Introduction
Depression is a common, distressing, and debilitating disorder that is frequently chronic and relapsing. Common treatments include antidepressant drugs, such as selective serotonin reuptake inhibitors (SSRIs), which influence monoamine transmission, and psychological treatments, such as cognitive behavioral therapy (CBT), which focus on encouraging patients to challenge their dysfunctional attitudes and negative automatic thought processes. Depression is the leading cause of disability worldwide in terms of total years lost to ill health,1 and is associated with absenteeism from work and presenteeism while at work (reduced productivity). In England in 2007, lost earnings due to depression amounted to £5.8 billion (∼$8.7 billion), and it has been estimated that lower productivity accounts for a further £1.7–£2.8 billion (∼$2.5–$4.2 billion).Reference McCrone, Dhanasiri, Patel, Knapp and Lawton-Smith2
Why should clinicians be interested in cognition in depression? First, depression is a cognitive disorder, as emphasized by the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV3) criteria for a major depressive episode (MDE). MDE Criterion 8 states that a depressed individual may have “diminished ability to think or concentrate, or indecisiveness”; MDE Criterion 2 is the cardinal symptom of anhedonia, defined as “markedly diminished interest or pleasure in all, or almost all, activities most of the day, nearly every day”; MDE Criterion 5 includes objective psychomotor retardation. Thus, depression fundamentally alters the perception of and interaction with the environment, including the social environment, and information processing. Second, it is this cognitive impact that primarily affects the ability to function, whether in the workplace, at school, or at home. Moreover, disrupted cognition may prevent severely ill patients from deriving full benefit from psychological treatments. Third, marked cognitive impairment predicts poor response to antidepressant medication, independent of symptom severity.Reference Potter, Kittinger, Wagner, Steffens and Krishnan4 Finally, in some depressed patients, cognitive abnormalities may not resolve completely upon remission, and are also observed in first-degree relatives, suggesting that they may be trait markers (predisposing factors). Hence, cognitive abnormalities may serve as useful avenues of research in the search for the neurobiological underpinnings of the disorder,Reference Hasler, Drevets, Manji and Charney5 as well as in the identification of at-risk individuals.
“Cold” Cognition in Depression
“Cold” cognition refers to information processing in the absence of any emotional influence (Figure 1). Theoretically, cold cognition is engaged on tests where the stimuli are emotionally neutral and the outcome of the test is not motivationally relevant (though motivational influences could conceivably turn a cold test “hot”; see Might “Cold” Cognition Be Turned “Hot” in Depression? below). Examples of neuropsychological tests usually considered cold include standardized pencil-and-paper assessments commonly used to assess function in neurological patients, for example the California Verbal Learning Test, the Trail-Making Test, and the Wisconsin Card Sort Test. Reliable impairments on such neuropsychological tests were observed from the 1970s onward in depressed patients. Some early studies adopted a classical neuropsychological case series approach,Reference Cavenar, Maltbie and Austin6 comparing the performance of individual depressed patients against population norms, identifying deficits of a magnitude judged to be clinically significant in several patients. More frequently, case-control designs were employed, and by the mid-1990s numerous studies comparing specific cognitive measures between groups of depressed patients and comparison subjects had been reported, particularly on memory tests.

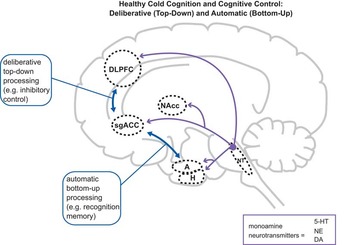
Figure 1 Normal “cold” cognition in nondepressed individuals. “Cold” (emotion-independent) cognition is instantiated via a complex set of circuits, including interactions (blue arrows) between the dorsolateral prefrontal cortex (DLPFC), the dorsal anterior cingulate cortex (ACC), and the hippocampus (H). Limbic structures connected to DLPFC, ACC, and H, such as the amygdala (A), the nucleus accumbens (NAcc), and the subgenual portion of the ACC (sgACC) also may also be activated during cold cognition, but are more strongly engaged during “hot” (emotion-laden) cognition (Figure 2). Monoamine neurotransmitter (NT) projections (purple arrows) emanating from the brainstem, including serotonin (5-HT), norepinephrine (NE), and dopamine (DA), may influence cold cognition via modulatory actions in cortical and subcortical regions. Note that most circuit nodes and connections are excluded in this and later figures for clarity, and that some connections may be indirect.
