INTRODUCTION
Competition between species that require similar resources can often lead to the local exclusion of one of the species (Begon et al., Reference Begon, Harper and Townsend1990). However, coexistence can also occur where species with overlapping habitat preferences meet in a common habitat (Rosenzweig, Reference Rosenzweig1981; Morris, Reference Morris1999). In patchy habitats, competitively inferior species can escape from competition by finding empty habitats not yet colonized by the superior competitor (e.g. Shorrocks et al., Reference Shorrocks, Atkinson and Charlesworth1979; Atkinson & Shorrocks, Reference Atkinson and Shorrocks1981; Kareiva, Reference Kareiva, Diamond and Case1986; Tilman, Reference Tilman1994; Lehman & Tilman, Reference Lehman, Tilman, Tilman and Kareiva1997). That is, community assemblages in patchy habitats can be influenced by the impacts of interspecific competition on habitat selection.
The dispersive larval phase and mobility in juvenile and adult phases of coral reef fish enable them to colonize a variety of patchy habitats. Highly heterogeneous spatial patterning of reef-building corals results in seascapes with complex topography over a range of spatial scales (Boström et al., Reference Boström, Pittman, Simenstad and Kneib2011; Pittman et al., Reference Pittman, Kneib and Simenstad2011). These can often lead to high species richness of fish communities. Coral reefs may consist of small patch reefs, contiguous large reefs and a variety of intermediate types, providing reef fish with patchy habitats of different sizes. Habitat selection and coexistence of species that require similar resources has been observed in coral reef fish (e.g. Sweatman, Reference Sweatman1983, Reference Sweatman1985; Robertson, Reference Robertson1996; Ceccarelli et al., Reference Ceccarelli, Jones and McCook2001; Ceccarelli, Reference Ceccarelli2007; Gardiner & Jones, Reference Gardiner and Jones2010). However, the influence of size of patchy habitat on their habitat use and coexistence has not been clarified, except for some anemonefish that coexist using the same host sea anemone with different sizes (Hattori, Reference Hattori1995, Reference Hattori2002).
Species–area relationships (SARs) of coral reef fish have been examined in patchy habitats of various sizes (McGuinness, Reference McGuinness1984; Sales & Steel, Reference Sale and Steel1986; McClanahan, Reference McClanahan1994; Ault & Johnson, Reference Ault and Johnson1998a, Reference Ault and Johnsonb; Chittaro, Reference Chittaro2002; Belmaker et al., Reference Belmaker, Ben-Moshe, Ziv and Shashar2007). However, the effects of species competition on SARs have not been explicitly detected. Observed SARs are often only explained by stochastic recruitment because of the highly dispersive larval phase and high mobility of fish (Sale & Steel, Reference Sale and Steel1986; Ault & Johnson, Reference Ault and Johnson1998a; Belmaker et al., Reference Belmaker, Ben-Moshe, Ziv and Shashar2007), although they have also been linked to microhabitat diversity, with higher microhabitat richness in larger patches (e.g. Ault & Johnson, Reference Ault and Johnson1998b; Chittaro, Reference Chittaro2002). However, behavioural observations of direct species competition and habitat use on patch reefs have not been conducted in relation to SARs.
Hattori & Shibuno (Reference Hattori and Shibuno2010) examined SARs of damselfish assemblages on 84 small patch reefs located close to one another in a shallow back reef of Ishigaki Island, Okinawa, Japan. Species richness increased linearly with log-transformed patch reef area, and was much lower on the two largest reefs than expected from random placement model simulations (RPMS); RPMS based on patch reef area predicted that 19 and 17 species would be found on the largest and second largest patch reefs, respectively, but 13 and 12 species were actually found on these reefs. Coral cover did not differ substantially among the 84 patch reefs. Their statistical analyses suggested that some of the territorial herbivores influence SARs. However, the pattern of habitat use and abundance of the herbivores in relation to patch reef size was not analysed. How do the herbivores affect SARs? In the present study, we observed direct species competition and habitat use of the herbivores on patch reefs.
