Introduction
Among Nereididae de Blainville, Reference de Blainville1818, Perinereis Kinberg, Reference Kinberg1865 is the second most species-rich genus with 89 valid taxa (Bonyadi-Naeini et al., Reference Bonyadi-Naeini, Rastegar-Pouyani, Rastegar-Pouyani, Glasby and Rahimian2017; Park & Kim, Reference Park and Kim2017; Read & Fauchald, Reference Read and Fauchald2018a; Villalobos-Guerrero, Reference Villalobos-Guerrero2019). The species have a broad ecological distribution ranging from supra-littoral (Wu et al., Reference Wu, Sun and Yang1985) to abyssal zones (deepest record about 3900 m depth; Faulwetter et al., Reference Faulwetter, Simboura, Katsiaras, Chatzigeorgiou and Arvanitidis2017), although they mainly inhabit shallow waters with sandy to muddy bottoms, dwelling among sessile organisms, rock crevices (Hutchings et al., Reference Hutchings, Reid and Wilson1991; Tanaka, Reference Tanaka2016), and fouling communities (Villalobos-Guerrero & Tovar-Hernández, Reference Villalobos-Guerrero and Tovar-Hernández2014).
Perinereis is a polyphyletic genus and cannot currently be accurately diagnosed (Bakken & Wilson, Reference Bakken and Wilson2005). Many authors have provided instead artificial groupings to assist identification of species. The number and type of paragnaths on areas V, VI, or both, were the primary characters proposed for groups in Perinereis, followed by the dorsal ligule development in posterior parapodia, the size of dorsal cirri, or the number of paragnaths on area I (e.g. Kinberg, Reference Kinberg1865; Grube, Reference Grube1878; Horst, Reference Horst1889). Nonetheless, the most recent proposal on Perinereis species groups considered the number of transverse bars on area VI (Groups 1–3) and the expansion of the dorsal ligule in posterior chaetigers (subgroups A and B) (Hutchings et al., Reference Hutchings, Reid and Wilson1991). These groupings by Hutchings et al. (Reference Hutchings, Reid and Wilson1991) have been followed ever since (e.g. Wilson & Glasby, Reference Wilson and Glasby1993; de León-González & Goethel, Reference de León-González and Goethel2013; Darbyshire, Reference Darbyshire2014).
Perinereis species in Groups 2 and 3 sensu Hutchings et al. (Reference Hutchings, Reid and Wilson1991) (hereafter abridged as G2 and G3, respectively) present more distinct differences to the type species P. novaehollandiae Kinberg, Reference Kinberg1865 [= P. amblyodonta (Schmarda, Reference Schmarda1861) fide Ehlers, Reference Ehlers1904]. Those species in G3, viz. the ‘Perinereis nuntia’ species complex, have been pointed out in detail (Wilson & Glasby, Reference Wilson and Glasby1993; Glasby & Hsieh, Reference Glasby and Hsieh2006; Villalobos-Guerrero, Reference Villalobos-Guerrero2019). G2 is characterized by having species with two bar-shaped paragnaths on area VI and dorsal ligules either not greatly (subgroup 2A) or greatly (subgroup 2B) expanded in posterior parapodia (Hutchings et al., Reference Hutchings, Reid and Wilson1991). Subgroup 2A species is the best represented in G2 and more widely distributed, in contrast to subgroup 2B initially proposed without members (Hutchings et al., Reference Hutchings, Reid and Wilson1991) but encompassing a few species known nowadays only from Tropical America (see de León-González et al., Reference de León-González, Villalobos-Guerrero, Conde-Vela, de León-González, Bastida-Zavala, Carrera-Parra, García-Garza, Salazar-Vallejo, Solís-Weiss and Tovar-Hernández2020). In the Eastern and South-eastern Asian seas, four G2 species were proposed: Perinereis aibuhitensis (Grube, Reference Grube1878) from the Philippines, P. linea (Treadwell, Reference Treadwell1936) from China, P. singaporiensis (Grube, Reference Grube1878) from Singapore, and P. vancaurica (Ehlers, Reference Ehlers1868) from the Nicobar Islands.
The present study aims to review the Perinereis species of artificial group G2 sensu Hutchings et al. (Reference Hutchings, Reid and Wilson1991) from the Eastern and South-eastern Asian regions and identify additional species of Perinereis and Neanthes Kinberg, Reference Kinberg1865 which belong in this group. Several species have been redescribed and their generic classification re-assessed. Where molecular data were available, the species identity of some problematic taxa have also been re-evaluated.
Materials and methods
Morphological observation
The type material examined in this study are deposited in the following zoological museums or institutions: Museum für Naturkunde, Berlin, Germany (ZMB); National Institute of Biological Resources, Incheon, Korea (NIBR); National Museum of Natural History, Smithsonian Institution, Washington DC, USA (USNM); National Museum of Nature and Science, Tsukuba Research Departments, Tsukuba, Japan (NSMT); Phyletisches Museum Jena, Friedrich-Schiller-Universität, Jena, Germany (PMJ).
Total length (LT), length from the distal end of prostomium to chaetiger 15 (L15), and body width at chaetiger 15 excluding parapodia (W15) were measured, and the total number of chaetigers was counted for complete specimens. Paragnaths of paired and unpaired areas in the pharynx and the number of teeth on the jaws were counted. Paired areas in pharynx were indicated as ‘a’ for left and ‘b’ for right. The observation of features on non-everted pharynx required, when permitted, a longitudinal dissection in the mid-ventral oral region. Parapodia were dissected and mounted on glass slides to examine parapodial features. Decimal numbers were used for practical purposes when measurements between two structures exceeded one unit (e.g. 1.2 times, 2.5 times, twice); whereas, written fractions were used when those measurements were less than one unit (e.g. half, two-thirds, four-fifths).
Light microscopy observations were made using both stereo and compound microscopes. Specimens were photographed using a digital camera (Canon EOS T6i), which was mounted on each of the microscopes with a portable microscope adaptor; around 15–20 photos were stacked to improve the depth of field using Helicon Focus® 6 (Method C). The figures' background was cleaned, darkened or lightened, and the final figures were assembled in plates using Adobe Photoshop® CS6. Drawings were prepared with a camera lucida attached to a stereoscopic microscope (Nikon SMZ1500). Parapodia were shown in anterior views unless otherwise stated.
In the descriptions, the described specimen's character information was given first, followed by variation values in parentheses for the remaining examined material. The relative extension of parapodial structures and the relative width of ligules and lobes were described following recent studies (Conde-Vela & Salazar-Vallejo, Reference Conde-Vela and Salazar-Vallejo2015; Villalobos-Guerrero & Carrera-Parra, Reference Villalobos-Guerrero and Carrera-Parra2015; Conde-Vela, Reference Conde-Vela2018). However, the length of dorsal cirri was measured in comparison with the full length of the proximal lobe of dorsal ligules (hereinafter proximal dorsal ligule); whereas, the length of the distal lobe of dorsal ligules (hereinafter distal dorsal ligule) was measured regarding the length of proximal dorsal ligules (Villalobos-Guerrero, Reference Villalobos-Guerrero2019).
The atoke and epitoke nereidid parapodial terminology by Villalobos-Guerrero & Bakken (Reference Villalobos-Guerrero and Bakken2018), which was modified from Hylleberg et al. (Reference Hylleberg, Nateewathana and Bussarawit1986) and Bakken & Wilson (Reference Bakken and Wilson2005), is followed. The standardized definitions of the articulations of chaetae proposed by Villalobos-Guerrero & Bakken (Reference Villalobos-Guerrero and Bakken2018) were used. The size of falcigers' blade (b/a ratio) and the length of its serrated edge concerning the total blade length were described following Bakken & Wilson (Reference Bakken and Wilson2005) and Glasby & Hsieh (Reference Glasby and Hsieh2006), respectively. In epitoke specimens, when available, the first natatory chaetiger was determined by the starting chaetiger with an additional parapodial lobe, particularly the lower lobe of ventral cirri cirrophore; other structures such as the natatory chaetae or the expanded neuropodial postchaetal lobe appear later.
The ridges' arrangement at dorsal areas of the oral ring of pharynx, i.e. areas VI–V–VI ridge pattern, is based from Villalobos-Guerrero (Reference Villalobos-Guerrero2019). Jansonius & Craig's (Reference Jansonius and Craig1971) nomenclature terminology of jaws and paragnaths by Bakken et al. (Reference Bakken, Glasby and Wilson2009) were used. The scheme of describing the paragnaths' arrangement on areas VII–VIII, and the partially readapted terminology of bar-shaped paragnaths proposed by Conde-Vela (Reference Conde-Vela2018) is followed. Four types of rectangular-base paragnaths are recognized: (1) Smooth bars, (2) shield-shaped bars, (3) pointed bars (P-bars) and (4) crescent-shaped bars (Bakken et al., Reference Bakken, Glasby and Wilson2009; Conde-Vela, Reference Conde-Vela2018). We adopt the improved terminology of paragnaths' parts (Conde-Vela, Reference Conde-Vela2018) but propose an additional term for describing the different shapes and stoutness of bars on area VI of the Perinereis G2 members exclusively, the (5) broad-petite bars (Figures 1A–L). This bar-shaped paragnath is stout, with a base ovoid to ellipsoid (up to 3 times wider than long; Figure 1B, E, F, H, K, L) and straight in its inferior edge (Figure 1A, C, D, G, I, J); the body has adjacent sides of similar size (Figure 1A, D, G, J) or sometimes slightly skewed to a flank; and the tip can be pointed (Figure 1B, C) or blunt (Figure 1E, F, H, I, K, L). This broad-petite bar sometimes has a conical appearance in anterior view (Figure 1A, C) with an ovoid base in superior view (Figure 1B). It has been usually confused with the conical paragnaths of Neanthes species (see Horst, Reference Horst1924; Wu et al., Reference Wu, Sun and Yang1985; Hutchings et al., Reference Hutchings, Reid and Wilson1991; Lee et al., Reference Lee, Je and Choi1992), which has rendered genus misplacements and species misidentifications. It is likely that variations of these paragnaths, such as the wear of tip, depend on the specimens' maturity or the utilization of the paragnaths themselves during feeding or digging behaviour. The broad-petite bars may have a melted base (Figure 1F, L) similar to that described by Bakken et al. (Reference Bakken, Glasby and Wilson2009) and redefined by Glasby et al. (Reference Glasby, Wilson and Bakken2011); both atoke and epitoke individuals may present this melting, and it is thus unrelated to the reproductive stage.

Fig. 1. Different broad-petite bar-shaped paragnaths on area VI of some Perinereis species belonging to Group 2A: (A–C) P. belawanensis (Pflugfelder, Reference Pflugfelder1933) comb. nov.; (D–F) P. vitabunda (Pflugfelder, Reference Pflugfelder1933) comb. nov.; (G–L) P. linea (Treadwell, Reference Treadwell1936) ((G–I), holotype of Nereis (Neanthes) orientalis Treadwell, Reference Treadwell1936; (J–L) holotype of Nereis (Neanthes) linea). (A, C, D, G, I, J) Anterior view of broad-petite paragnaths; (B, E, F, H, K, L) superior view of broad-petite paragnaths. Drawings indicate the tip (dark brown), body (light brown) and base (yellow) of broad-petite paragnaths. Arrows: white, tip of bar; black, base of bar. Scale bars: (C) 0.3 mm; (F) 0.2 mm; (I, L) 1 mm; remaining figures without scales.