In 1995 Burt etal. Reference Burt, Zembar and Niederehe7 performed the first systematic review of this literature, identifying nearly 100 case-control reports examining memory performance in depressed patients. Their meta-analysis identified deficits in patients of standardized effect sizes (Cohen's d) in the range 0.27 (small) to 0.67 (medium-to-large), varying across outcome measures. Surprisingly, patients who were younger exhibited greater deficits. However, several of the studies included subjects with organic neurological illness, and did not match the groups on important demographic variables such as age and educational level, making it difficult to draw firm conclusions. A later meta-analysis by Veiel,Reference Veiel8 which utilized more stringent inclusion criteria, identified higher effect sizes for memory, in the range 0.83–0.97 (large), and additionally reported differences in other domains of cognitive function, with only tests in the domain “attention and concentration” apparently relatively spared in depressed patients (however, see next paragraph). This latter result does appear surprising, given Criterion 8 for an MDE: “diminished ability to … concentrate.” Importantly, impairments on paper-and-pencil tests have been observed reliably in unmedicated samples.Reference Porter, Gallagher, Thompson and Young9
The advent of theoretically based, computerized cognitive tests in the 1990s provided an important methodological advance in understanding cognition in depression. One example of this approach is in the use of the Cambridge Neuropsychological Test Automated Battery (CANTAB; http://www.cantab.com). Broadly consistent with the results from pencil-and-paper studies described above, impairments were noted on a wide variety of CANTAB tests, including not only memory and executive function,Reference Elliott, Sahakian and McKay10 but also attentional measures.Reference Swainson, Hodges and Galton11 These later studies employed computerized continuous performance tests (for example the CANTAB Rapid Visual Information Processing test, RVP) to assess sustained attention, in which subjects must detect specific targets presented in a train of hundreds of successively presented stimuli, separated by a sub-second time interval, over several minutes.Reference Swainson, Hodges and Galton11 The marked difference in results from previous studies exemplifies one advantage of utilizing a computerized testing system, which allows stimuli to be presented with greater flexibility and temporal precision than traditional paper-and-pencil assessments, thereby furnishing tests with higher cognitive specificity. Other advantages of computerized testing include automated data collection, resulting in lower inter-administrator variability, as well as standardized recording and scoring of results.
This approach contrasts with earlier non-computerized studies, in which continuous performance paradigms such as the RVP were impractical to administer routinely. As such, the only measures of attention available in the meta-analysis of VeielReference Veiel8 were variants of the digit-span test from the Wechsler Adult Intelligence Scale, which does not require a high degree of sustained concentration, and in a recent meta-analyses of executive function in depression was found to be relatively unimpaired.Reference Snyder12 Several studies identified cognitive impairment during remission,Reference Beats, Sahakian and Levy13 though it is possible that some of this continued impairment might be explained in part by residual subclinical symptoms, as meta-analyses have reported small-to-moderate (r values in the range 0.1–0.5) relationships between the degree of cognitive impairment and symptom load.Reference McDermott and Ebmeier14
Later work confirmed the clinical significance of cognitive deficits during a depressive episode, at least in elderly patients (reviewed in Pimontel etal. Reference Pimontel, Culang-Reinlieb, Morimoto and Sneed15). For example, Potter and colleaguesReference Potter, Kittinger, Wagner, Steffens and Krishnan4, Reference Story, Potter, Attix, Welsh-Bohmer and Steffens16 reported that more cognitively impaired elderly depressed patients improved less following treatment with antidepressant medication. Executive function deficits appear to be particularly reliable predictors of poor treatment response,Reference McLennan and Mathias17 and some studies have found that severely cognitively impaired depressed patients may benefit from psychological therapy specifically tailored to boosting problem solving.Reference Arean, Raue and Mackin18 This is consistent with complementary evidence from trials using the cognitive enhancer modafinil as an adjunct to SSRI treatment to improve response,Reference Abolfazli, Hosseini and Ghanizadeh19 though the cognitive mechanisms underlying this potentially important finding remain to be clarified.
Might “Cold” Cognition Be Turned “Hot” in Depression?