Our preliminary observations indicated that three dominant territorial herbivorous damselfish (Pisces: Pomacentridae), bluntsnout gregory (Stegastes lividus Forster 1801), dusky farmerfish (Stegastes nigricans Lacepède 1802) and lagoon damselfish (Hemiglyphidodon plagiometopon Bleeker 1852), coexisted only on large patch reefs. All of them require large areas for grazing on filamentous algae (Sammarco, Reference Sammarco1983; Hata & Kato, Reference Hata and Kato2002, Reference Hata and Kato2006; Jan et al., Reference Jan, Ho and Shiah2003; Hata et al., Reference Hata, Watanabe and Kato2010). The three species seemed to interact aggressively with each other and with other damselfish species, with possible segregated three-dimensional (3-D) territories on the largest patch reef. In the present study, we first re-examined the distribution patterns of the three focal species on the 84 patch reefs in relation to patch reef height, as well as patch reef area. Second, we examined their intra- and interspecific aggressive behaviours on the two large patch reefs (the largest complex patch reef and the large flat patch reef), where the three focal species coexisted. Finally, we described the 3-D territories of the focal species on the largest complex patch reef.
MATERIALS AND METHODS
Study site and species
The field study was conducted during June and August 2007, March and May 2008, and July 2011 in the shallow nearshore zone (1.5–2.5 m depth) on the back reef of Shiraho Reef, Ishigaki Island (24°22′18.22N 124°15′13.82E), Japan. Bluntsnout gregory (Stegastes lividus), dusky farmerfish (Stegastes nigricans) and lagoon damselfish (Hemiglyphidodon plagiometopon) vigorously defend their territories, which consist of small patches of filamentous algae growing on dead corals, and the two Stegastes species form conspecific aggregations (e.g. Sammarco, Reference Sammarco1983; Allen, Reference Allen1991; Hata & Kato, Reference Hata and Kato2002, Reference Hata and Kato2006; Jan et al., Reference Jan, Ho and Shiah2003; Hata et al., Reference Hata, Watanabe and Kato2010). Shiraho Reef comprises a continuous large reef and many patch reefs of various sizes (0.05–45.4 m2), which are inhabited by many damselfish with high density, and the three species examined were the most abundant territorial herbivorous damselfish at this study site (Hattori & Shibuno, Reference Hattori and Shibuno2010). Patch reefs were defined as small natural reefs that are reflected in high-resolution aerial photographs (OKC-94-13, 1/10000, 95 Ishigaki C15-34, taken in 1995 by the Geographical Survey Institute, Ministry of Land, Infrastructure and Transport, Japan). The smallest patch reef was 0.25 m wide and 0.35 m tall, and all patch reefs comprised live and/or dead coral (e.g. coral patches, coral bomboras and micro-atolls (see Hattori & Shibuno, Reference Hattori and Shibuno2010)).
Distribution and abundance of focal species in relation to reef size
The distribution and abundance of the three focal species on 84 patch reefs were examined in relation to patch reef size. Original data of six censuses conducted in 2007 by Hattori & Shibuno (Reference Hattori and Shibuno2010) were used for this analysis. The area of each patch reef was measured on high-resolution aerial photographs and calculated on a computer using the public domain software Image J 1.33 (Rasband, Reference Rasband1997; see Hattori & Shibuno, Reference Hattori and Shibuno2010). We refer to a group of three or more individuals whose territories were contiguous to each other as ‘an aggregation’. In the present study, we do not use the term ‘colony’ because we claim neither obvious function nor social unit as a whole. Small juveniles (<20 mm in total length (TL)) were excluded from the analysis because they did not have territories. As our preliminary observations on the largest patch reef implied that the focal species separate their territories in vertical axis on the patch reef, the height of each reef was measured to the nearest 5 cm in the field. The statistical software R 3.0.1 (R Development Core Team, 2006) was used for data analyses in the present study. Because the total number of the individuals of territorial herbivores increased almost linearly with patch reef area (Hattori & Shibuno, Reference Hattori and Shibuno2010), we used the Pearson product moment correlation coefficient to analyse the relationships between abundance of the focal species and patch reef size. Non-parametric tests (i.e. Mann–Whitney U-test and Kruskal–Wallis test) were applied for reef size comparisons because of the small sample size of a reef category (the ‘Wilcox. Exact’ and ‘Kruskal. Test’ functions of R 3.0.1 were used).