DNA extraction, PCR amplification and molecular analysis
Partial sequences of two DNA barcoding gene regions: mitochondrial cytochrome c oxidase subunit I (COI) and mitochondrial 16S ribosomal DNA (16S rDNA), were obtained to examine the genetic distance between Perinereis linea and P. aibuhitensis. Topotype specimens of P. aibuhitensis and non-type specimens of P. linea were used for DNA sequencing. Another four Perinereis species were also included for in-group comparison: Perinereis anderssoni Kinberg, Reference Kinberg1865 from Brazil, P. euiini Park & Kim, Reference Park and Kim2017 from Korea, P. vallata (Grube & Kröyer in Grube, Reference Grube1858) from Australia and P. vancaurica (Ehlers, Reference Ehlers1868) from Australia, whereas Hediste atoka Sato & Nakashima, Reference Sato and Nakashima2003 from Japan was utilized as outgroup. Several sequences of COI and 16S rDNA genes utilized in the present study are newly sequenced from specimens deposited in NIBR (Table 1). In contrast, a few others were mined from GenBank based upon the following criteria: (1) DNA sequence was obtained from type locality or at least close to the type locality of each species, (2) DNA sequence was obtained from specimens identified by nereidid taxonomists (Table 1).
Table 1. Information on voucher specimens and GenBank accession numbers for molecular analysis

*Taxon used as outgroup for rooting the tree.
Genomic DNA was extracted from the ventral part of the worm's soft tissue using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA) following the manufacturer's instructions. PCR amplifications were conducted using gene-specific primer sets (Table 2). PCR thermal cycling condition for COI followed Park & Kim (Reference Park and Kim2017) and 16S rDNA followed Tosuji et al. (Reference Tosuji, Nishinosono, Hiseh, Glasby, Sakaguchi and Sato2019). Amplified PCR products were purified using the QIAquick PCR purification Kit (Qiagen, Valencia, CA, USA). The sequencing reaction was conducted with BigDye Terminator ver. 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) using each of the same primers. The product was then analysed using an ABI 3730 sequencer (Applied Biosystems, Foster City, CA, USA). Sequences obtained were aligned using MUSCLE implemented in Geneious Prime 2020.1.2. Pairwise distances were calculated using the Kimura-2-parameter model (Kimura, Reference Kimura1980). Dendrograms were constructed using neighbour-joining (NJ) with 1000 times bootstrap resampling in MEGA ver. 10.1.8. for macOS (Stecher et al., Reference Stecher, Tamura and Kumar2020).
Table 2. Information on primers used in this study

Literature review
The list of valid species of Neanthes Kinberg, Reference Kinberg1865 available in the World Polychaeta Database (Read & Fauchald, Reference Read and Fauchald2018b) was examined and compared with those lists formerly performed (Hartman, Reference Hartman1959; Fauchald, Reference Fauchald1972; Wilson, Reference Wilson1984) or with other more recent literatures to discover those Perinereis G2 species currently hidden in Neanthes. The original descriptions, redescriptions, or both, of the 79 currently valid species of Neanthes were gathered and analysed. These literatures were only taken into account to disregard possible misidentifications based upon non-type materials.
Results
The definition of a novel type of short bar-shaped paragnath in this study, i.e. broad-petite bars, has permitted a re-evaluation of some Neanthes species' generic placement traditionally considered as having two ‘conical’ paragnaths on area VI in a single transverse row.
Two semi-terrestrial Neanthes species from the Strait of Malacca, N. belawanensis (Pflugfelder, Reference Pflugfelder1933) and N. vitabunda (Pflugfelder, Reference Pflugfelder1933), and three marine species recently described from Taiwan, N. babuzai Hsueh, Reference Hsueh2019, N. kinmenensis Hsueh, Reference Hsueh2019 and N. shigungensis Hsueh, Reference Hsueh2019, were found with the features of the Perinereis subgroup 2A sensu Hutchings et al. (Reference Hutchings, Reid and Wilson1991): two bar-shaped paragnaths on area VI and proximal dorsal ligule not expanded. Interestingly, all these species also resemble each other by sharing (1) proximal dorsal ligule subequal or becoming shorter towards posterior end, (2) dorsal cirri short, and (3) blades of heterogomph falcigers straight with long terminal tooth forming a tendon. Therefore, P. babuzai comb. nov., P. belawanensis comb. nov., P. kinmenensis comb. nov., P. shigungensis comb. nov. and P. vitabunda comb. nov. are thus transferred to Perinereis and placed within the newly proposed ‘P. aibuhitensis’ species group, which also comprises other species, particularly the stem species P. aibuhitensis and P. linea. They were all compared with other Perinereis species of the subgroup 2A based upon several pharyngeal and parapodial features. Perinereis belawanensis comb. nov. and P. vitabunda comb. nov. are redescribed in detail and distinguished from the similar species P. aibuhitensis; whereas a diagnosis of Hsueh's species transferred to Perinereis is provided based on the original descriptions.
Moreover, P. linea that has been misidentified in the literature with P. aibuhitensis was also redescribed. Morphological comparisons based on the type specimens, non-type materials, or both, reveal that the species can be distinguished from P. aibuhitensis by the patterns of the ridge of areas VI–V–VI, the arrangement of bands of paragnaths on areas VII–VIII, the presence of lateral groups of paragnaths on area III, and the arrangement of paragnaths on area II. Both sequences of COI and 16S rDNA genes newly obtained or mined from GenBank for six Perinereis species demonstrate distinctive clusters for each species in the neighbour-joining tree construction (Figure 2; Table 1, 3). Perinereis linea forms a genetically different cluster with interspecific distances ranging from 20–26.8 for COI and 8.5–15.6 for 16S rDNA (Table 3), supporting it as a distinct species. Hence, we confirm P. linea as a valid species based on this morphological and molecular evidence, and regard N. (Neanthes) orientalis and P. vancaurica tetradentata as junior synonyms of P. linea instead of P. aibuhitensis because of the similar morphology of the type material.

Fig. 2. Neighbour-joining (NJ) tree using partial sequences of COI (A) and 16S rDNA (B) genes for six Perinereis species. Hediste atoka (Hatoka) used as outgroup for rooting the tree. The numbers next to the branches indicate bootstrap support with 1000 replications.
Table 3. Pairwise sequence divergence (%) ranges of partial COI (below diagonal) and 16S rRNA (upper diagonal) among six Perinereis species

a , intraspecific divergence; N, number of specimens; numbers in parentheses indicate ranges of mean divergence.
Nucleotide sequence divergence were based on Kimura 2-parameter model.
Systematics
Phylum ANNELIDA Lamarck, Reference Lamarck1802
Class PLEISTOANNELIDA Struck, Reference Struck2011
Subclass ERRANTIA Audouin & Milne-Edwards, Reference Audouin and Milne-Edwards1832
Order PHYLLODOCIDA Dales, Reference Dales1962
Family NEREIDIDAE de Blainville, Reference de Blainville1818
Genus Perinereis Kinberg, Reference Kinberg1865
Perinereis Kinberg, Reference Kinberg1865: 175; Reference Kinberg1910: 52.
Type species
Perinereis novaehollandiae Kinberg, Reference Kinberg1865, by subsequent designation (fide Hartman, Reference Hartman1949). Currently regarded as a junior synonym of P. amblyodonta Schmarda, Reference Schmarda1861 (Ehlers, Reference Ehlers1904; Hartman, Reference Hartman1959).
Remarks
Perinereis is considered a non-monophyletic genus (Bakken & Wilson, Reference Bakken and Wilson2005; Glasby et al., Reference Glasby, Wei and Gibb2013; Liu et al., Reference Liu, Liu, Wang, Guan and Ge2013). The presence of transverse bars on area VI, or more restrictively smooth bars as stated in subsequent studies (Hutchings et al., Reference Hutchings, Reid and Wilson1991; Bakken et al., Reference Bakken, Glasby and Wilson2009), has been traditionally regarded as the main feature to recognize Perinereis species (e.g. de Saint-Joseph, Reference de Saint-Joseph1898; Gravier, Reference Gravier1902; Fauvel, Reference Fauvel1923; Fauchald, Reference Fauchald1977). This feature is not unique in the genus since it is also shared with species of Eunereis Malmgren, Reference Malmgren1865 (Bakken & Wilson, Reference Bakken and Wilson2005). Furthermore, the type species P. novaehollandiae Kinberg, Reference Kinberg1865 [= P. amblyodonta (Schmarda, Reference Schmarda1861) fide Ehlers, Reference Ehlers1904] has different bars on area VI – shield-shaped (sensu Bakken et al., Reference Bakken, Glasby and Wilson2009) or crescent-shaped (sensu Conde-Vela, Reference Conde-Vela2018) (see Knox, Reference Knox1951). Perinereis differs from the polyphyletic genus Neanthes Kinberg, Reference Kinberg1865 by the presence of bars on area VI (absent in Neanthes sensu Bakken & Wilson, Reference Bakken and Wilson2005), Pseudonereis Kinberg, Reference Kinberg1865 by the absence of both P-bars and comb-like rows on areas II–IV (present in Pseudonereis sensu Conde-Vela, Reference Conde-Vela2018 and Villalobos-Guerrero & Idris, Reference Villalobos-Guerrero and Idris2020), and Eunereis by the presence of paragnaths on the maxillary ring (absent in Eunereis sensu Bakken & Wilson, Reference Bakken and Wilson2005). In the broad sense, the members of Perinereis are characterized by having paragnaths well-separated and mostly conical on both pharyngeal rings and bar-shaped paragnaths on area VI. However, a comprehensive revision of this polyphyletic genus is needed to restrict the genus definition, detect reliable generic features, and remove disparate species. The genus definition followed here is based on the phylogenetic study of Nereidinae sensu Fitzhugh (Reference Fitzhugh1987) by Bakken & Wilson (Reference Bakken and Wilson2005).
‘Perinereis aibuhitensis' species group
Perinereis subgroup 2A: Hutchings et al., Reference Hutchings, Reid and Wilson1991: 271–273 (partim).
