INTRODUCTION
Understanding variation in the infection levels of parasites in hosts through time, relative to biotic and abiotic factors, has become a critical issue due to the potential effect of Global Climate Change (GCC) (Benedetti-Cecchi et al. Reference Benedetti-Cecchi, Acunto, Bulleri and Cinelli2000; Zander, Reference Zander2003, Reference Zander2004, Reference Zander2005; Abell et al. Reference Abell, Gadeka, Pearceb and Congdon2006). There is no clear proof that climate change will increase climate variability and there are also some disputes on the increase of extreme climate events. However, there is evidence that climate variability (such as El Niño Southern Oscillation, ENSO) (Oliva et al. Reference Oliva, Barrios, Thatje and Laudien2007; Soniat et al. Reference Soniat, Hofmann, Klinck and Powell2009), extreme weather events (Easterling et al. Reference Easterling, Evans, Groisman, Kunkel and Ambenje2000; Martínez and Merino, Reference Martinez and Merino2011) (such as hurricanes) (Lafferty, Reference Lafferty2009; Aguirre-Macedo et al. Reference Aguirre-Macedo, Vidal-Martínez and Lafferty2011), and seasonality (Kennedy, Reference Kennedy1993; Simková, Reference Simková2005; Kerans et al. Reference Kerans, Stevens and Lemmon2005; Poulin and Mouritsen, Reference Poulin and Mouritsen2006; Krasnov et al. Reference Krasnov, Korallo-Vinarskaya, Vinarski, Shenbrot, Moullot and Poulin2008; Luque and Poulin, Reference Luque and Poulin2008; Knipes and Janovy, Reference Knipes and Janovy2009) can influence the life cycles of human and animal parasites in both terrestrial and aquatic environments producing phenological or physiological as well as distributional changes (Githeko et al. Reference Githeko, Lindsay, Confalonieri and Patz2000; Parmesan and Galbraith, Reference Parmesan and Galbraith2004; Patz et al. Reference Patz, Campbell-Lendrum, Holloway and Foley2005; Poulin and Mouritsen, Reference Poulin and Mouritsen2006; Marcogliese, Reference Marcogliese2008; Mas-Coma et al. Reference Mas-Coma, Valero and Bargues2009; Ujvari et al. Reference Ujvari, Andersson, Brown, Shine and Madsen2010).
For temperate latitudes, the majority of studies on temporal variation of parasites in aquatic hosts has been undertaken over the short term or annually, due to seasonality of temperature being a key variable causing fluctuations ranging from annual cycles (Kennedy, Reference Kennedy1993; Simková, Reference Simková2005; Kerans et al. Reference Kerans, Stevens and Lemmon2005; Poulin and Mouritsen, Reference Poulin and Mouritsen2006; Krasnov et al. Reference Krasnov, Korallo-Vinarskaya, Vinarski, Shenbrot, Moullot and Poulin2008; Luque and Poulin, Reference Luque and Poulin2008; Knipes and Janovy, Reference Knipes and Janovy2009). For notifiable human diseases, few long-term studies have shown the lack of temporal variability associated to large-scale weather changes as described by the North Atlantic Oscillation (NAO) (Húbalek et al. Reference Hubálek, Halouzka and Juricová2003; Húbalek, Reference Jiménez-García and Vidal-Martínez2005).