Intra- and interspecific interactions of focal species on the two large patch reefs
We observed behaviours of the focal species on the two large patch reefs where they coexisted (Figure 1: see also figure 1b in Hattori & Shibuno (Reference Hattori and Shibuno2010)). The largest complex patch reef had an area of 45.4 m2, three projections of Porites rock (two were 2.0 m in height), coral cover of 32.7%, and the average species richness of damselfish assemblage was 12.7 for six censuses. The large flat patch reef had an area of 23.9 m2, flat plane (1.5 m in height), coral cover of 37.3%, and average species richness of damselfish assemblage of 11.8 for six censuses. The abundances of each damselfish species found on the two patch reefs, which were obtained from the original data of six censuses in 2007 by Hattori & Shibuno (Reference Hattori and Shibuno2010), are listed in Table 1. With the exception of the time of lowest tides or rough surface conditions, we could observe damselfish easily from the water surface while snorkelling without disturbing them, as in our previous studies (Kobayashi & Hattori, Reference Kobayashi and Hattori2006; Hattori & Shibuno, Reference Hattori and Shibuno2010; Hattori, Reference Hattori2012). Between March and May 2008, we traced the swimming track of each adult-size individual (>40 mm in TL, personal observations), for 10 min on maps from above (Figure 2), and individuals were recognized based on the locations of their home ranges and relative body size. Thirty-four and 24 individuals of S. nigricans were randomly selected as target individuals in the largest complex patch reef and the large flat patch reef, respectively (Figure 3A); 35 and 15 individuals of S. lividus were randomly selected in the largest complex patch reef and the large flat patch reef, respectively (Figure 3B). We recorded the frequency of their attacking behaviours, such as rushing against and biting other individuals, and feeding frequency, which is expected to decrease under conditions of intense competition. Non-parametric tests were used for all behavioural analyses. Only four and three fish of H. plagiometopon were found on the largest complex patch reef and the large flat patch reef, respectively. We observed them eight times on the largest patch reef and three times on the flat patch reef. We pooled data to calculate average values of their behaviours (Figure 3C). Statistical comparisons were not conducted for H. plagiometopon because of the small sample size.

Fig. 1. Schematic and photograph of the two large patch reefs (the largest complex patch reef and the large flat patch reef). The photograph in Figure 3 was taken from the position indicated by the thick arrow.

Fig. 2. Example of a map for observations at the largest complex patch reef. Home ranges of Stegastes nigricans and Stegastes lividus are shown. Home ranges of Hemiglyphidodon plagiometopon could not be described on the map from immediately above because they used the overhang space at the base of the reef (see Results). The photograph in Figure 3 was taken from the position indicated by the thick arrow.

Fig. 3. (A) Photograph taken from the position indicated by the thick arrow in Figures 1 & 2, which was used for the observation of three-dimensional territories. Projection of massive Porites coral is shown. The yellow line represents a scale bar of 2 m. (B) Three-dimensional territories of Stegastes nigricans (top, yellow), Stegastes lividus (bottom left, green) and Hemiglyphidodon plagiometopon (bottom right, blue), determined by four 15 min observation periods on the photograph.
Table 1. Mean number of individuals (six censuses) of damselfish observed on the largest complex patch reef and the large flat patch reef.

Three-dimensional territories and habitat use of focal species on the largest complex patch reef
To confirm preliminary observations of 3-D spacing patterns and habitat use of the three focal species on the largest complex patch reef, we described their territories. Underwater photographs were taken 2 m from the reef in a down-current location (Figures 1 & 2). The home ranges of the individuals that inhabited the part of the reef reflected in each photograph were recorded by one snorkeller, who traced the swimming tracks of each individual onto the waterproof print of the photograph for 15 min at high tide with a calm surface (Figure 3A). Observations were repeated four times within a week (July 2011). The home ranges of all individuals were overlaid to detect their territories, with the boundaries of their territories denoted by a line encircling all tracks.