Diagnosis
Prostomium with anterior margin complete. Four eyes, lenticulate. Antennae present. Palpophores with marked transverse groove. Four pairs of tentacular cirri with distinct cirrophores. Apodous anterior segment greater than length of chaetiger 1. Jaws denticulate, two canals emerging from pulp cavity. Maxillary and oral pharyngeal rings with paragnaths only (Figure 3A–D), rarely absent on area V. Conical paragnaths on all areas, except on area VI (rarely one); bar-shaped paragnaths only on area VI, two (rarely one, occasionally 3–4) in a transverse row on each side (Figure 3A, B); area IV without merged paragnaths. Pharyngeal areas VI–V–VI ridge pattern λ-shaped or π-shaped. Paired oesophageal caeca present. Glandular patches present in dorsal ligule. Notopodia well-developed. Dorsal cirri short, conical at least in middle and posterior parapodia, attached medially to dorsal ligule. Proximal dorsal ligule similar in size throughout body, or slightly enlarged in posterior parapodia. Distal dorsal ligule subequal throughout or becoming shorter towards posterior end. Notopodial prechaetal lobe absent, sometimes as acicular process in anterior chaetigers. Neuropodial postchaetal lobe absent. Neuropodial superior and inferior lobes blunt, present at least in anterior parapodia. Ventral ligule present throughout. Ventral cirri single. Notoaciculae absent in first two chaetigers, thereafter present. Aciculae black. Notochaetae all homogomph spinigers, throughout. Supracicular neurochaetae with homogomph spinigers and heterogomph falcigers, both throughout. Subacicular neurochaetae with heterogomph spinigers and heterogomph falcigers, both throughout. Blades of falcigers straight, with incurved terminal tooth markedly elongated forming distinct tendon (Figure 3E). Anal cirri with cirrophore.
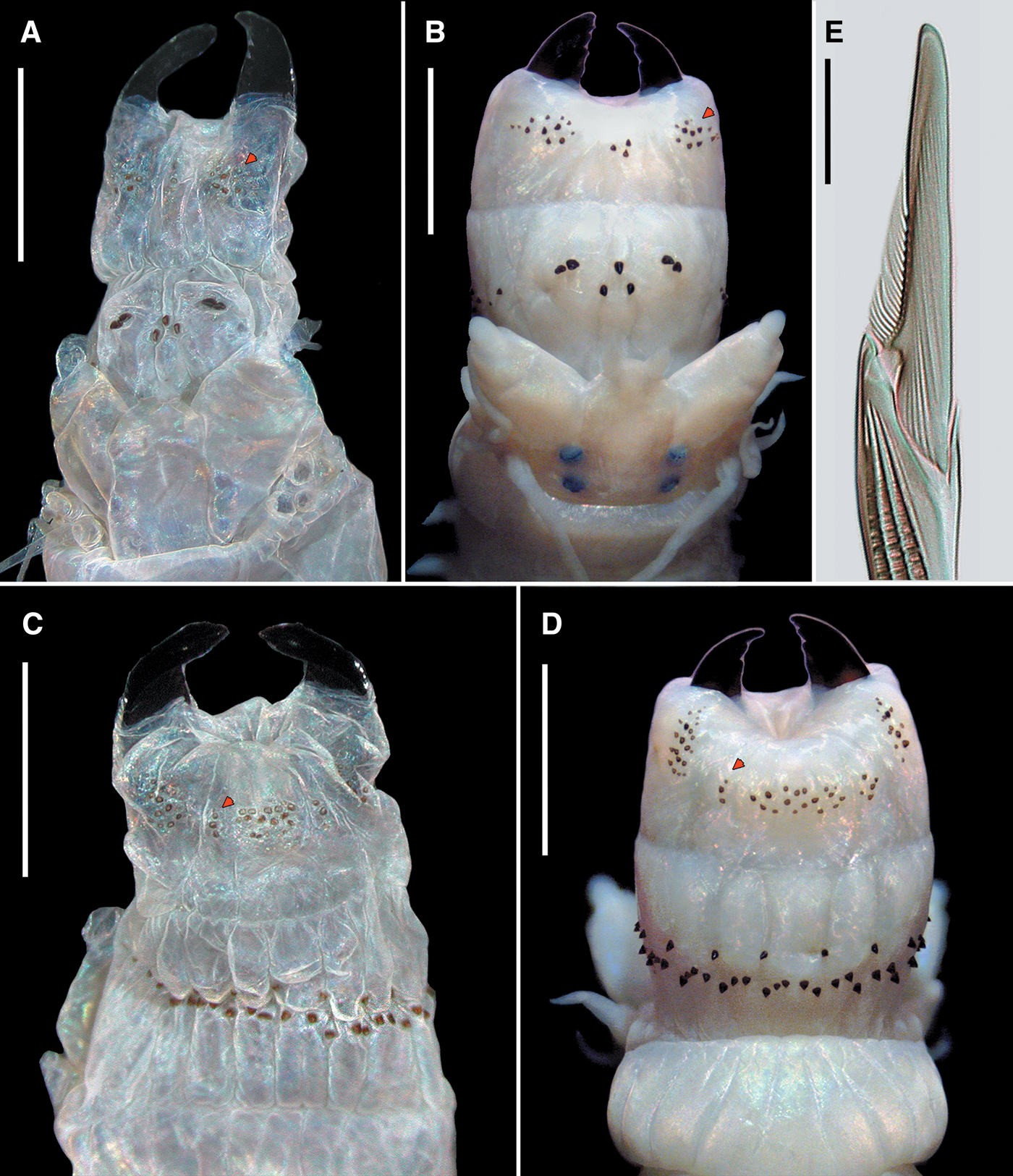
Fig. 3. Perinereis aibuhitensis (Grube, Reference Grube1878). (A, C, E) Paralectotype of Nereis (Perinereis) aibuhitensis (ZMB Q.3440), Aibukit village, Palau, atoke; (B, D) topotype of P. aibuhitensis, Melekeok, Palau, atoke. (A, B) Anterior region in dorsal view (arrows pointing distal edge of rows on area II); (C, D) anterior region in ventral view (arrows pointing lateral isolated paragnaths on area III); (E) heterogomph falciger from neuropodial supracicular fascicle (anterior chaetiger). Scale bars: A–D, 2 mm; E, 20 μm.
Remarks
According to Hutchings et al. (Reference Hutchings, Reid and Wilson1991), Perinereis species of the subgroup 2A are characterized by having area VI with two bar-shaped paragnaths and dorsal ligules not greatly expanded in posterior parapodia. In the present study, we noticed that most subgroup 2A species also share short dorsal cirri (not projecting beyond the end of distal dorsal ligule in medial parapodia) and blade of heterogomph falcigers straight with incurved terminal tooth markedly elongated forming a distinct tendon.
A major morphological species group is, therefore, here proposed for 11 species that share all the morphological features mentioned above: Perinereis aibuhitensis, P. brevicirrata (Treadwell, Reference Treadwell1920), P. linea, P. rookeri de León-González & Goethel, Reference de León-González and Goethel2013, P. singaporiensis (Grube, Reference Grube1878), P. vancaurica (Ehlers, Reference Ehlers1868), and other five species previously in Neanthes but here transferred: Perinereis babuzai comb. nov., P. belawanensis comb. nov., P. kinmenensis comb. nov., P. shigungensis comb. nov. and P. vitabunda comb. nov. The remaining species of the subgroup 2A have long dorsal cirri, extending further beyond distal dorsal ligule in medial parapodia, and blades of heterogomph falcigers with incurved terminal tooth short and inconspicuous tendon. These species are P. camiguinoides (Augener, Reference Augener and Skottsberg1922), P. horsti Gravier, Reference Gravier1899, P. jascooki Gibbs, Reference Gibbs1972, P. kuwaitensis Mohammad, Reference Mohammad1970 and P. variodentata (Augener, Reference Augener, Michaelsen and Hartmeyer1913). All these species can be morphologically distinguished as stated in the key (see below).
The most representative species in subgroup 2A is P. aibuhitensis (Grube, Reference Grube1878), originally described from Palau. This species is widely studied due to its commercial value in both aquaculture and recreational fisheries (Gu et al., Reference Gu, Jiang and Zheng2002; Deng et al., Reference Deng, Ma, Niu, Dong and Su2007), as a biological indicator of marine pollution (Wang et al., Reference Wang, Zhou, Zhang and Zhang2008; Yang et al., Reference Yang, Zhou, Zhao, Zhou, Sun, Wang and Yuan2012; Tian et al., Reference Tian, Liu, Wang, Zhou and Tang2014), as a model in ecotoxicology studies (Yuan et al., Reference Yuan, Chen, Zhou, Liu and Yang2010; Leung & Chan, Reference Leung and Chan2018), and even applications in traditional and modern medicine (Gu et al., Reference Gu, Jiang and Zheng2002; Pan et al., Reference Pan, Liu, Ge, Han and Zheng2004; Li et al., Reference Li, Li, Liu, Wang, Zhou, Cheng, Feng, Cheng, Liu and Chen2017). The species has also been subjected to several taxonomic studies using specimens from different geographic regions (e.g. Horst, Reference Horst1924; Fauvel, Reference Fauvel1932, Reference Fauvel1953; Wu et al., Reference Wu, Sun and Yang1985; Hylleberg et al., Reference Hylleberg, Nateewathana and Bussarawit1986; Lee et al., Reference Lee, Je and Choi1992; Khlebovich, Reference Khlebovich1996; Sun & Yang, Reference Sun and Yang2004), and combining them in a single redescription with the type material (Hutchings et al., Reference Hutchings, Reid and Wilson1991). Hence, we have selected P. aibuhitensis to name the species group.
Perinereis babuzai (Hsueh, Reference Hsueh2019) comb. nov.
Neanthes babuzai Hsueh, Reference Hsueh2019: 174–177, figs 1, 2, table 2.
Diagnosis (based upon Hsueh, 2019)
Species of subgroup 2A belonging to ‘P. aibuhitensis’ species group. Specimens with broad-petite bars on area VI; areas VI–V–VI ridge pattern λ-shaped; distal dorsal ligule anteriorly conical, posteriorly distinctly short; neuroacicular ligule posteriorly subequal to median ligule; falcigers with camerated shaft divided into two partitions; postero-dorsal tentacular cirri extending to chaetigers 5–9.
Remarks
Neanthes babuzai Hsueh, Reference Hsueh2019 is here transferred to Perinereis based on having bar-shaped paragnaths on area VI. The species resembles P. linea by having broad-petite bars on area VI, distal dorsal ligule conical in anterior parapodia, and areas VI–V–VI ridge pattern λ-shaped. Some relevant characters such as the presence of laterally isolated paragnaths on area III and the number of rows in the anterior band of areas VII–VIII were not mentioned nor illustrated in the original description, and the number of divisions in the camerated shaft of falcigers is unclear, and thus remain unknown. However, the length of distal dorsal ligule in posterior parapodia can distinguish both species. In P. babuzai comb. nov., the distal dorsal ligule is distinctly short in posterior parapodia, projecting barely beyond notoaciculae; whereas, in P. linea the distal dorsal ligule is of medium length in posterior parapodia, projecting markedly beyond notoaciculae.
Habitat
Intertidal muddy bottom.
Reproduction
Unknown.
Type locality
Xianxi, Changhua County, Taiwan.
Distribution
The species is known only from the type locality in Changhua County (Taiwan).
Perinereis belawanensis (Pflugfelder, Reference Pflugfelder1933) comb. nov.