The precise theoretical significance of these reliable group differences on cold cognitive measures has been a matter of debate. Some investigators interpret these effects as reflecting a core feature of depression, likely of central importance in its etiology with the potential to be used as endophenotypes in molecular genetic studies.Reference Glahn, Curran and Winkler20 Others have wondered whether the poor performance observed might reflect a motivational deficit, caused by depressed patients treating task feedback differently to controls.Reference Elliott, Sahakian, Herrod, Robbins and Paykel21 This latter interpretation has also received some empirical support. In a study using the CANTAB in depression, Beats etal. Reference Beats, Sahakian and Levy13 identified a pattern of responding they termed “catastrophic response to perceived failure.” When depressed patients made an error on a test, they were proportionately more likely than controls to make an error on the subsequent trial. This pattern was confirmed in several studies,Reference Elliott, Sahakian and McKay10, Reference Murphy, Michael, Robbins and Sahakian22 including one in which comparison patient groups were included, that were matched for overall performance.Reference Elliott, Sahakian, Herrod, Robbins and Paykel21
These studies raise the possibility that at least some of the poor performance on neuropsychological tests in depression might be due to altered “hot,” ie, emotion-dependent, cognition (Figures 2 and 3). In other words, ostensibly cold cognitive tasks, especially those featuring explicit feedback, may take on an emotional quality in depressed individuals, and instead of using negative feedback to improve performance, depressed individuals may become discouraged. It is also possible that depressed individuals do not experience positive feedback as intensely as controls, further reducing the motivation to perform well. However, it is important to note that cold cognitive impairments have been observed on tests that do not feature explicit task feedback, and also in individuals who were fully recovered from depression. Therefore both hot and cold cognitive mechanisms are likely to contribute to poor neuropsychological test performance in depressed individuals (Figure 3).
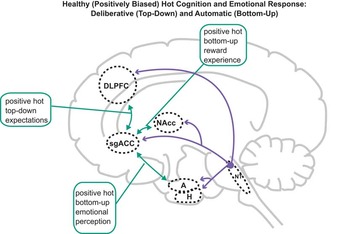
Figure 2 Normal positively biased “hot” (emotion-laden) cognition in nondepressed individuals. Hot cognition is instantiated by circuits including interactions (green arrows) between limbic regions including A, NAcc, and sgACC, the activity in which is profoundly modulated by 5-HT, NE, and DA. These regions share reciprocal connections with DLPFC, ACC, and H, and consequently hot and “cold” (emotion-independent) cognition necessarily interact (for example, motivation alters ostensibly cold cognitive test performance). Nondepressed individuals exhibit positive (green arrows) bottom-up (perceptions/experience) and top-down (expectation) biases, providing resilience to adverse events. Abbreviations and colors as in Figure 1.

Figure 3 Negatively biased hot and impaired cold cognition in depression. Top-down cold cognitive control and monoamine transmission are both compromised (dashed arrows). Depressed individuals exhibit negative (red arrows) bottom-up biases (emotional perceptions and reward experiences) due to disrupted monoamine transmission. They also have negative top-down biases (expectations), resulting in dysfunctional and self-perpetuating negative schemata, which give rise to depressive symptoms. Abbreviations and colors as in Figure 1.
“Hot” Cognition in Depression
In the past decade, several groups have reported that depressed individuals exhibit more negatively biased responses on tests of emotional processing. These are typically variants of cold cognitive tests that were adapted to include emotionally valenced stimuli. A common finding is that never-depressed individuals exhibit a positive bias (Figure 2), possibly reflecting resilience to negative emotional information, and that this is either attenuated or reversed in depressed individuals (Figure 3; see Roiser etal. Reference Roiser, Elliott and Sahakian23 for a review). Such a negatively biased pattern of responding in depressed individuals, both medicated and unmedicated, has been reported on tests of perception,Reference Joormann and Gotlib24 memory,Reference Matt, Vazquez and Campbell25 attention,Reference Gotlib and Joormann26 and working memory.Reference Joormann and Gotlib27 For example, on the CANTAB Affective Go/No-Go test, on which subjects must respond to one word-type while inhibiting responses to another, Murphy etal. demonstrated that while control individuals responded slightly more quickly to positive than negative target words, the converse was true for depressed patients.Reference Murphy, Sahakian and Rubinsztein28 A similar negative bias was identified using the same test in unmedicated depressed individuals.Reference Erickson, Drevets and Clark29
While studies of emotional bias in depression were first conducted many decades ago, abnormalities in another type of hot cognition, reward and punishment processing, have started to receive attention only relatively recently (see Eshel and RoiserReference Eshel and Roiser30 for a comprehensive review and studies cited therein for further details). This dearth of studies is surprising, given that anhedonia, which is closely related to reward processing, is one of the cardinal symptoms of a depressive episode. Moreover, experimental animal models of depression used for drug discovery frequently focus on behavioral constructs related to reward and punishment processing,Reference Berton, Hahn and Thase31 including learned helplessness, behavioral despair, sucrose preference, and intracranial self-stimulation.