For tropical latitudes, most studies on the temporal variability of the infection parameters of parasites of aquatic organisms have been carried out over the short term (e.g. Leong, Reference Leong1986; Coley and Aide, Reference Coley, Aide, Price, Lewinshom, Fernades and Benson1991; Fiorillo and Font, Reference Fiorillo and Font1999; Steinauer and Font, Reference Steinauer and Font2003; Vincent and Font, Reference Vincent and Font2003; Martin et al. Reference Martin, Pless, Svoboda and Wikelski2004; Violante-Gonzalez et al. Reference Violante-González, Aguirre-Macedo and Vidal-Martínez2008). A further complication in the tropics for the study of the temporal dynamics of the infection parameters of aquatic parasites is its climate variability due to the presence of phenomena that occur at time scales longer than a year such as El Niño 3–5 years (Ghil, Reference Ghil, MacCracken and Perry2002), and hence long-term observations are necessary. Recently, Pech et al. (Reference Pech, Aguirre-Macedo, Lewis and Vidal-Martínez2010) and Aguirre-Macedo et al. (Reference Aguirre-Macedo, Vidal-Martínez and Lafferty2011) have shown over a relatively long term of 9 years that rainfall and hurricanes rather than temperature are key variables in driving the proportion of snails and fish hosts infected in the Peninsula of Yucatan (tropical Mexico). In both cases, these authors combined the data of several trematode species into a single index called the ‘percentage of infected hosts’ (PIH). This strategy is useful to address general patterns of infection over the long term, but without determining the effect of rainfall or other environmental variables on the infrapopulations of specific parasite species. Apart from the work of Jiménez-García and Vidal-Martínez (Reference Jiménez-García and Vidal-Martínez2005) on the larval digenean Oligogonotylus manteri, there is a lack of information on parasite infrapopulations in aquatic hosts in tropical latitudes.
Therefore in the present investigation, the infrapopulations of the daniconematid (dracunculoid) nematode Mexiconema cichlasomae were analysed in both the definitive fish (Cichlasoma urophthalmus) and intermediate crustacean (Argulus yucatanus) hosts from a coastal lagoon in south-eastern Mexico. The effects of environmental factors on the temporal dynamics of M. cichlasomae in natural populations of its fish and crustacean host would clearly contribute to our understanding of how parasites might respond to extreme environmental changes in the light of GCC. Therefore, the aim of the present study was to determine the potential influence of rainfall or/and temperature from 2003 to 2010 on long-term fluctuations of the prevalence and mean abundance of M. cichlasomae in both its intermediate and definitive hosts in Celestun, a tropical coastal lagoon from Yucatan, south-eastern Mexico.
MATERIALS AND METHODS
Study site and sampling procedures
Celestun is a karstic tropical lagoon located at the northwest corner of the Yucatan Peninsula (20°52′47.50″N; 90°21′13.10″O) characterized by the input of freshwater from groundwater discharges that vary according to the rainfall regime (Herrera-Silveira et al. Reference Herrera-Silveira, Martín and Díaz-Arce1999). Celestun coastal lagoon has been described as a dynamic ecosystem (Herrera-Silveira et al. Reference Herrera-Silveira, Martín and Díaz-Arce1999), demonstrating natural significant intra-annual variability in its water column characteristics, which are due to seasonal rainfall, winds and temperature regimes prevailing in the zone (Pech et al. Reference Pech, Ardisson and Hernández-Guevara2007). Although the regional climatic conditions are not always well defined in the study area, 3 seasons occur namely a dry season with low precipitation levels (March to May, 0–50 mm and 14 °C–38 °C), a rainy season (June to October >500 mm and 21 °C–35 °C) and a winter frontal storm ‘Nortes’ season (November to February, 20–60 mm). The latter is characterized by relatively strong winds (>60 km/h), marked and rapid decreases in air temperature (31 °C–8 °C) and a small amount (10–60 mm), of cool (9 °C–18 °C) rainfall (Hernández-Guevara et al. Reference Hernández-Guevara, Pech and Ardisson2008).
