to Michael Toffolo, which included a Europe Research Stay at the Weizmann Institute of Science. Part of the FTIR measurements were performed on instrumentation funded by a grant from the Deutsche Forschungsgemeinschaft (MI 1748/1-1). Sample preparation for 14C dating was funded by the Exilarch’s Foundation for the Dangoor Research Accelerator Mass Spectrometer and by the Max Planck-Weizmann Center for Integrative Archaeology and Anthropology. Micromorphological analysis was conducted by RSG at the Kimmel Center for Archaeological Science, Weizmann Institute of Science. The authors are grateful to Israel Finkelstein and Mario Martin, co-directors of the Megiddo Expedition, for granting access to the site, and to Heinrich Taubald for performing stable isotopes analysis. CB wishes to thank Frieder Lauxmann for performing µ-Raman measurements. MBT wishes to thank Francesca Strappini for her continued support during laboratory analyses and writing, Steve Weiner and Yotam Asscher for useful discussions, and Ilit Cohen-Ofri for assistance during TGA analysis.