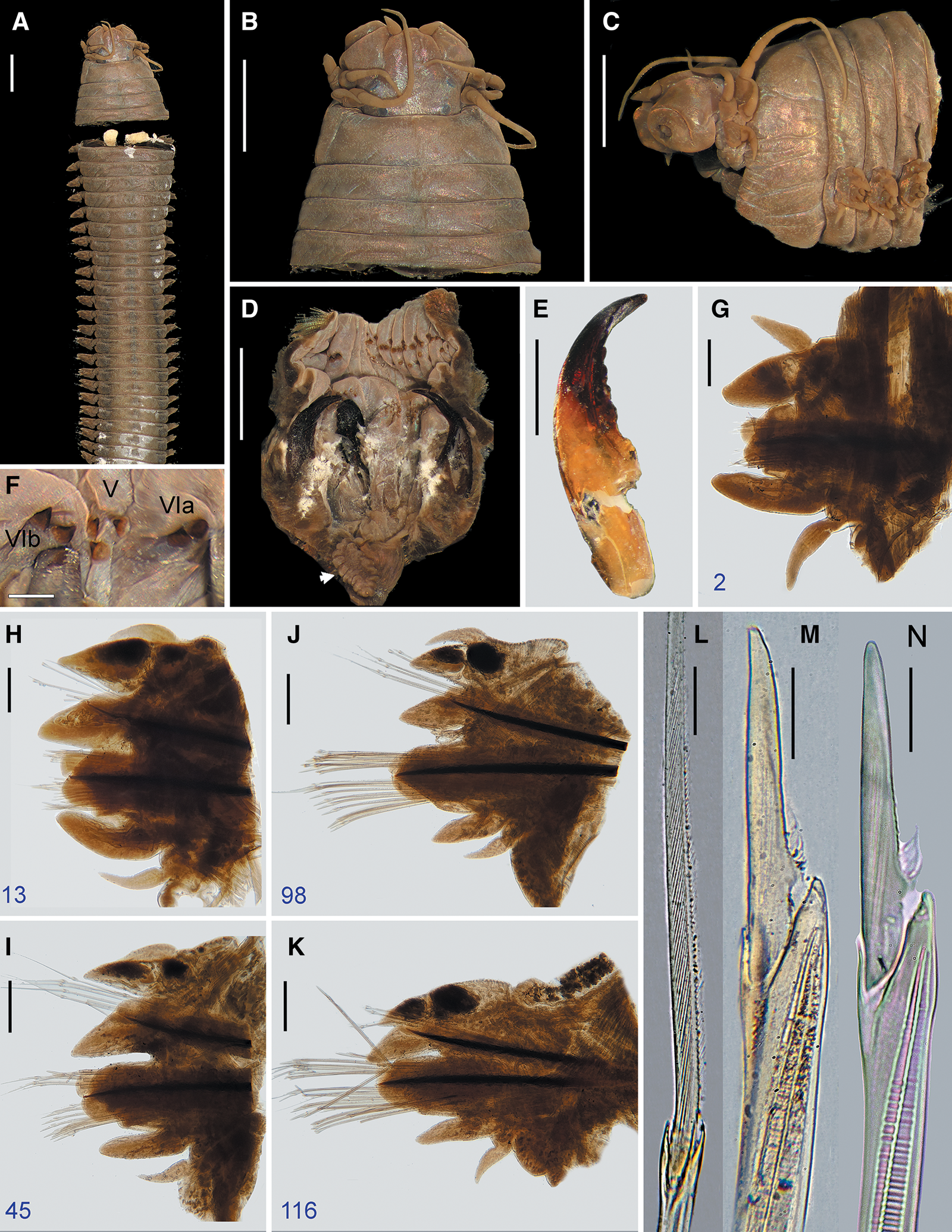
Fig. 4. Perinereis belawanensis (Pflugfelder, Reference Pflugfelder1933) comb. nov. Holotype (PMJ Ann-168), Belawan, Sumatra, atoke: (A) anterior region in dorsal view; (B) prostomium in dorsal view; (C) anterior region in lateral view; (D) non-everted pharynx in ventral view (arrow pointing caecal gland); (E) right jaw in dorsal view; (F) oral ring in ventral view; (G–K) parapodia, numbers refer to the chaetiger; (L) homogomph spiniger from notopodia (chaetiger 60); (M) heterogomph falciger from neuropodial supracicular fascicle (chaetiger 116); (N) heterogomph falciger from neuropodial subacicular fascicle (chaetiger 98). Scale bars: A–D, 2 mm; E, 1 mm; F, 0.5 mm; G–K, 0.2 mm; L–N, 20 μm.
Nereis belawanensis Pflugfelder, Reference Pflugfelder1933: 72–73, fig. 13A–D; Harms, Reference Harms1934: 29–30 (habitat); Wesenberg-Lund, Reference Wesenberg-Lund1958: 29 (species list); Salazar-Vallejo et al., Reference Salazar-Vallejo, Carrera-Parra, Muir, de León-González, Piotrowski and Sato2014: 23 (species list).
Neanthes belawanensis: Hartman, Reference Hartman1959: 250; Fauchald, Reference Fauchald1972: 409; Wilson, Reference Wilson1984: 225 (all species list).
Neanthes succinea: Hartman, Reference Hartman1974: 618 (non Leuckart, Reference Leuckart, Frey and Leuckart1847).
Neanthes belewanensis (sic): Glasby et al., Reference Glasby, Timm, Muir and Gil2009: 14.
Type material
Holotype: PMJ Ann-168, Belawan, Sumatra, Indonesia, coll. J. W. Harms, 1927 or 1929.
Material examined
One specimen: PMJ Ann-167a, Belawan, Sumatra, Indonesia, coll. J. W. Harms, 1927 or 1929, atoke, in good condition.
Diagnosis
Species of subgroup 2A belonging to ‘P. aibuhitensis’ species group. Specimens with broad-petite bars on area VI; areas VI–V–VI ridge pattern π-shaped; area III with laterally isolated paragnaths; areas VII–VIII with anterior band of paragnaths consisting of two rows; neuroacicular ligule markedly projected; distal dorsal ligule distinctly short; falcigers with camerated shaft divided into two partitions; postero-dorsal tentacular cirri extending to chaetiger 4.
Description
Holotype atoke, incomplete, posterior part missing, in good condition except already cut off into two parts at level of third and fourth chaetigers, 75 (95) mm LT, 13 (13.8) mm L15, 3.5 (3.2) mm W15, with 133 (140) chaetigers. Body colour brownish (Figure 4A), with three darkish longitudinal lines of tegument on dorsum of segments: one mid-dorsal line and two dorsolateral lines present throughout; body covered by salt granules in mid-anterior dorsal part.
Prostomium campanulate, faintly stretching in middle, as long as wide; anterior end broad, distally complete; anterolateral gap aside palpophore broad, 1.5 times as wide as basal diameter of antennae (Figure 4B). Nuchal organs deeply embedded, medium size, subequal to diameter of posterior pair of eyes.
Palpophores sub-conical, thick, as long as wide, as long as three-quarters of entire prostomium; sub-distal transverse groove distinct, deeply embedded (Figure 4B, C). Palpostyles oval, one-quarter as wide as diameter of palpophore.
Antennae tapered, thick, short; extending forwards to tip of palpophore and posteriorly to distal quarter of length of prostomium; antennae separated with gap as wide as basal diameter of antennae (Figure 4B).
Paired eyes blackish, arranged in a trapezoid form; gap between both pairs twice diameter of posterior pair of eyes (Figure 4B); anterior pair of eyes oval, subequal to basal diameter of antennae, gap between both eyes as wide as 5.5 times diameter of eyes, with lens distinct, whitish, covering 85% of eye; posterior pair of eyes rounded, three-quarters as wide as basal diameter of antennae, with lens distinct, purplish, placed in middle of eye and covering 50% of it.
Apodous anterior segment 3.5 times wider than long, 1.7 times as long as chaetiger 1 (Figure 4A–C), with even anterior margin, dorsum without marked transverse wrinkles.
Tentacular cirri slender, smooth (Figure 4B, C); postero-dorsal cirri extending posteriorly to chaetiger 4, 2.3 times as long as antero-dorsal cirri; antero-dorsal cirri extending posteriorly to chaetiger 1; postero-ventral cirri extended over first quarter of prostomium; antero-ventral cirri as long as three-quarters of postero-ventral cirri and slightly smaller than palpophore; cirrophores cylindrical, postero-dorsal cirrophores longest, postero-ventral cirrophores narrowest.
Pharynx not everted, previously dissected with pharyngeal bulb and its surrounding muscle removed from body, separated in vial. Jaws (Figure 4D, E) reddish in distal half, remaining amber, with eight slightly developed and blunt denticles; pulp cavity as long as three-fifths of jaw, with two thick canals. Pharynx with paragnaths brownish on maxillary ring (Figure 4D) and reddish paragnaths on oral ring (Figure 4D, F), consisting of uniform-base cones, except broad-petite bars on area VI; merged paragnaths and plate-like basements absent. Area I: 2, longitudinal row of cones, distal one smaller; areas IIa: 15 (10) and IIb: 12 (11), three irregular rows of uneven cones in ovoid slightly curved patch, medial cones larger; area III: 25 (18), four slightly regular rows of uneven cones in sub-rounded patch, distal cones larger, with distinct isolated lateral groups; areas IVa: 19 (16) and IVb: 15, five regular transverse rows of uneven cones in sub-oval patch, distal-most and most proximal cones shorter; area V: 3, triangular patch of coarse cones of similar size, two proximal cones in transverse row and single distal cone on same level as distal-most paragnath on area VI; areas VIa: 2 and VIb: 2, one oblique row of even, coarse broad-petite bars with pointed tip, barely separated (Figures 1A–C, 4F); areas VII–VIII: 36 (35), two well-separated bands of coarse and uneven cones, with anterior band consisting of two transversely aligned rows (furrow row with one stout paragnath on each region, ridge row with a slightly shorter cone only on ridge regions A and paired B), and posterior band with two transverse rows displaced from each other (furrow row proximal with one cone on each region, ridge row distal with two cones on each region). Areas VI–V–VI ridge pattern, π-shaped. Gap between area VI and areas VII–VIII broad, as wide as palpophore.
Paired oesophageal caeca present (Figure 4D).
Parapodia with blackish, glandular notopodial patches, more distinct in posterior chaetigers (Figure 4K).
Notopodia consisting of dorsal cirrus, dorsal ligule (distal and proximal), and median ligule in biramous parapodia; notopodial prechaetal lobe or notoacicular process not developed throughout.
Dorsal cirri conical, thick, short (Figure 4G–K), extending up to three-quarters of distal dorsal ligule throughout; dorsal cirri longer than proximal dorsal ligule in anteriormost parapodia (Figure 4F), subequal in anterior parapodia (Figure 4H), shorter in following parapodia (Figure 4I–K); dorsal cirri inserted basally to dorsal ligules in anteriormost parapodia, one-third in anterior parapodia, medially in following parapodia.