RESULTS
Distribution and coexistence of focal species in relation to patch reef size
There was a very high correlation between patch reef area and the abundance of both Stegastes lividus (R = 0.955, T s = 29.3, P < 0.0001) and Hemiglyphidodon plagiometopon (Pearson product moment correlation coefficient, R = 0.920, T s = 21.2, P < 0.0001). However, S. nigricans did not show such a high correlation (R = 0.663, T s = 8.0, P < 0.0001). For both S. lividus and H. plagiometopon, there was a weak correlation between patch reef height and abundance (S. lividus, R = 0.368, T s = 3.6, P = 0.0006; H. plagiometopon, R = 0.393, T s = 3.9, P = 0.0002). In contrast, the abundance of S. nigricans was highly correlated with patch reef height (R = 0.736, T s = 9.8, P < 0.0001). For both S. lividus and H. plagiometopon, there was a very high correlation between ‘patch reef area × patch reef height’ and abundance (S. lividus, R = 0.957, T s = 29.9, P < 0.0001; H. plagiometopon, R = 0.921, T s = 21.4, P < 0.0001). However, S. nigricans did not show such a high correlation (R = 0.639, T s = 7.5, P < 0.0001).
Aggregations of S. nigricans and S. lividus coexisted only on the two large patch reefs (Table 2). While reefs with aggregations of only S. nigricans were abundant (N = 15), reefs with those of only S. lividus were very rare (N = 2), with the former reefs (mean = 2.9 m2) significantly larger than the latter (mean = 0.8 m2, Mann–Whitney U-test, U = 0, P = 0.015). All S. lividus individuals in the two patch reefs were smaller than 40 mm in TL. There were significant differences in patch reef area among reefs that had aggregations of both species (mean = 34.6 m2, N = 2), those with only aggregations of S. nigricans or S. lividus (mean = 2.6 m2, N = 17) and those without aggregations of both species (mean = 0.8 m2, N = 65, Kruskal–Wallis test, H = 28.2, P < 0.001). In the two large patch reefs, S. lividus was more abundant than S. nigricans (mean = 50.0 individuals ind. versus 18.5 ind. for six censuses, U-test, U = 0, P = 0.002). However, in the total number of all 84 patch reefs, S. lividus was less abundant than S. nigricans (mean = 70.8 ind. versus 133.8 ind. for six censuses, U-test, U = 0, P = 0.002).
Table 2. Coexistence pattern of the non-aggregational territorial herbivore (Hemiglyphidodon plagiometopon, Hp) and the two aggregational territorial herbivores (Stegastes nigricans, Sn and S. lividus Sl) in relation to patch reef size (area and height). Data on small individuals (< 20 mm total length) are excluded. Data on temporal visitors of H. plagiometopon are also excluded.
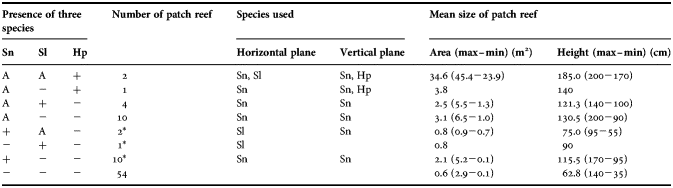
A, aggregation (≥3 territorial individuals); +, non-aggregational individuals; −, no individuals;*. All individuals were smaller than 40 mm total length on patch reef.
Reefs inhabited by at least one individual of H. plagiometopon (i.e. ≥ 1 ind. on average for six censuses) were larger than 3.7 m2 (N = 3). These reefs were taller than the other patch reefs (mean = 163.3 cm versus 80.7 cm, U-test, U = 12, P = 0.008). Ten reefs inhabited by H. plagiometopon, which included temporary visitors (i.e. > 0 ind. on average for six censuses), were significantly larger than the other 74 reefs (mean = 9.4 m2 versus 1.0 m2, U-test, U = 83, P < 0.001). Small juveniles (<2 cm TL) were very cryptic and rarely found.
Intra- and interspecific interactions of focal species on the two large patch reefs
Stegastes nigricans attacked 18 species in total, including five damselfish on the largest complex patch reef, and eight damselfish on the large flat patch reef (Figure 4A). On the largest complex patch reef, there was no significant difference between frequencies of intra- and interspecific interactions (Mann–Whitney U-test, U = 493, P = 0.283, N = 34). Conversely, on the flat patch reef, interspecific interactions were observed more frequently than intraspecific interactions (U-test, U = 120.5, P < 0.001, N = 24), where S. nigricans often attacked Amblyglyphidodon curacao (mean = 3.1 per 10 min) and Dischistodus prosopotaenia (mean = 2.3 per 10 min). Feeding frequency of S. nigricans was significantly higher on the largest complex patch reef than on the large flat patch reef (U-test, U = 142.5, P < 0.001).