Biological aspects of the host-parasite system under study
Cichlasoma urophthalmus is an euryhaline species, abundant in freshwater canals and brackish waters of the Atlantic watersheds from the Rio Coatzacoalcos basin southward through Mexico, including the Yucatan Peninsula up to Nicaragua (Martínez-Palacios and Ross, Reference Martinez-Palacios and Ross1992; Greenfield and Thomerson, Reference Greenfield and Thomerson1997; Trexler et al. Reference Trexler, Loftus, Jordan, Lorenz, Chick and Kobza2000). This fish species survives in a wider range of temperature (24 °C–38 °C) and salinities (4–40·3 ppt) (Martinez-Palacios, Reference Martínez-Palacios1987). They are primarily carnivorous, feeding mainly on invertebrate (i.e. microcrustacea, mollusks, isopods and polychaetes) (Martínez-Palacios and Ross, Reference Martínez-Palacios and Ross1988; Miller et al. Reference Miller, Winckley and Norris2005). Breeding occurs throughout the year, with a reproductive peak between May and September (rainy season). The maximum age reached by C. urophthalmus is 7 years old (Faunce et al. Reference Faunce, Patterson and Lorenz2002). Argulus yucatanus is a crustacean ectoparasite infecting C. urophthalmus in coastal lagoons along the Peninsula of Yucatan. This ectoparasite has a proboscis to penetrate the host's skin, feeding on blood (Avenant-Oldewage et al. Reference Avenant-Oldewage, Swanepoel and Knight1994). Argulus yucatanus is known to be a vector of the nematode larvae of Mexiconema cichlasomae (Moravec et al. 1999).
This parasite is a dracunculoid (Family Daniconematidae). The nematodes are genetically related to Skrjabillanids (Mejia-Madrid and Aguirre-Macedo, Reference Mejia-Madrid and Aguirre-Macedo2011) and used as intermediate host to A. yucatanus. In the fish M. cichlasomae develop into mature male and female, the gravid females are approximately 2–3 times as long as male and produce thousands of larvae (Moravec et al. 1992). However, in spite of this information there are many aspects of their life cycle still unknown, such as recognition of their different stages of maturation in both hosts and the parasite´s larval morphogenesis.
Sampling procedure
Specimens of C. urophthalmus were collected by hook and line in the middle zone of Celestun lagoon (20°52′46.68″N; 90°21′15.4″O), on a monthly basis between March 2003 and December 2010 with a sample size of 15 fish per month. Three factors were considered when determining the sample size for detecting infections with M. cichlasomae and Argulus yucatanus. First, the prevalence was based on that of 43% by of M. cichlasomae Moravec et al. (Reference Moravec, Vidal-Martínez and Salgado-Maldonado1992) and 63% by Salgado-Maldonado and Kennedy (Reference Salgado-Maldonado and Kennedy1997). The other two factors included the accepted level of risk, α = 0·05 in this case, and the sensitivity of stereomicroscopy as a method for parasite identification. We assumed that such a diagnostic method has a sensitivity of 75% due to human error. Thus, assuming a Poisson distribution for the probability of identifying M. cichlasomae, the monthly sample size was obtained using the formula n = 4/prev, where n is the fish sample size, the number 4 originated from –Ln (α = 0·05*sensitivity of the diagnostic method) and prev is the prevalence in the fish population (des Clers, Reference Des Clers1994). In the case of larvae of M. cichlasomae in A. yucatanus due to low prevalence values (1·29% for n = 155 A. yucatanus; Moravec et al. Reference Moravec, Vidal-Martinez and Aguirre-Macedo1999), a statistically representative sample size with this method cannot be guaranteed. Nevertheless, we decided to present the data on these larvae to further our understanding of the biology of M. cichlasomae.
For the first 6 years of study of this host–parasite system, each month the fish were captured and taken together in a 200 L tank with lagoon water to the laboratory. Once there, the body surface of each fish was examined under a stereomicroscope especially for A. yucatanus. Certainly, this method can present some error to represent statistically the prevalence of this ectoparasite due to the detachment from the fish. Thus, in the last 2 years at the very moment of a capture, each fish is placed in an individual lagoon water-filled container and its external surface immediately examined. Argulus yucatanus were collected with the aid of forceps and allocated in the same container, numbered and transported to the laboratory with lagoon water. We did not find significant differences between the old and new techniques for prevalence (G-test; G = 2·74 P > 0·05) or mean abundance (W = 275·50 P > 0·05). Thus, we concluded that the data from both the ‘old’ and ‘new’ methods can be reliably compared.