Though the literature on reward processing in depression is much smaller than that on emotional biases, some consistent findings have emerged. One is the confirmation of the finding discussed above that depressed patients are hypersensitive to negative feedbackReference Elliott, Sahakian and McKay10; this finding was determined using tasks that were designed explicitly to assess this process. During a probabilistic reward and punishment reversal learning task featuring two stimuli (one more often associated with positive feedback and the other more often associated with negative feedback), medicated depressed patients showed a pronounced tendency to switch their choice following misleading negative feedbackReference Murphy, Michael, Robbins and Sahakian22—a result that was later replicated in unmedicated patients.Reference Taylor Tavares, Clark and Furey32 Other studies report hyposensitivity to positive feedback, for example, a failure to liberalize response bias on a difficult task when more points are gained for correct responses than are lost for incorrect responses,Reference Henriques and Davidson33 or reduced learning from rewarding stimuli.Reference Robinson, Cools, Carlisi, Sahakian and Drevets34
The above tasks rely on the learning of stimulus–outcome associations, while other tests have probed the impact of explicitly providing reward or punishment information about choices on decision-making in depression. One of the first studies to examine this question used the CANTAB Cambridge Gambling Task,Reference Rogers, Everitt and Baldacchino35 which requires participants initially to choose which of two outcomes they think will occur, with the probability of being correct varying, and then to stake points on their decision. A consistent finding across medicated, unmedicated, and even remitted samples is that depressed individuals increase their stake with increasingly better odds (termed “risk adjustment”) to a lower extent than controls,Reference Murphy, Rubinsztein and Michael36, Reference Rawal, Collishaw, Thapar and Rice37 which possibly reflects ambivalence to winning points. Other studies have used effort-based tasks where subjects must respond quickly in order to achieve rewards, demonstrating reduced motivation in depression.Reference Treadway, Bossaller, Shelton and Zald38 Interestingly, similar findings have been reported in subjects with schizophrenia,Reference Roiser, Stephan and den Ouden39, Reference Murray, Clark and Corlett40 who also experience anhedonia.
An important recent development in this field is the application of formal computational models to understand reward- and punishment-driven behavior. These models can dissect out specific aspects of reward processing behavior (eg, learning, points value, randomness) more precisely than analyses that use measures based on raw data.Reference Montague, Dolan, Friston and Dayan41 For example, using such a computational approach, Chase etal. demonstrated that reward learning was particularly poor in highly anhedonic depressives.Reference Chase, Frank and Michael42 Another recent study used computational modeling to demonstrate more random choices in subjects who scored high on depressive symptom rating scales (though without a categorical diagnosis) when using stimuli of known reward associations to guide decision making.Reference Kunisato, Okamoto and Ueda43
A Cognitive Neuropsychological Model of Depression
What are the theoretical implications of hot and cold cognitive abnormalities in depression? In our view, they mandate a reframing of the classic cognitive model of depression proposed by Beck,Reference Beck44 which proposes that depression results from stable, self-reinforcing, dysfunctional negative schemata, and is the inspiration for talking therapy approaches such as CBT. Beck's cognitive model predicts the presence of abnormal hot processing in depression (ie, negative emotional biases) on the basis of “top-down” influences, or what we conceptualize as “negative expectations” (Figure 3). In other words, depressed individuals may exhibit slower responses to happy wordsReference Erickson, Drevets and Clark29 or misinterpret facial expressions as sadReference Joormann and Gotlib24 precisely because they expect to encounter such negative information in the environment. These negative expectations, which include dysfunctional attitudes and negative attributional styles, and give rise to thought processes characteristic of depression, such as negative automatic thoughts and rumination, can be considered a form of “top-down” hot cognition (Figure 3). They are the targets of psychological interventions such as cognitive therapy, which could be conceptualized as training depressed individuals to exert cold cognitive control over their top-down negative biases, for example through working memory, inhibition, and problem solving (Figure 4).
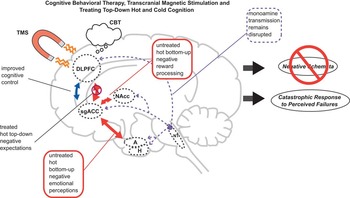
Figure 4 Treating hot and cold top-down cognition in depression. Psychotherapies such as cognitive behavioral therapy (CBT) train patients to challenge their top-down hot biases (negative expectations) and thereby resolve dysfunctional negative schemata. Transcranial magnetic stimulation (TMS) directly activates DLPFC and interconnected structures, improving cold top-down cognitive control and emotional regulation. However, dysfunctional monoamine transmission and bottom-up negative biases (negative perceptions) are not treated directly by CBT or TMS, and may thus persist. Abbreviations and colors as in Figure 1.