Fig. 4. Frequencies of intra- and interspecific interactions of (A) Stegastes nigricans, (B) Stegastes lividus, and (C) Hemiglyphidodon plagiometopon, shown as average frequencies (±SD) of each interaction per 10 min period. Interspecific behaviour indicates agonistic interactions between individuals of the target species and other damselfish species. Feeding frequencies are also shown. Species of damselfish that inhabited the two large patch reefs are listed in Table 1.
Stegastes lividus attacked 12 species in total, including four damselfish on the largest complex patch reef, and six damselfish on the large flat patch reef (Figure 4B). On the largest complex patch reef, there was no significant difference between frequencies of intra- and interspecific interactions (U-test, U = 544, P = 0.2963, N = 35). However, on the flat patch reef, interspecific interactions were observed more frequently than intraspecific interactions (U-test, U = 26.5, P < 0.001, N = 15), where S. lividus slightly attacked Dascyllus aruanus (mean = 0.8 per 10 min) and S. nigricans (mean = 0.6 per 10 min). There was no significant difference in feeding frequency of S. lividus between the two patch reefs (Figure 4B, U-test, U = 234.5, P = 0.561).
Hemiglyphidodon plagiometopon attacked 11 species in total, including only three damselfish on the largest complex patch reef and three damselfish on the large flat patch reef. Almost no intraspecific interactions and very few interspecific interactions were observed on both reefs (Figure 4C): H. plagiometopon seldom attacked Acanthus nigrofuscus (mean = 0.6 per 10 min) and S. nigricans (mean = 0.4 per 10 min).
Intra- and interspecific territories of focal species on the largest complex patch reef
Four individuals of S. nigricans, six individuals of S. lividus and two individuals of H. plagiometopon had 3-D segregated territories (Figure 3B). The H. plagiometopon territories were around the bases of Porites rock projections. During 15 min observations, H. plagiometopon sometimes visited neighbouring small reefs that were 2–3 m away from their territories. Territories of S. lividus and aggregations of their conspecifics covered the flat part of the reef. Stegastes nigricans had territories and aggregations of conspecifics around the vertical planes of the tops of Porites rock projections.
DISCUSSION
The territorial herbivorous damselfish, Stegastes lividus, S. nigricans and Hemiglyphidodon plagiometopon, need large areas of hard substratum to maintain their territories (e.g. Sammarco, Reference Sammarco1983; Allen, Reference Allen1991; Hata & Kato, Reference Hata and Kato2002, Reference Hata and Kato2006; Jan et al., Reference Jan, Ho and Shiah2003; Hata et al., Reference Hata, Watanabe and Kato2010). With the exception of small juveniles (<20 mm in TL), these species never inhabit patch reefs smaller than 0.6 m2 (Hattori & Shibuno, Reference Hattori and Shibuno2010). In the present study, no aggregations of S. lividus or S. nigricans were found on patch reefs smaller than 1 m2, except for individuals smaller than 40 mm in TL (see Table 2). The non-aggregational H. plagiometopon never had territories on patch reefs smaller than 3.8 m2, although some temporary visitors (i.e. <0.1 ind. on average for six censuses) were found on such reefs. These observations indicated that there is a minimal patch reef area to hold an aggregation for the two Stegastes and to hold a territory for H. plagiometopon. On medium-sized patch reefs (1–7 m2), aggregations of S. nigricans were abundant but no aggregations of S. lividus were found even on the reefs that were unoccupied by S. nigricans. In addition, the two species rarely attacked each other on the two large patch reefs. Therefore, S. lividus appeared not to be directly excluded by S. nigricans, but they could not colonize medium-sized patch reefs. All three focal species coexisted on the two large patch reefs. Thus, their coexistence pattern was largely dependent on patch reef area.