Proximal dorsal ligule even towards posterior end; subequal to distal dorsal ligule in anterior parapodia (Figure 4H), becoming longer than distal dorsal ligule from medial parapodia (Figure 4I), twice as long distal dorsal ligule in posterior parapodia (Figure 4J, K); one massive, sub-oval glandular patch throughout, larger in posterior parapodia (Figure 4K).
Distal dorsal ligule becoming gradually shorter towards posterior end, extending beyond end of notoaciculae throughout, slightly in posterior parapodia (Figure 4I–K); distal dorsal ligule conical throughout (Figure 4H–K), shorter than median ligule throughout, except in anteriormost parapodia; one massive, sub-oval glandular patch throughout, larger than that in proximal dorsal ligule in anterior parapodia, becoming smaller in medial and posterior parapodia (Figures 4J, K).
Median ligule bluntly conical in anteriormost and anterior parapodia (Figure 4H, I), conical and becoming slightly shorter and narrower in following parapodia (Figure 4J, K).
Neuropodia consisting of neuroacicular ligule with superior and inferior lobes, ventral ligule, and ventral cirrus; neuropodial postchaetal lobe reduced throughout.
Neuroacicular ligule shorter than ventral ligule in anteriormost parapodia (Figure 4G), subequal in anterior parapodia, distinctly longer in following parapodia (Figure 4I–K); neuroacicular ligule twice as wide as ventral ligule in anteriormost and anterior parapodia, 2.5 times as wide in medial and posterior parapodia.
Superior lobe rounded, subequal to inferior lobe and neuroacicular ligule in anterior and medial parapodia (Figure 4G–J), reduced in posterior parapodia from chaetiger 118.
Inferior lobe rounded, slightly longer than neuroacicular ligule in first 34 chaetigers (Figure 4G, H), becoming shorter and narrower in following parapodia.
Ventral ligule digitiform and subequal to median ligule in anteriormost and anterior parapodia (Figure 4G, H), slightly tapering and becoming shorter in following parapodia (Figure 4I–K).
Ventral cirri cirriform and thick in anteriormost and anterior parapodia (Figure 4G, H), becoming conical and narrower in following parapodia; ventral cirri as long as two-thirds of ventral ligule, except one-half of ventral ligule in posterior parapodia.
Pygidium missing but topotype with regenerating posterior end, anal cirri incomplete, as long as last two chaetigers.
Aciculae black, with basal end uncoloured. Notoaciculae absent in chaetigers 1 and 2 (Figure 4G). Neuroaciculae markedly extending beyond distal end of notoaciculae throughout (Figure 4H–K); neuroaciculae shorter than median ligule in anteriormost and anterior parapodia, subequal to median ligule in medial and posterior parapodia.
Notochaetae all homogomph spinigers; 11–13 spinigers present in anterior parapodia, 6–10 spinigers in medial parapodia, 3–5 spinigers in posterior parapodia.
Supracicular neurochaetae consisting of homogomph spinigers and heterogomph falcigers, both present throughout; 1–2 spinigers present in anteriormost and anterior parapodia, 3–4 spinigers in medial parapodia, 2–3 spinigers in posterior parapodia; 7–9 falcigers present in anteriormost and anterior parapodia, 5–7 falcigers in medial and posterior parapodia.
Subacicular neurochaetae consisting of heterogomph spinigers and heterogomph falcigers, both present throughout; 1–2 spinigers present in anteriormost parapodia, 3–4 spinigers in anterior parapodia, 1–2 spinigers in medial and posterior parapodia; 14–16 falcigers in anteriormost and anterior parapodia, 8–10 falcigers in medial parapodia, 11–13 falcigers in posterior parapodia.
Blades of both homogomph (Figure 4L) and heterogomph spinigers finely serrated towards toothed edge, evenly spaced, long with high b/a ratio (9–16.5). Blades of heterogomph falcigers long with low b/a ratio (2–2.5), slender, straight, distal end digitiform with incurved terminal tooth very long forming distinct tendon (equalling about two-fifths of total blade length: 0.42–0.47); blades of falcigers partially serrated, with serrations capilliform, curved, looking upwards, present in about half (0.49–0.52) of total blade length (Figure 4M, N); vertex between distal and basal end on serrated edge markedly prominent, sub-conical. Shaft of falcigers camerated, with cavity divided sub-distally into two distinct longitudinal partitions (Figure 4M, N).
Remarks
Perinereis belawanensis (Pflugfelder, Reference Pflugfelder1933) comb. nov. belongs to the P. aibuhitensis species group characterized by having short dorsal cirri throughout the body and blade of heterogomph falcigers straight with incurved terminal tooth markedly elongated and forming a distinct tendon. Perinereis belawanensis comb. nov. resembles P. aibuhitensis, P. rookeri and P. vitabunda comb. nov. by sharing areas VI–V–VI ridge pattern π-shaped, area VI with broad-petite bars, and area III with distinct laterally isolated paragnaths. Nonetheless, P. belawanensis comb. nov. is separated from P. aibuhitensis and P. vitabunda comb. nov. by having an anterior band of areas VII–VIII with two transverse rows on furrows and ridges (Figure 4D) and the camerated shaft of falcigers divided sub-distally into two partitions (Figure 4M) (only furrow row on the anterior band of areas VII–VIII and three partitions in the shaft of falcigers in the latter two species; Figure 3C–E). Likewise, P. belawanensis comb. nov. can be distinguished from P. aibuhitensis and P. rookeri because the neuroacicular ligule is projecting markedly beyond ventral ligule in posterior parapodia (Figure 4K), the distal dorsal ligule is distinctly shorter than median ligule throughout the body (Figure 4H–K), and the proximal dorsal ligule is longer than distal dorsal ligule in medial and posterior parapodia (Figure 4J, K); whereas in P. aibuhitensis and P. rookeri the neuroacicular ligule is subequal to or slightly shorter than ventral ligule, the distal dorsal ligule is subequal to or barely shorter than median ligule, and both proximal and distal dorsal ligules are subequal in medial and posterior parapodia. Perinereis belawanensis comb. nov. can also be distinguished from P. rookery because the blade of the heterogomph falciger is evenly slender towards the distal end, whereas in P. rookery the proximal end of the blade is more expanded than the distal end.
Perinereis belawanensis comb. nov. is more similar to P. vitabunda comb. nov. in terms of habitat and locality; however, they are different in several respects. In P. belawanensis comb. nov., the distal dorsal ligules exceed the distal end of notoaciculae, whereas in P. vitabunda comb. nov. they are subequal to or slightly shorter than notoaciculae. In P. belawanensis comb. nov., the postero-dorsal tentacular cirri are longer (reaching chaetiger 4) than in P. vitabunda comb. nov. (reaching chaetiger 1). In P. belawanensis comb. nov., the camerated shaft of falcigers has cavity divided sub-distally into two longitudinal partitions, although in P. vitabunda comb. nov. it is divided into three partitions. In P. belawanensis comb. nov., the paragnaths on area VI are arranged obliquely, area III has 25 (18) paragnaths, and area IV has 15–19 paragnaths; whereas in P. vitabunda comb. nov., the paragnaths on area VI are arranged transversally, area III has 36 paragnaths, and area IV has 31 paragnaths. Finally, in P. belawanensis comb. nov., the nuchal organs are subequal to the diameter of posterior eyes, which in P. vitabunda comb. nov. are distinctly shorter than the diameter of the same eyes.
Perinereis belawanensis comb. nov. is a semi-terrestrial species from Sumatra originally described as a Nereis by Pflugfelder (Reference Pflugfelder1933). The species was transferred to Neanthes and recognized there without further information (Hartman, Reference Hartman1959; Fauchald, Reference Fauchald1972; Wilson, Reference Wilson1984; Glasby et al., Reference Glasby, Timm, Muir and Gil2009). Moreover, Hartman (Reference Hartman1974) synonymized the species with Neanthes succinea (Leuckart, Reference Leuckart, Frey and Leuckart1847) (currently in Alitta Kinberg, Reference Kinberg1865) from the North Sea with unknown justification. However, both species are markedly different, and the synonymy has prevailed ever since and listed as such in recent works either in Neanthes or in Alitta (e.g. Salazar-Vallejo et al., Reference Salazar-Vallejo, Carrera-Parra, Muir, de León-González, Piotrowski and Sato2014; Villalobos-Guerrero & Carrera-Parra, Reference Villalobos-Guerrero and Carrera-Parra2015; Read & Fauchald, Reference Read and Fauchald2019). After re-examining the type material, the species is here transferred to Perinereis.
Perinereis belawanensis comb. nov. has not been recorded since the original description. The specimens used by Pflugfelder (Reference Pflugfelder1933) were collected by Jürgen W. Harms in 1927 or 1929, who provided a detailed description of the habitat of the species (Harms, Reference Harms1934).
Habitat
Semi-terrestrial. Dwelling in burrows dug within almost-dried, sandy-clay soil in 20–30 cm depth, which are only partially rinsed by water when tides are high, usually living along with its congener P. vitabunda comb. nov. (Pflugfelder, Reference Pflugfelder1933).
Reproduction
Unknown.
Type locality
Belawan, Sumatra, Indonesia.
Distribution
The species is known only from the type locality, Belawan (Indonesia).
Perinereis kinmenensis (Hsueh, Reference Hsueh2019) comb. nov.
Neanthes kinmenensis Hsueh, Reference Hsueh2019: 183–185, figs 9, 10, table 2.
Diagnosis (based upon Hsueh, Reference Hsueh2019)
Species of subgroup 2A belonging to ‘P. aibuhitensis’ species group. Specimens with broad-petite bars on area VI; areas VI–V–VI ridge pattern π-shaped; area III with laterally isolated paragnaths; areas VII–VIII with anterior band consisting of one row; distal dorsal ligule anteriorly subulate, subequal in size throughout; neuroacicular ligule posteriorly subequal to median ligule; falcigers with camerated shaft divided into three partitions; postero-dorsal tentacular cirri extending to chaetiger 2.
Remarks
Neanthes kinmenensis Hsueh, Reference Hsueh2019 is here transferred to Perinereis based on the presence of bar-shaped paragnaths on area VI. The species resembles P. aibuhitensis by having broad-petite bars on area VI, distal dorsal ligule projecting markedly beyond notoaciculae, areas VI–V–VI ridge pattern π-shaped, areas VII–VIII with the anterior band having only one furrow row, and area III with distinct laterally isolated paragnaths. However, both species can be distinguished by the shape of ligules in anterior parapodia, the division of camerated shaft of falcigers, and the length of postero-dorsal tentacular cirri. In P. kinmenensis comb. nov., the ligules in anterior parapodia are slender and acuminate, whereas those in P. aibuhitensis are thicker with a blunt tip. Finally, in P. kinmenensis comb. nov. the postero-dorsal tentacular cirri extend posteriorly to chaetiger 2, whereas in P. aibuhitensis they extend to chaetiger 4–5.
Habitat
Intertidal soft bottom.
Reproduction
Unknown.
Type locality
Yangshan, Kinmen County, Fujian, China.