In the laboratory each specimen (A. yucatanus) was transferred to a slide with a drop of lagoon water and a coverslip, for examination under a compound microscope (10X). Following a post-mortem examination of each fish the body cavity, mesenteries, swim bladder and kidney were examined thoroughly as these are the preferred microhabitats of M. cichlasomae (Vidal-Martínez et al. 2001). Each organ was compressed between two glass slides of 15 × 15 cm (0·5 cm thick) for observation under the stereomicroscope. Each nematode was placed in a Petri dish containing 0·7% saline solution and later fixed in 4% formalin at 80 °C (see Vidal-Martínez et al. Reference Vidal-Martínez, Scholz, Aguirre-Macedo, Gonzalez-Solis and Mendoza-Franco2001). The recognition of each stage of maturity of M. cichlasomae was based on previous work by Moravec et al. (Reference Moravec, Vidal-Martínez and Salgado-Maldonado1992), Moravec (Reference Moravec1994), and Caspeta-Mandujano and Monjica (Reference Caspeta-Mandujano and Mejía-Mojica2004).
Additionally we obtained the water temperature (°C) monthly data from 2003 to 2010 ourselves, directly from site, with the aid of multiparameter Ysi model 85. The monthly rainfall data were obtained from the Climatologic Station (CELYC) of CNA (National Water Commission at Celestún, Yucatán, México), localized to 20°51′29″N; 90°22′59″O approximately to 2 km of the study site.
Data analysis
The prevalence and mean abundance, referred to as infection parameters, of M. cichlasomae in both the definitive and intermediate hosts were calculated according to the method of Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997). A single spectral analysis by Fourier series (Legendre and Legendre, Reference Legendre and Legendre1998) was used to extract variability patterns and periodical cycles of the infection parameters of M. cichlasomae in both hosts, together with monthly cycles of rainfall and temperature, (Statistica v. 6 Statsoft©). This analysis requires data points equally spaced in time (Press et al. Reference Press, Teukolsky, Vatterling and Flannery1996), and our data sets fulfilled this requirement. Each temporal data set was transformed into sine curves of the same amplitude or harmonic frequencies of different amplitudes and phases that collectively smooth the original time series (Scharlemann et al. Reference Scharlemann, Benz, Hay, Purse, Tatem, Wint and Rogers2008) and were represented in a periodogram. Here harmonic frequencies, measured as spectral densities (strength of the frequency signal), represent a temporal scale of maximum variability in the temporal distribution (Platt and Denman, Reference Platt and Denman1975). Any marked frequency peaks were interpreted as temporal scales of maximum variability to show trends of infection and environmental variables (temperature and rainfall).
Spectral density values of parasite infection parameters and environmental variables were compared using cross-correlation coefficients. Cross-correlation quantifies temporal associations between variables and provides a measure of the similarity between 2 different data sets at different time lags and determines the extent to which data sets exhibit correlated periodic variations. Time lags refer to delayed responses of dependent variables following fluctuations in independent variables (Olden and Neff, Reference Olden and Neff2001). The time lag with the highest correlation coefficient is taken as the accurate time lag between the 2 time series (Wei, Reference Wei1990), and the cross-correlation coefficients were calculated for a significance of P < 0·05 (Thiel et al. Reference Thiel, Romano, Schwarz, Kurths and Timmer2004).
RESULTS
A total of 825 specimens of C. urophthalmus, based on monthly samples of 15 fish from the Celestun coastal lagoon, were collected between March 2003 and December 2010, except in August 2007, when sampling was not possible due to the hurricane Dean.
Rainfall and temperature showed marked temporal fluctuations throughout the study period (Fig. 1A). The water temperature data showed 3 peaks every 12, 21 and 41 months (Fig. 1C) with the minimum at 25·2 °C and maximum at 29·2 °C and high frequency peaks of rainfall occurred every 12 months (Fig. 1D). Data on temperature and rainfall from Fig. 1A–D are also repeated in Figs 2A–D and 3A–D to provide an overall effect of these two environmental factors on changes in the infection parameters of both M. cichlasomae and A. yucatanus.