On the two large patch reefs (45.4 and 23.9 m2), S. lividus were more abundant than S. nigricans. In contrast, among the 84 patch reefs, S. nigricans were more abundant than S. lividus. On medium-size patch reefs, S. nigricans used both horizontal and vertical planes, but used the vertical plane only on the largest complex patch reef, where S. lividus occupied the horizontal plane. In the largest complex patch reef, the two Stegastes species had 3-D segregated territories. On the large flat patch reef, there was a greater frequency of interspecific agonistic behaviours of S. nigricans against damselfish than of intraspecific agonistic behaviours. In contrast, on the largest complex patch reef, there was no significant difference in the frequencies of the two behaviours. These results suggest that the vertical planes of patch reefs provide less competitive space for S. nigricans. Indeed, the abundance of S. nigricans was more closely correlated with patch reef height than patch reef area or area × height. Although much is known regarding the distribution and abundance of S. nigricans from several areas (Done et al., Reference Done, Dayton, Dayton and Steger1991; Jones et al., Reference Jones, Santana, McCook and McCormick2006), the effects of reef height on their distribution and abundance have not been reported. For S. lividus and H. plagiometopon, there was a very strong correlation between ‘patch reef area × patch reef height’ and abundance. The present study suggested that not only patch reef area but also patch reef height can affect the pattern of species coexistence. On the largest complex patch reef, H. plagiometopon used the overhang space near the base. This unique microhabitat was not used by other damselfish (Hattori & Shibuno, Reference Hattori and Shibuno2010). The overhang space existed only in some large and/or tall reefs. The abundance of H. plagiometopon was more strongly correlated with ‘patch reef area × patch reef height’ than with reef area or height. It is likely that H. plagiometopon does not need sunlit space because it mainly feeds on detritus within its territory rather than filamentous algae (Wilson & Bellwood, Reference Wilson and Bellwood1997; Ceccarelli, Reference Ceccarelli2007). Indeed, interspecific interactions were very rarely observed on its territories. It should be important for other territorial herbivorous damselfish to cultivate filamentous algae on sunlit flat planes as food sources. Thus, the vertical plane of patch reefs may provide less competitive space for less superior competitors.
An interspecific trade-off between competitive ability and dispersal (=colonization) ability of species that require similar resources often explains why so many ecologically similar species can coexist in a patchy environment (Tilman, Reference Tilman1994; Lehman & Tilman, Reference Lehman, Tilman, Tilman and Kareiva1997). In marine fish, dispersal ability is usually high because of the drifting larval phase and mobility after recruitment; it may be inferred from settlement patterns of the drifting larvae and movement patterns after settlement. Competitive ability may be inferred from patterns of habitat use. For example, two coral reef anemonefish, Amphiprion clarkii and Amphiprion perideraion, are known to coexist using the same host sea anemone, Heteractis crispa, which are sparsely and patchily distributed (Hattori, Reference Hattori1995, Reference Hattori2002, Reference Hattori2006). Amphiprion perideraion is a superior competitor that uses large hosts only, which are necessary for reproduction of the two species, but cannot use small hosts because its drifting larvae cannot settle on small hosts irrespective of the presence of A. clarkii and both adults and juveniles cannot move between neighbouring hosts. In contrast, A. clarkii is a superior disperser that can settle on large hosts without A. perideraion and the small hosts, and both juveniles and adults move to find neighbouring larger hosts. Eventually, A. perideraion takes over the large hosts after growth (Hattori, Reference Hattori1995, Reference Hattori2002). In the present study site, S. lividus may be a superior competitor because it formed larger aggregations on the large patch reefs than S. nigricans occupying the horizontal plane, but may be an inferior disperser because it did not colonize medium-size patch reefs. In contrast, S. nigricans is a superior disperser because it was more abundant on medium-size patch reefs, but must be an inferior competitor because it used the vertical plane in the largest complex patch reefs where S. lividus was abundant. While adult S. nigricans can move between neighbouring patch reefs to find larger mates for spawning (Karino & Nakazono, Reference Karino and Nakazono1993), adult S. lividus may be unable to do so. On removal of S. lividus from the largest complex patch reef, S. nigricans would form new aggregations on the horizontal plane. On removal of S. nigricans from a medium-size patch reef, in contrast, S. lividus would not form new aggregations on the horizontal plane because of its low colonization ability (low mobility and/or low dispersive ability). We could not have conducted such experiments because Shiraho Reef was designated as a Marine Protected Area in 2007. Further studies on settlement patterns of drifting larvae, as well as removal experiments, are needed to confirm the patterns of interspecific trade-offs between competitive ability and dispersal (=colonization) ability of the two Stegastes species that use patchy habitats.