Distribution
The species is known only from the type locality, Yangshan in Fujian (China).
Perinereis linea (Treadwell, Reference Treadwell1936)
(Figures 1G–L, 5–9)
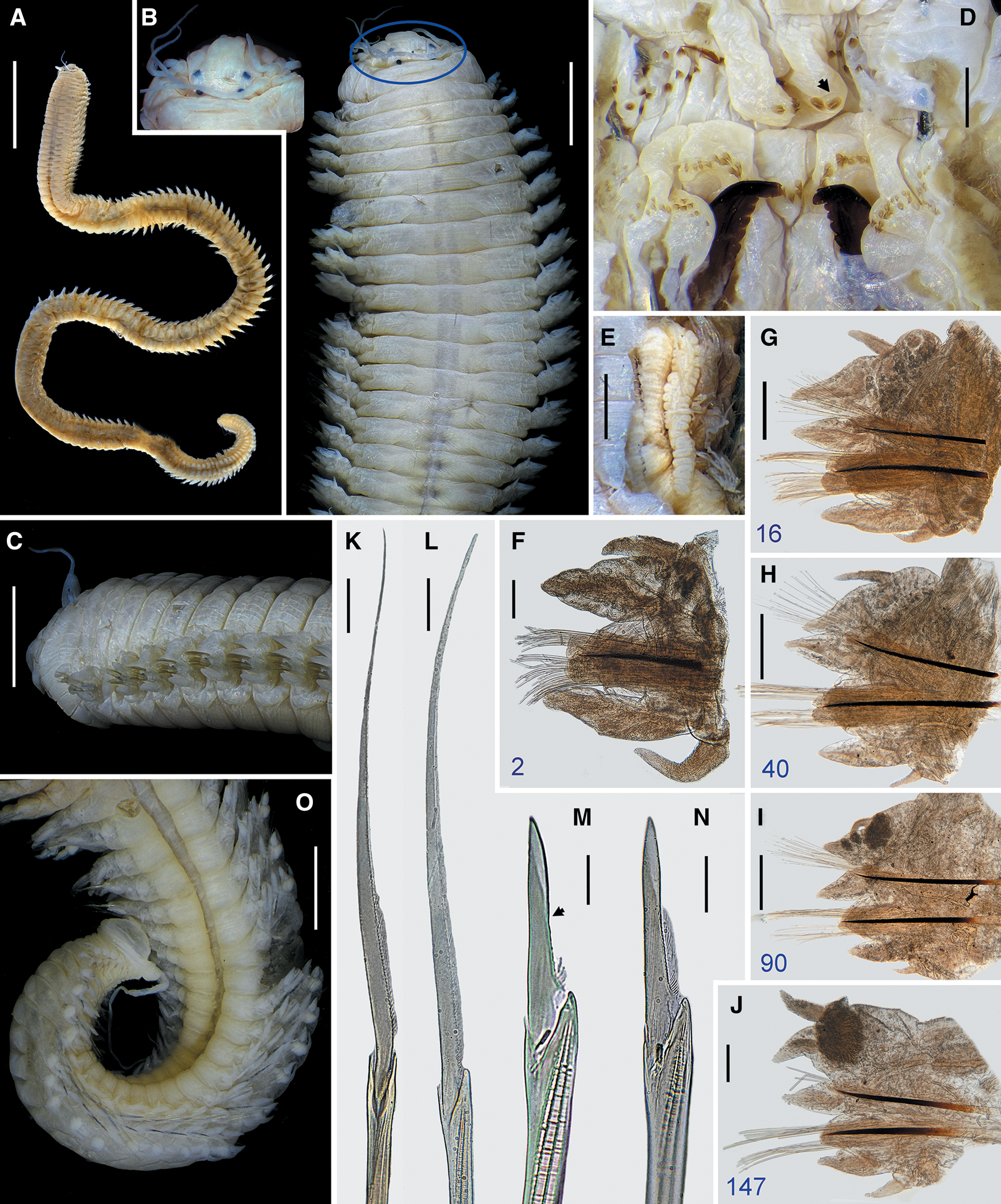
Fig. 5. Perinereis linea (Treadwell, Reference Treadwell1936). Holotype (USNM 20115), Amoy (Xiamen), Fujian, China, atoke: (A) whole body in dorsal view; (B) anterior region in dorsal view (frame showing prostomium); (C) anterior region in lateral view; (D) non-everted pharynx in ventral view (arrow pointing broad-petite bar); (E) paired oesophageal glands in ventral view; (F–J) parapodia, numbers refer to the chaetiger; (K) homogomph spiniger from neuropodial supracicular fascicle with medial and distal serrations broken (chaetiger 16); (L) heterogomph spiniger from neuropodial subacicular fascicle (chaetiger 40); (M) heterogomph falciger from neuropodial supracicular fascicle with most serrations broken (chaetiger 16, arrow indicates end of serration); (N) heterogomph falciger from neuropodial subacicular fascicle (chaetiger 16). Scale bars: A, 15 mm; B, C, F, J, O, 2 mm; D, G–I, 0.5 mm; E, 1 mm; K–N, 20 μm.
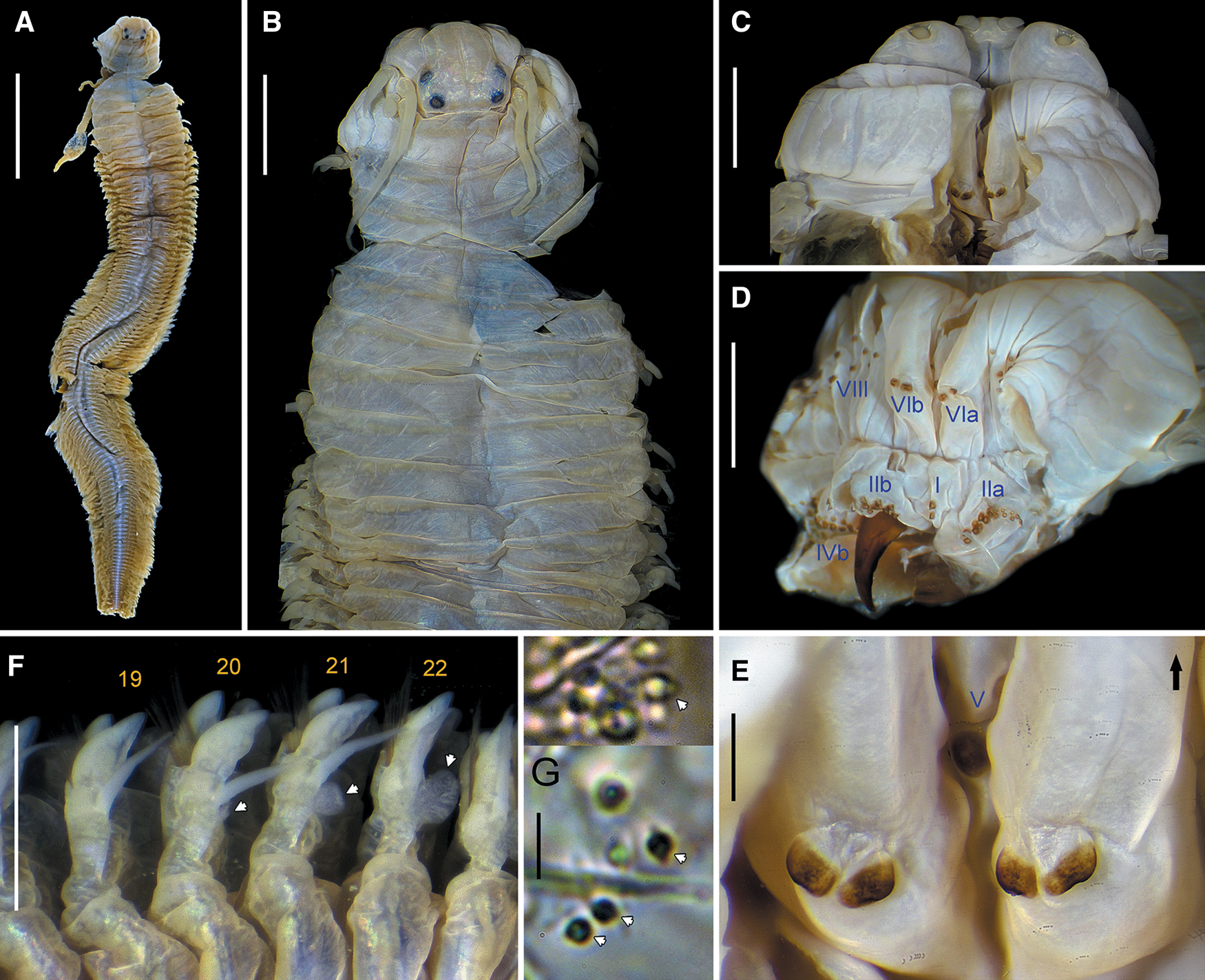
Fig. 6. Perinereis linea (Treadwell, Reference Treadwell1936). Holotype of Nereis (Neanthes) orientalis Treadwell, Reference Treadwell1936 (USNM 20116), Amoy (Xiamen), Fujian, China, epitoke male: (A) whole body in dorsal view; (B) anterior region in dorsal view; (C) buccal region in ventral view; (D) everted pharynx in dorso-lateral view; (E) dorsal areas of oral ring of non-everted pharynx (arrow pointing orientation of prostomium); (F) right parapodia from chaetigers 19–22 in dorsal view (arrows pointing upper lobe of dorsal ligule); (G) mature sperm pointed by arrows. Scale bars: A, 10 mm; B, D, 3 mm; C, E, F, 2 mm; G, 5 μm.

Fig. 7. Perinereis linea (Treadwell, Reference Treadwell1936). Holotype of Nereis (Neanthes) orientalis Treadwell, Reference Treadwell1936 (USNM 20116), Amoy (Xiamen), Fujian, China, epitoke male: (A) left parapodia from chaetigers 19–26 in ventral view (arrows pointing lower lobe of ventral cirri cirrophore); (B–H) parapodia, numbers refer to the chaetiger (black arrow pointing inferior lobe; blue arrow pointing lower secondary lobe of median ligule; green arrow pointing upper secondary lobe of ventral cirri cirrophore; grey arrow pointing upper secondary lobe of ventral ligule; red arrow pointing upper secondary lobe of distal dorsal ligule); (I) basal end of notoacicula (left) and neuroacicula (right) (chaetiger 40); (J) heterogomph falciger from neuropodial subacicular fascicle (chaetiger 2). Scale bars: A, 1 mm; B-G, 0.5 mm; H, I, 0.2 mm; J, 20 μm.

Fig. 8. Perinereis linea (Treadwell, Reference Treadwell1936). (A–C) Holotype of P. vancaurica tetradentata Imajima, Reference Imajima1972 (NSMT-Pol-H78), Tokyo, Japan, atoke. (A) Anterior region in dorsal view (arrows pointing concave edge of rows on area II); (B) anterior region in ventral view; (C) heterogomph falciger in neuropodial supracicular fascicle (anterior chaetiger). Scale bars: A, B, 2 mm; C, 20 μm.