Fig. 1. Argulus yucatanus ectoparasite from the fish Cichlasoma urophthalmus in a Celestun (México) coastal lagoon from 2003 to 2010. (A) Shows temporal fluctuations in the infection parameters. Temperature (°C) and rainfall (mm) of Celestún Yucatan. (B) The prevalence (black line), mean abundance ± S.D. (dotted line) of Argulus yucatanus in Cichlasoma urophthalmus. (C) Spectral density of the prevalence of A. yucatanus (black line) and temperature (°C) (dotted line). (D) Spectral density of the mean abundance of A. yucatanus (black line) and rainfall (mm) (dotted line) by Fourier series. (E) Cross-correlations between the prevalence and mean abundance of A. yucatanus relative to temperature (°C), rainfall (mm).

Fig. 2. Mexiconema cichlasomae larvae from ectoparasite Argulus yucatanus in a Celestun (México) coastal lagoon from 2003 to 2010 showing temporal fluctuations in the infection parameters. (A) Temperature (°C) and rainfall (mm) of Celestun Yucatan. (B) The prevalence (black line), mean abundance ± S.D. (dotted line) of Mexiconema cichlasomae larvae in Argulus yucatanus. (C) Spectral density of prevalence M. cichlasomae larvae (black line) and temperature (°C) (dotted line). (D) Spectral density of mean abundance of M. cichlasomae larvae (black line) and rainfall (mm) (dotted line) by Fourier series. (E) Cross-correlations between the prevalence and mean abundance of M. cichlasomae larvae relative to temperature (°C), rainfall (mm).

Fig. 3. Dracunculoid nematode Mexiconema cichlasomae from the fish Cichlasoma urophthalmus in a Celestun (México) coastal lagoon from 2003 to 2010 showing temporal fluctuations in the infection parameters. (A) Temperature (°C) and rainfall (mm) of Celestun Yucatan. (B) The prevalence (black line), mean abundance ± S.D. (dotted line) of Mexiconema cichlasomae in Cichlasoma urophthalmus. (C) Spectral density of the prevalence of M. cichlasomae (black line) and temperature (°C) (dotted line) (D) spectral density of the mean abundance of M. cichlasomae (black line) and rainfall (mm) (dotted line) by Fourier series. (E) Cross-correlations between the prevalence and mean abundance of M. cichlasomae in C. urophthalmus relative to temperature (°C), rainfall (mm).
Argulus yucatanus in Cichlasoma urophthalmus
Argulus yucatanus was found to infect C. urophthalmus between 2003 and 2010 (94 sampling temporal points) with prevalence and mean abundance values from 6 to 100%, and 4·12 ± 2·84 respectively (Fig. 1B).
The spectral analysis of prevalence and mean abundance showed 2 peaks of maximum variability occurring every 12 and 45 months (Fig. 1C, D). Significant cross-correlation coefficients between prevalence or mean abundance of A. yucatanus and rainfall or temperature were observed at lag 0, suggesting an immediate response to infection to changes in these environmental variables. Additionally, significant cross-correlation coefficients between prevalence, abundance and rainfall were observed at lag (6) (1 lag = 1 month), suggesting a delay in response to infection (Fig. 1E).
Mexiconema cichlasomae larvae in Argulus yucatanus
The prevalence of larval M. cichlasomae fluctuated between 0 to 100% and the average number of larvae was 0·13 ± 0·36 throughout the entire study (Fig. 2B). The periodograms of prevalence and mean abundance showed peaks of maximum variability every 12 and 40 months (Fig. 2C, D). From the cross-correlation analysis, the prevalence, mean abundance and rainfall correlated significantly at lag 1 and lag 6, whereas the prevalence and mean abundance correlated significantly with temperature at lag 1 and lag 2 (Fig. 2E).