However, a complementary view that has gained convincing empirical support over the past decade is that disrupted neurotransmission in systems targeted by antidepressant drugs, such as serotonin, norepinephrine, and dopamine, alters the “bottom-up” processing of emotional stimuli, instantiating “negative perceptions” (Figure 3). Importantly, a large body of evidence suggests that manipulating monoamine transmission experimentally can alter reward and emotional processing biases, in both healthy volunteers and depressed individualsReference Harmer, O'Sullivan and Favaron45–Reference Roiser, Levy and Fromm47 (Figure 5). According to this view, negative biases occur due to compromised monoamine modulation of the neural circuits that process emotional stimuli.Reference Harmer, Goodwin and Cowen48

Figure 5 Treating hot bottom-up cognition in depression. Antidepressant medications target the monoamine systems (5-HT, NE, and DA), which may be disrupted in some depressed patients, thereby resolving bottom-up hot biases (negative perceptions). Deep brain stimulation (DBS) in the sgACC may influence circuits instantiating bottom-up negative biases directly, via connections with other regions in limbic circuits such as A. However, top-down negative biases (negative expectations) are not treated directly by antidepressant medications or DBS, and may thus persist. Abbreviations and colors as in Figure 1.
Our cognitive neuropsychological model of depressionReference Roiser, Elliott and Sahakian23 (Figure 6) proposes an integrated approach, accommodating both the traditional psychological framework (Figure 4) and more recent psychopharmacological findings (Figure 5). We propose that bottom-up biases (negative perceptions), caused by disrupted monoamine transmission, play a causal role in the development of dysfunctional negative schemata, but that the latter can themselves engender top-down biases (negative expectations), thus maintaining negative schemata. This model also proposes a central role for a type of top-down cold cognition, cognitive control, in depression, suggesting that negative perceptions may feed into dysfunctional negative schemata particularly when cognitive control is impaired (Figure 3). Importantly, these different cognitive processes (negative perceptions, negative expectations, and cognitive control) are likely instantiated via the dysfunctional operation of separate, but interacting, neural circuits (Figures 1–3). Being able to identify signals from and manipulate these circuits may enable researchers to better parse the mechanistic heterogeneity of depression, and provide novel approaches to treatment (Figures 4 and 5).

Figure 6 A cognitive neuropsychological model of depression. Red boxes/text indicate factors contributing to the development and maintenance of depressive symptoms. Green boxes/text indicate factors contributing to the treatment of and recovery from depression. 5-HTTLPR: serotonin transporter linked polymorphic region. Reprinted from Roiser etal. Reference Roiser, Elliott and Sahakian23 with permission.
Understanding Novel Treatments Through Cognition
The findings reviewed in this article, together with associated neuroimaging results, provide an important basis for the understanding of two novel brain circuit-based intervention strategies in treatment-resistant depression. First, repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) is a non-invasive method of stimulation, by which a neural circuit implicated in cognitive control can be manipulated directly. Although rTMS for depression was first attempted in the early 1990s (see George etal. Reference George, Taylor and Short49 for a review), the most convincing evidence for efficacy has come from two large double-blind trials,Reference George50, Reference O'Reardon, Solvason and Janicak51 both of which reported that in treatment-resistant patients, 2–3 times as many subjects who were administered active stimulation remitted (∼15%) compared to those receiving sham stimulation.
The mechanisms underpinning rTMS in the treatment of depression remain to be completely clarified, but may relate to top-down cold cognition—specifically cognitive control—and its interaction with top-down hot cognitionReference Roiser, Elliott and Sahakian23 (Figure 4). Several neuroimaging studies have found that depressed subjects exhibit exaggerated prefrontal cortex responses during difficult working memory tasks, interpreted as reflecting prefrontal “inefficiency,” which is not altered by SSRI treatment.Reference Walsh, Williams and Brammer52 By contrast, rTMS to the DLPFC has been reported to boost performance on tests requiring cognitive control in depressed subjects,Reference Martis, Alam and Dowd53 and to improve cognitive control over distracting negative information in healthy volunteers.Reference Leyman, De Raedt, Vanderhasselt and Baeken54 Some preliminary studies have linked these effects to symptomatic relief directly, reporting that treatment-resistant depressed patients who respond to two weeks of rTMS exhibit improved attentional control over neutral information after a single stimulation session,Reference Vanderhasselt, De Raedt, Leyman and Baeken55 when mood effects were not yet apparent, and that treatment response is associated with better inhibition of negative distracting information after 10 days of treatment,Reference Leyman, De Raedt, Vanderhasselt and Baeken56 when symptoms had started to remit. Future studies should explore in greater detail whether rTMS exerts its beneficial effects in depression by boosting cognitive control.