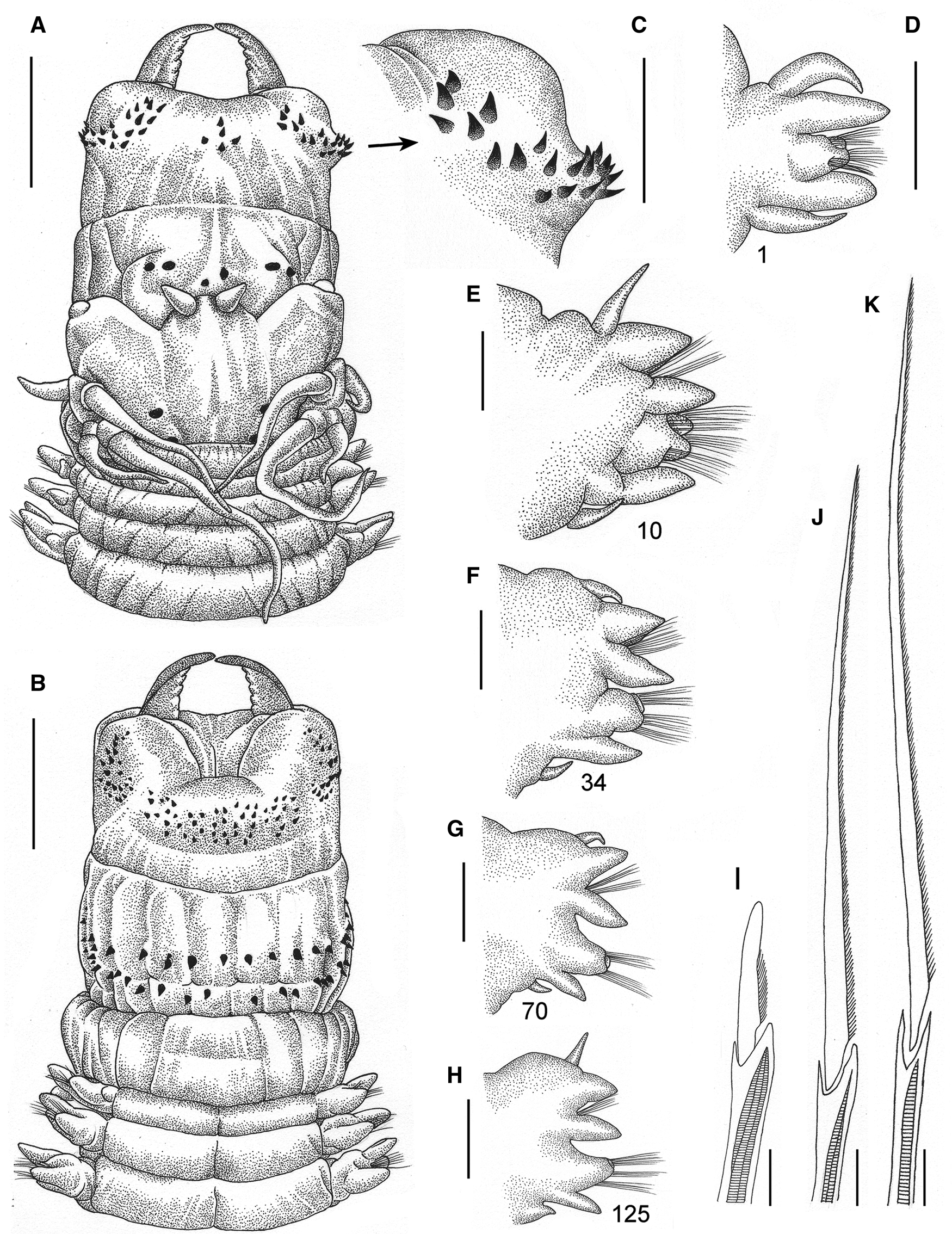
Fig. 9. Perinereis linea (Treadwell, Reference Treadwell1936). Non-types from Ganghwado Island, Korea: (A–C) NIBRIV0000783811; (D–K) NIBRIV0000783812. (A) Anterior region in dorsal view; (B) anterior region in ventral view; (C) right flank of maxillary ring (note curved paragnaths on area II); (D–H) parapodia, numbers refer to the chaetiger; (I) heterogomph falciger from neuropodial supracicular fascicle (chaetiger 34); (J) heterogomph spiniger from neuropodial subacicular fascicle (chaetiger 70); (K) homogomph spiniger from notochaetae (chaetiger 34). Scale bars: A, B, 2 mm; C–H, 1 mm; I–K, 20 μm.
Perinereis aibuhitensis: Fauvel, Reference Fauvel1933: 25–26; Hartman, Reference Hartman1938: 15; Khlebovich & Wu, Reference Khlebovich and Wu1962: 39, 50–51; Wu et al., Reference Wu, Sun and Yang1985: 189–193, figs 107–109 (partim, non records from Hainan Island); Lee et al., Reference Lee, Je and Choi1992: 1–10, figs. 2–3; Imajima, Reference Imajima1996: 131, fig. 104A–H; Khlebovich, Reference Khlebovich1996: 147, pl. 46, figs 1–7 (partim, only records from the Yellow Sea); Sun & Yang, Reference Sun and Yang2004: 180–183, figs 101–103 (partim, non records from Hainan Island) (non Grube, Reference Grube1878).
Nereis aibuhitensis: Monro, Reference Monro1934: 361–362 (non Grube, Reference Grube1878).
Nereis (Neanthes) linea Treadwell, Reference Treadwell1936: 268–270, figs 19A–E.
Nereis (Neanthes) orientalis Treadwell, Reference Treadwell1936: 270–272, figs 19F–I.
Perinereis linea: Wu, Reference Wu1967: 68–69, figs 10a–d; Sato, Reference Sato, Motokawa and Kajihara2017: 492–493, table 19.1 (Perinereis vancaurica tetradentata jun. syn.).
Perinereis vancaurica tetradentata Imajima, Reference Imajima1972: 86–88, fig. 23; Paik, Reference Paik1975: 7, pl. 6, figs 44–46; Reference Paik1977: 172–174, figs 16A–F; Reference Paik1989: 309–311, text figs 72A–E, pl. 24, figs 62A (1–2), pl. 25, fig. 62A–3; Sato, Reference Sato, Motokawa and Kajihara2017: 493.
Neanthes virens: Paik, Reference Paik1975: 412–413, pl. 3, figs 16–24; Reference Paik1977: 200–202, figs 29A–F; Reference Paik1982: 789, pl. 14, figs J–L, Reference Paik1989: 339–341, text figs 89A–H, pl. 32, figs 78(1–2), pl. 33, fig. 78–3 (partim, non figures in plates, non Sars, Reference Sars1835).
Type material
Holotype: Nereis (Neanthes) linea Treadwell, Reference Treadwell1936, USNM 20115, Amoy (Xiamen), Fujian, China, coll. T. Y. Chen.
Holotype: Nereis (Neanthes) orientalis Treadwell, Reference Treadwell1936, USNM 20116, Amoy (Xiamen), Fujian, China, coll. T. Y. Chen.
Holotype: Perinereis vancaurica tetradentata Imajima, Reference Imajima1972, NSMT-Pol-H78, Sumida-gawa River, Tokyo, Japan, coll. A. Izuka (?), 20 July 1908.
Comparative material examined
Nereis (Perinereis) aibuhitensis Grube, Reference Grube1878. Paralectotype: ZMB Q.3440, Aibukit village, Ngebuked, Babeldaob Island, Palau, coll. C. G. Semper, any date between end of March 1862 to January 1863 (sensu Semper, Reference Semper1873). Topotypes: Four specimens (NIBRIV0000787926), Melekeok, Palau (7°28′5″N 134°36′42″E), coll. T. Park, 5 August 2013, mangrove, fixed in 80% ethanol. Non-types: Twenty-four specimens (NIBRIV0000787927), Koh Kong, Cambodia, Gulf of Thailand, coll. T. Park, 6 September 2011, mangrove, fixed in 70% ethanol; one specimen (NIBRIV0000787924), Koh Rung Samloem Island, Cambodia (10°37′20″N 103°17′37″E), Gulf of Thailand, coll. T. Park, 29 April 2012, sandy beach, fixed in 80% ethanol.
Other material examined
Fourteen specimens (NIBRIV0000783811, 1 ind.; NIBRIV0000783812, 1 ind.; NIBRIV0000783813, 1 ind.; NIBRIV0000783814, 11 inds), Ganghwado Island, Dongmak-ri, Hwadomyeon, Ganghwa-gun, Incheon-si, Korea, coll. T. Park, 12 May 2013, muddy tidal flat, fixed in 10% formalin. Nine specimens (NIBRIV0000317216, 3 inds; NIBRIV0000317217, 2 inds; NIBRIV0000317218, 2 inds; NIBRIV0000317220, 2 inds), Daehang-ri, Byeonsan-myeon, Buan-gun, Jeollabuk-do, Korea (35°41′55.3″N 126°33′8.4″E), coll. H.-K. Choi, muddy tidal flat, 13 August 2014. Four specimens (NIBRIV0000129004), Sangam-ri, Buan-myeon, Gochang-gun, Jeollabuk-do, Korea, coll. Y. Eun & S.-S. Hong, muddy tidal flat, 24 July 2007. One specimen (NIBRIV0000262307), Songnim-ri, Janghang-eup, Seocheon-gun, Chungcheongnam-do, Korea, coll. S.-H. Kim, 9 August 2010. One specimen (NIBRIV0000521098), Dongho-ri, Haeri-myeon, Gochang-gun, Jeollabuk-do, Korea (35°31′18″N 126°29′8.84″E), coll. H.-K. Choi, 3 May 2015. One specimen (NIBRIV0000282343), Jungsan-dong, Jung-gu, Incheon-si, Korea (37°31′45.19″N 126°35′25.83″E), coll. S.-Y. Wang, 7 March 2012. Five specimens (NIBRIV0000810291), Jeongok Harbor, Jeongok-ri, Hwaseong-si, Gyeonggi-do, Korea (37°11′12″N 126°38′3″E), coll. P. G. Lee and H. P. Lee, 8 March 2012, muddy tidal flat, fixed in 80% ethanol. Seven specimens (NIBRIV0000866077), Dalian, Liaoning, China, coll. R. Sun, no further data. Five specimens (NIBRIV0000810299), fishing bait shop, Shirahama, Nishimuro-gun, Wakayama, Japan, 8 January 2006, fixed in 99% ethanol by Ko Tomikawa.
Diagnosis
Species of subgroup 2A belonging to ‘P. aibuhitensis’ species group. Specimens with broad-petite bars on area VI; areas VI–V–VI ridge pattern λ-shaped; area III without laterally isolated paragnaths; areas VII–VIII with anterior band consisting of two rows; distal dorsal ligule anteriorly conical, subequal in size throughout; falcigers with camerated shaft divided into three partitions; postero-dorsal tentacular cirri extending to chaetiger 5–6.