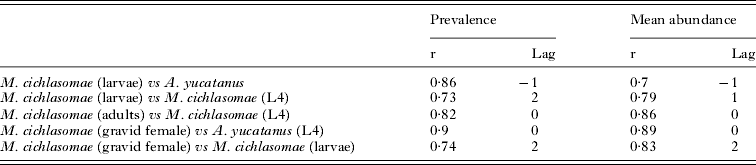
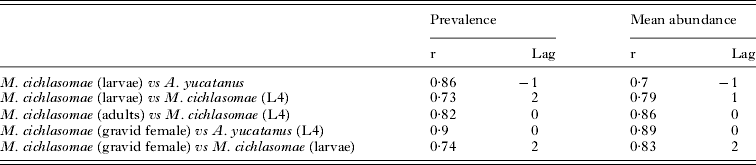
In addition, cross-correlation analyses between the infection parameters of M. cichlasomae and those of A. yucatanus in C. urophthalmus showed significant negative correlations (Table 1). Positive cross-correlations were observed between the mean abundance of both larval and adult nematodes in C. urophthalmus (Table 1).
Table 1. Cross-correlations between the prevalence and mean abundance of Mexiconema cichlasomae including L1 and L4 larvae, adult worms (male and female), gravid females and Argulus yucatanus
Mexiconema cichlasomae in Cichlasoma urophthalmus
Adult nematodes of M. cichlasomae (males, mature females, gravid females) and L4 larvae were present throughout the entire sampling period, with prevalences ranging from 20 to 100% and a mean abundance of 3·74 ± 4·40 (Fig. 3B). Spectral analysis of prevalences showed very similar patterns for all developmental stages, with peaks of maximum variability for prevalence and mean abundance occurring every 24–27 months (Fig. 3C), and 12 and 24 months respectively (Fig. 3D). Cross-correlation analyses between the prevalence and mean abundance of M. cichlasomae and rainfall showed significant associations at lags 4 and 6 (Fig. 3E). Similar significant results were found between the prevalence, mean abundance of M. cichlasomae and the temperature at lags 2 and 4 (Fig. 3E). The spectral density analysis of prevalence and mean abundance of M. cichlasomae L4 showed similar peaks of maximum variability to those of adults every for 10 and 27 months (data not included). The prevalence and mean abundance of both adults and L4 showed significant cross-correlations at lag 0 (Table 1). Furthermore, significant associations were observed between the prevalence and mean abundance of gravid females and larvae of M. cichlasomae in A. yucatanus at lag 2 respectively (Table 1).
DISCUSSION
The present results showed significant annual peaks of both prevalence and mean abundance of M. cichlasomae in A. yucatanus and bi-annual peaks of maximum variability of M. cichlasomae in the definitive fish host associated with temporal patterns of rainfall and temperature, despite slight asynchronies in the transmission peaks of some stages in the life cycle of M. cichlasomae.
The maximum variability of both prevalence and abundance of A. yucatanus in C. urophthalmus were observed every 12 and 45 months. The first peak correlated with seasonal fluctuations in both rainfall and temperature at lag 0 suggesting that both environmental factors (rainfall and temperature) were acting simultaneously to trigger the infection levels of A. yucatanus in C. urophthalmus. This result is in contrast to that reported by Pech et al. (Reference Pech, Aguirre-Macedo, Lewis and Vidal-Martínez2010) who suggested that rainfall was the single key environmental factor affecting the percentage of infected hosts (snails and C. urophthalmus) at Celestun. It is clear that the effects of both environmental factors were evident either immediately (lag 0) or with delay (lag 6), reflecting periods of increases in both temperature and rainfall (the rainy season) or only in rainfall (the winter frontal storm).