The observed SARs of coral reef fish can be explained by stochastic recruitment (Sale & Steel, Reference Sale and Steel1986; Ault & Johnson, Reference Ault and Johnson1998a; Belmaker et al., Reference Belmaker, Ben-Moshe, Ziv and Shashar2007) and microhabitat diversity, with greater microhabitat richness in larger patches (e.g. Ault & Johnson, Reference Ault and Johnson1998b; Chittaro, Reference Chittaro2002). Hattori & Shibuno (Reference Hattori and Shibuno2010) examined SARs in shallow waters less than 2.5 m deep, where species richness increased linearly with log-transformed patch reef area but was much lower on large reefs than expected from RPMS. They suggested that some of the territorial herbivores negatively influence species richness on large patch reefs. While large patch reefs may be tall allometrically in deeper waters, they are not sufficiently tall in shallow back reefs. As a result, large flat patch reefs may not have the high species richness expected from RPMS based on patch reef area. If SARs had been examined in deeper waters in our previous study (Hattori & Shibuno, Reference Hattori and Shibuno2010), there might have been a negligible influence of species competition on species richness due to the increased height of the patch reefs. The effects of species competition on SARs have not been explicitly detected (Sale & Steel, Reference Sale and Steel1986; Ault & Johnson, Reference Ault and Johnson1998a, Reference Ault and Johnsonb; Chittaro, Reference Chittaro2002; Belmaker et al., Reference Belmaker, Ben-Moshe, Ziv and Shashar2007), which may be because studies on SARs of reef fish were mostly conducted in relatively deep waters. Patch reef height should be incorporated into studies on SARs of coral reef fish, because some species may be able to use the vertical plane (and the overhang space near the base) of patch reefs.
Land reclamation is common in shallow back reefs of densely populated coral reef islands, such as Okinawa, Japan (Spalding et al., Reference Spalding, Ravilious and Green2001; Tsuchiya et al., Reference Tsuchiya, Nadaoka, Kayanne and Yamano2004). The associated habitat loss is one of the most serious anthropological drivers of reduced marine biodiversity in shallow near-shore habitats (Roff & Zacharias, Reference Roff and Zacharias2011). While stochastic recruitment to habitat patches was believed to strongly affect coral reef fish communities, recent studies suggested that reef fish populations in patchy environments are actually closed or semi-closed metacommunities, despite the high dispersal ability of fish (e.g. Almany et al., Reference Almany, Berumen, Thorrold, Planes and Jones2007; Planes et al., Reference Planes, Jones and Thorrold2009; Pinsky et al., Reference Pinsky, Palumbi, Andréfouët and Purkis2012). We suggest that shallow back reefs with large and tall patch reefs are critical parts of semi-closed metacommunities, even with low coral cover, and can contribute to maintain local metacommunities with high species richness. This is because small patch reefs and vertical planes of large and tall patch reefs may be able to provide less competitive space for less superior competitors, which are expected to have high mobility and high dispersal ability. Recently, Harborne et al. (Reference Harborne, Mumby and Ferrari2012) suggested that coral-generated meso-scale rugosity is an important factor influencing species abundance and diversity of coral reef fish. It was suggested that tall patch reefs (>0.5 m) can be better habitats in terms of high carrying capacity for some coral reef fish than flat patch reefs of the same total area in shallow back reefs (Hattori & Kobayashi, Reference Hattori and Kobayashi2007). Values of ‘patch reef area × patch reef height’ can be a useful indicator for designating effective marine protected areas in shallow near-shore habitats. Extracting the total area of ‘tall’ patch reefs on coral reef seascapes, which are well reflected in high-resolution ‘stereoscopic’ colour aerial photographs, can also be a useful tool.
ACKNOWLEDGEMENTS
We are grateful to Yasunobu Yanagisawa, David Price, Laura Wicks, and the anonymous referees for their valuable advice regarding the manuscript. Fumitoshi Iwasaki provided valuable information on references.
FINANCIAL SUPPORT
This work was supported in part by grants-in-aid for Science Research from the Japan Ministry of Education, Culture, Sports, Science and Technology (grant numbers 23570022, 21520791, 20570020). A.H. is an Affiliated Scientist at the Center for Ecological Research, Kyoto University (Japan).