Second, for highly treatment-resistant depressed patients, invasive deep brain stimulation (DBS), particularly to the subgenual anterior cingulate cortex (sgACC), has been found to be effective in open-label trials.Reference Mayberg, Lozano and Voon57 The rationale for targeting this brain region is the reliable finding from neuroimaging studies that it is over-active in depressed individualsReference Drevets58 and increases in metabolism during negative mood.Reference Mayberg, Liotti and Brannan59 The sgACC plays an important role in hot cognition and regulates activity in the amygdala,Reference Vidal-Gonzalez, Vidal-Gonzalez, Rauch and Quirk60 in which responses to negative stimuli are exaggerated in depression and normalized with SSRI treatmentReference Victor, Furey, Fromm, Ohman and Drevets61 (Figures 3 and 5). Importantly, responses to negative stimuli in the sgACC are blunted in both depressed patientsReference Grimm, Boesiger and Beck62 and healthy individuals at genetic risk for depression,Reference O'Nions, Dolan and Roiser63 thus supporting its role in the instantiation of emotional biases. As with rTMS, the mechanism underlying the beneficial effects of DBS is not completely clear, but may be related to resolving negative bottom-up biases (Figure 5) via altered activity in the sgACC and other interconnected regions, for example the amygdala and orbitofrontal cortex.Reference Mayberg, Lozano and Voon57 Future studies should test directly whether stimulation in this region alters bottom-up negative biases in depression.
Conclusion
We have reviewed evidence that supports a central role for both hot (emotion-laden) and cold (emotion-independent) cognition in the pathophysiology and treatment of depression. Depressed patients exhibit reliable impairments on cold neuropsychological tests, and the presence of such impairments during remission suggests that these are not simply epiphenomena of illness. In the domain of hot cognition, negative emotional and reward biases are commonly reported in depression, and the finding that these can be altered by pharmacological intervention suggests that they result from bottom-up, as well as top-down, influences. However, hot and cold cognition are by no means independent, and there is good evidence for heightened responses to negative feedback in depression, which may impair performance on ostensibly cold cognitive tasks. Specifically, informative negative feedback may take on a highly emotive quality, and positive feedback may fail to exert an appropriate motivational influence in depressed patients, thus influencing task performance. Such negative feedback biases may play a particularly important role in disrupting functioning in the workplace or at school.
Our neuropsychological model of depression (Figure 6)Reference Roiser, Elliott and Sahakian23 provides an integrated account of disrupted hot and cold cognition in depression (Figure 3). It also has implications for understanding common treatments such as psychotherapy and medication. For example, SSRIs may assist patients toward the goal of recovery by resolving bottom-up negative biases (Figure 5), but this goal may only be achieved if they use that assistance to work to improve their cognitive and functional outcome by challenging top-down biases (Figure 4), as suggested by the superior treatment efficacy of combined psychotherapy and antidepressant medication relative to each in isolation.Reference Hollon, Jarrett and Nierenberg64 In other words, good mental health is an active process, and we should encourage patients to understand that they may have to work to get better, even while taking antidepressants.
Future studies should focus on the early detection of abnormalities in hot cognition,Reference Owens, Goodyer and Wilkinson65 since 75% of mental health disorders start before the age of 24.Reference Kessler, Berglund and Demler66 This would facilitate earlier treatment or even prevention of depression, stopping it from becoming a lifelong disorder and robbing people of their mental capacity and well-being.Reference Beddington, Cooper and Field67 As a society, we know that we have to work to maintain our physical health by making an effort to eat healthily and exercise, and these messages are reinforced from the start of our formal education. Our view is that society and governments should consider good brain health in exactly the same way.
Disclosures
Jonathan Roiser has the following disclosure: Cambridge Cognition, consultant, consulting fees, retainer. Barbara Sahakian has the following disclosures: Cambridge Cognition, consultant, consulting fees; Janssen Pharmaceuticals, research support, grant; CeNeS Pharmaceuticals, shareholder.