A peak every 45 months or approximately 4 years appears to be a consequence of the processes acting at a larger temporal scale, suggesting the influence of natural disturbances such as El Niño Southern Oscillation (ENSO), which occurs every 3–7 years (Stenseth et al. Reference Stenseth, Ottersen, Hurrell, Mysterud, Lima, Chan, Yoccoz and Ådlandsvik2003). This climatic phenomenon, which produces strong rainfall in both the Atlantic and Pacific coasts in the intertropical region (Stenseth et al. Reference Stenseth, Ottersen, Hurrell, Mysterud, Lima, Chan, Yoccoz and Ådlandsvik2003), is likely to be a key environmental variable in this host-parasite system at relative intermediate temporal scales. Similar interpretations have been made on the occurrence of disease outbreaks in humans (e.g. dengue, malaria) and aquatic animals such as Perkinsus marinus in oysters to ENSO (Hay et al. Reference Hay, Myers, Burke, Vaughn, Endy, Ananda, Shanks, Snow and Rogers2000; Húbalek, Reference Hubálek2005; Oliva et al. Reference Oliva, Barrios, Thatje and Laudien2007; Soniat et al. Reference Soniat, Hofmann, Klinck and Powell2009; Lafferty Reference Lafferty2009, Colón-Gonzalez et al. Reference Colón-González, Lake and Bentham2011).
The infection parameters of M. cichlasomae larvae in A. yucatanus followed a similar pattern to those of A. yucatanus in C. urophthalmus, with peaks of maximum variability every 12 and 40 months. The peak at 12 months is not surprising since A. yucatanus is the intermediate host of M. cichlasomae (Moravec et al. Reference Moravec, Vidal-Martinez and Aguirre-Macedo1999). The influence of rainfall and temperature on A. yucatanus also explains the significant cross-correlations found at lags 0, and 6, whereas the influence of ENSO explains the peak at 40 months.
The presence of larval M. cichlasomae in A. yucatanus is likely to be linked with the infection of C. urophthalmus with gravid females. In the present study we found a synchronic occurrence between the prevalence of gravid females of M. cichlasomae and both the prevalence and mean abundance of A. yucatanus in C. urophthalmus. This indicates that sufficient intermediate hosts had become infected with the larvae of M. cichlasomae, whereas the presence of gravid females throughout the year explains lags of up to 2 months in the occurrence of gravid females in the fish host and the maximum prevalence and mean abundance larval M. cichlasomae in A. yucatanus. This suggests a significant association between maturation of gravid females and the number of A. yucatanus. Such synchronicity is similar to that in other species in the Daniconematidae family, where the presence of gravid female nematodes occurs at the same time as the number of free-living copepod intermediate hosts increases (Moravec et al. Reference Moravec, Vidal-Martínez and Salgado-Maldonado1992).
A time lag of 2 months could be related to the time of transmission of larvae from the definitive cichlid fish host to A. yucatanus the intermediate host. Two possible methods of transmission could occur. First, May-Tec (Reference May-Tec2007), who found larvae of M. cichlasomae in the blood of C. urophthalmus, has suggested that species of Argulus become infected through ingestion of contaminated blood. Hence differences in the time lag between the presence of larvae in A. yucatanus and gravid females of M. cichlasomae may be attributed to the delay in the time taken for larvae of M. cichlasomae to migrate from the swim bladder to the peripheral blood of the cichlid fish host. Argulus yucatanus could therefore ingest the larvae during the course of a bloodmeal. This feeding behaviour is characteristic of life cycles in other nematode species of the family Skrjabillanidae, including Molnaria intestinalis and Skrjabillanus scardinii which use Argulus sp. as intermediate hosts (Tikhomirova, Reference Tikhomirova1980; Molnar and Székely, Reference Molnár and Székely1998; Smith et al. Reference Smith, Wootten and Sommerville2007).
A second possible scenario is that A. yucatanus containing larvae of M. cichlasomae could be ingested by C. urophthalmus during a symbiotic process of removing or ‘cleaning’ Argulus from its skin surface. Such ingestion of infected A. yucatanus could be higher during the breeding season of C. urophthalmus due to aggregation. This behaviour may also explain the low prevalence of infection of M. cichlasomae in A. yucatanus on the surface of C. urophthalmus, but further experimental approaches are needed to support such a transmission process.
Peaks of maximum variability every 12 and 24–27 months are more likely to be due to the accumulation of nematodes throughout time, as transmission occurs throughout the year. This accumulation can be related with the age of C. urophthalmus, which has a life span up to 7 years (Faunce et al. Reference Faunce, Patterson and Lorenz2002), i.e. enough time to accumulate nematodes as each individual fish grows. Support for this interpretation comes from the fact that in fish populations, intensity of infection by parasite increases with age or size of fish host (Poulin, Reference Poulin2000). Furthermore, a number of authors have reported that M. cichlasomae and other helminth parasites in tropical areas respond to seasonal changes on a yearly basis (Salgado-Maldonado, Reference Salgado-Maldonado1993; Jiménez-García and Vidal-Martínez, Reference Jiménez-García and Vidal-Martínez2005; Violante-González et al. Reference Violante-González, Aguirre-Macedo and Vidal-Martínez2008) but most of these studies have been undertaken over a short time scale of 1 year or less. However, the present results suggest that the occurrence of bi-annual peaks in the infection dynamics of M. cichlasomae is not only due to the accumulation of infective stages through time, but also to changes in both rainfall and temperature. This is in contrast to the work of Pech et al. (Reference Pech, Aguirre-Macedo, Lewis and Vidal-Martínez2010) where rainfall was found to be the key factor in triggering host infection parameters in parasite infracommunities in tropical aquatic hosts.
The present investigation has shown that rainfall and temperature influence the infection dynamics of M. cichlasomae in its definitive and intermediate hosts at different time scales. Within 12 months, there is a peak in the mean abundance of gravid females of M. cichlasomae in the definitive fish host, presumably due to first-stage larvae being released into the bloodstream and then appearing in A. yucatanus 2 months later. Ultimately, adult worms of M. cichlasomae, including gravid females, accumulate through time, reaching peaks of abundance every 24–27 months (Fig. 4). What happens with fish carrying a large number of nematodes every 2 years? There are 2 possibilities: the first being senescence of old nematodes, and the second being parasite-induced host mortality. Both options are being analysed elsewhere (Vidal-Martínez et al. manuscript in preparation). Meanwhile, we conclude that the temporal dynamics of M. cichlasomae showed yearly fluctuations in infection levels associated with annual seasonal increases in rainfall and temperature, and bi-annual peaks due to the accumulation of infective stages.

Fig. 4. Descriptive model of the temporal dynamics of Mexiconema cichlasomae in its intermediate host, the fish louse Argulus yucatanus and the Mayan cichlid Cichlasoma urophthalmus.
ACKNOWLEDGEMENTS
This study was part of the Ph. D. thesis of A.L.M.T., who is grateful to CONACYT-México (216405) for providing 4 years scholarship. Sincere and grateful thanks are extended to all at Laboratorio de Patología Acuática CINVESTAV-IPN Unidad Mérida including Clara Vivas-Rodríguez, Gregory Arjona-Torres, Nadia Herrera, Francisco Puc, Geny Aíl and Trinidad Sosa who helped with field and laboratory work at different times over the past 8 years.
FINANCIAL SUPPORT
This study was partly supported by grant No. 44590 ‘Invertebrados como hospederos intermediarios de helmintos de Lutjanus griseus y otros peces de importancia comercial en dos lagunas costeras de Yucatán’ given to M.L.A-M. by CONACyT-Mexico D 44590-Q. We are indebted to SEP-PROMEP for an award to support our project ‘Propuesta sobre Calentamiento Global y Cambio Climático de la Red Académica de Instituciones SEP-PROMEP del Sureste: área Sensibilidad Marina’ and to a FOMIX Yucatán award in support of the project ‘Sensibilidad y vulnerabilidad de los ecosistemas costeros del sureste de México ante el Cambio Climático Global’ YUC-2008-C06-108929.