Description
Holotype of Nereis (Neanthes) linea Treadwell, 1936
Atoke, complete, in good condition, 172 mm LT, 17 mm L15, 4.5 mm W15, with 158 chaetigers. Body colour yellowish (Figure 5A), lacking pigmentation.
Prostomium campanulate (Figure 5B), 1.3 times longer than wide; anterior end broad, distally complete; anterolateral gap aside palpophore broad, twice as wide as basal diameter of antennae. Nuchal organs deeply embedded, small, subequal to diameter of posterior pair of eyes.
Palpophores sub-conical, thick, 1.5 times longer than wide, as long as four-fifths of entire prostomium; sub-distal transverse groove distinct. Palpostyles oval, two-fifths as wide as diameter of palpophore.
Antennae tapered, thick, short; extending forwards to three-quarters of palpophore and posteriorly to distal quarter of length of prostomium; antennae separated with gap as wide as basal diameter of antennae.
Paired eyes blackish, arranged in trapezoid form; gap between both pairs 2.5 times as wide as diameter of posterior pair of eyes (Figure 5B); anterior pair of eyes reniform, as wide as basal diameter of antennae, gap between both eyes 5 times diameter of eyes, with lens distinct, whitish, covering 35% of eye; posterior pair of eyes rounded, three-quarters as wide as basal diameter of antennae, with lens distinct, whitish, placed mid-posteriorly in eyes and covering 60% of it.
Apodous anterior segment 5 times wider than long, 1.5 times as long as chaetiger 1 (Figure 5B), with even anterior margin, dorsum without marked transverse wrinkles.
Tentacular cirri markedly slender, smooth (Figure 5B); postero-dorsal cirri extending posteriorly to chaetiger 6, 1.5 times as long as antero-dorsal cirri; antero-dorsal cirri extending posteriorly to chaetiger 3; postero-ventral cirri extended over half of prostomium; antero-ventral cirri as long as three-fifths of postero-ventral cirri and extending to two-thirds of palpophore; cirrophores of anterior cirri ring-shaped, those in posterior cirri cylindrical, postero-dorsal cirrophores 1.5 times as long as antero-dorsal cirrophores, antero-ventral cirrophores broadest, postero-ventral cirrophores narrowest.
Pharynx not everted, previously dissected. Jaws with 10 slightly developed and blunt denticles; pulp cavity with two canals. Pharynx with paragnaths dusky-yellow amber on maxillary ring and brownish paragnaths on oral ring (Figure 5D), consisting of uniform-base cones, except broad-petite bars on area VI; merged paragnaths and plate-like basements absent. Area I: 4, cones of similar size in sub-rhomboidal patch, except proximal cone slightly longer; areas IIa: 17 and IIb: 19, two irregular rows of uneven cones in crescent and markedly curved patch, distal cones curved and longer; area III: 48, four irregular rows of cones with similar size in rectangular patch, without distinct isolated lateral groups; areas IVa: 16 and IVb: 14, four two irregular rows of uneven cones in crescent and markedly curved patch, medial cones longer; area V: 3, linear oblique row of coarse cones with similar size, distal-most cone on same level as paragnaths on area VI; areas VIa: 2 and VIb: 2, one transverse row of uneven, coarse broad-petite bars with blunt tip and melted base (Figure 1J–L, 5D), separated, inner bar slightly longer; areas VII–VIII: 55, two well-separated bands of coarse cones, with anterior band consisting of two transversely aligned rows (furrow row and ridge row with one cone on each region), and posterior band with two transverse rows slightly displaced from each other (furrow row proximal with one cone on each region, ridge row distal with two cones on region A and one or two cones in remaining regions). Areas VI–V–VI ridge pattern, λ-shaped. Gap between area VI and areas VII–VIII broad, as wide as palpophore.
Paired oesophageal caeca present (Figure 5E).
Parapodia with barely distinct, whitish glandular notopodial patches.
Notopodia consisting of dorsal cirrus, dorsal ligule (distal and proximal), and median ligule in biramous parapodia; notopodial prechaetal lobe or notoacicular process not developed throughout.
Dorsal cirri digitiform, thick, short (Figure 5F–J), extending up to three-quarters of distal dorsal ligule throughout; dorsal cirri longer than proximal ligule in anteriormost parapodia (Figure 5F), subequal in anterior parapodia (Figure 5G), shorter in following parapodia (Figure 5J); dorsal cirri inserted basally to dorsal ligules in anteriormost parapodia, one-third in anterior parapodia (Figure 5G), medially in medial and posterior parapodia (Figure 5H, I), sub-distally in posteriormost parapodia (Figure 5J).
Proximal dorsal ligule even towards posterior end except slightly enlarged in posterior parapodia; shorter than distal dorsal ligule in anteriormost and anterior parapodia, becoming longer than distal dorsal ligule from medial parapodia, 1.3 times as long as distal dorsal ligule in posterior parapodia (Figure 5I), twice as long as distal dorsal ligule in posteriormost parapodia (Figure 5J); glandular patch massive and sub-oval, more distinct in medial and posterior parapodia (Figure 5I, J).
Distal dorsal ligule extending markedly beyond end of notoaciculae throughout (Figure 5G–J); conical (Figure 5C, F–J), subequal or slightly smaller than median ligule throughout (Figure 5G–I), except slightly longer in anteriormost parapodia (Figure 5J); one whitish glandular patch throughout, much smaller than that in proximal dorsal ligule of medial and posterior parapodia (Figure 5J).
Median ligule conical throughout, becoming slightly shorter and narrower from medial parapodia towards posterior end (Figure 5G–J).
Neuropodia consisting of neuroacicular ligule with superior and inferior lobes, ventral ligule, and ventral cirrus; neuropodial postchaetal lobe reduced throughout.
Neuroacicular ligule subequal and twice as wide as ventral ligule throughout (Figure 5F–H), except slightly longer in posterior parapodia.
Superior lobe rounded, subequal to inferior lobe in anteriormost parapodia, becoming shorter than inferior lobe in following ones (Figure 5G, H), reduced in posterior parapodia from chaetiger 87.
Inferior lobe rounded, longer than neuroacicular ligule in first 125 parapodia (Figure 5G, H), becoming shorter and narrower towards posterior end from chaetiger 32.
Ventral ligule conical, thick and subequal to median ligule in anteriormost parapodia (Figure 5C), becoming shorter and slightly narrower in following parapodia (Figure 5I, J).
Ventral cirri digitiform and thick in anteriormost parapodia (Figure 5C, F), becoming conical in following ones; ventral cirri as long as two-thirds of ventral ligule in anteriormost parapodia, as long as one-quarter of ventral ligule in following parapodia.
Pygidium with anal cirri elongated, as long as last 25 chaetigers, with small cirrophores.
Aciculae black, with basal end uncoloured. Notoaciculae absent in chaetigers 1 and 2 (Figure 5F). Neuroaciculae extending beyond distal end of notoaciculae throughout; neuroaciculae as long as two-thirds of median ligule in anterior and medial parapodia, as long as one-half of median ligule in following parapodia (Figure 5J).
Notochaetae all homogomph spinigers; 20–22 spinigers present in anterior and medial parapodia, 15–16 spinigers in posterior parapodia, 3–5 in posteriormost parapodia.
Supracicular neurochaetae consisting of homogomph spinigers and heterogomph falcigers, both present throughout; 3–4 spinigers in anteriormost, anterior and medial parapodia, 7–8 spinigers in posterior parapodia, 4–5 spinigers in posteriormost parapodia; 7–8 falcigers present in anteriormost parapodia, 12–13 falcigers in anterior parapodia, 8–9 falcigers in medial parapodia, 6–7 falcigers in posterior parapodia, 4–5 falcigers in posteriormost parapodia.
Subacicular neurochaetae consisting of heterogomph spinigers and heterogomph falcigers, both present throughout; 5–6 spinigers in anteriormost and anterior parapodia, 3–4 spinigers in medial and posterior parapodia, 1–2 spinigers in posteriormost parapodia; 21–24 falcigers in anteriormost parapodia, 15–18 falcigers in anterior parapodia, 12–14 falcigers in medial parapodia, 9–10 falcigers in posterior parapodia, 6–7 falcigers in posteriormost parapodia.
Blades of both homogomph (Figure 5K) and heterogomph (Figure 5L) spinigers finely serrated towards toothed edge, evenly spaced, long with high b/a ratio (8.5–13). Blades of heterogomph falcigers long with low b/a ratio (2.3–3.3), slender, straight, distal end club-shaped with incurved terminal tooth very long forming distinct tendon (equalling about two-fifths of total blade length: 0.36–0.38); blades of falcigers partially serrated, with serrations capilliform, curved, looking upwards, present in about one-third (0.34–0.35) of total blade length (Figure 5M, N); vertex between distal and basal end on serrated edge markedly prominent, sub-conical. Shaft of falcigers camerated, with cavity divided sub-distally into three longitudinal partitions (Figure 5N).
Holotype of Nereis (Neanthes) orientalis Treadwell, 1936
Epitoke male, incomplete, possibly only a few posterior chaetigers missing, relatively in good condition, anterior region almost detached, strongly dissected at third and fourth chaetigers (parapodia missing), 74 mm LT, 15 mm L15, 6 mm W15, with 112 chaetigers. Body colour brownish (Figure 6A), with a transverse pale brown line on distal third of dorsum and venter of all chaetigers, whitish pigmentation in dorsum and venter of natatory chaetigers.
Prostomium campanulate, slightly wider than long (Figure 6B); anterior end broad, distally complete; anterolateral gap aside palpophore broad, twice as wide as basal diameter of antennae. Nuchal organs deeply embedded, small, subequal to diameter posterior pair of eyes.
Palpophores oval, thick (Figure 6C), slightly wider than long, as long as three-fifths of entire prostomium; sub-distal transverse groove distinct. Palpostyles oval, one-third as wide as diameter of palpophore.
Antennae tapered, thick, short (Figure 6C); extending forwards to tip of palpophore, posteriorly to distal quarter of length of prostomium; antennae separated, with gap as wide as basal diameter of antennae.
Paired eyes blackish, slightly enlarged, arranged in a trapezoid form; gap between both pairs as wide as diameter of posterior pair of eyes (Figure 6B); anterior pair of eyes rounded, 1.7 times as wide as basal diameter of antennae, gap between both eyes as wide as 3.5 times diameter of eyes, with lens distinct, whitish, covering 70% of eye; posterior pair of eyes rounded, as wide as diameter of anterior pair, with lens distinct, whitish, placed mid-posteriorly in eyes and covering 60% of it.
Apodous anterior segment 4 times wider than long, 1.5 times as long as chaetiger 1 (Figure 6B), with even anterior margin, dorsum without marked transverse wrinkles.
Tentacular cirri thickened, smooth (Figure 6B); postero-dorsal cirri extending posteriorly to chaetiger 5, 1.5 times as long as antero-dorsal cirri; antero-dorsal cirri extending posteriorly to chaetiger 2; postero-ventral cirri extended over half of prostomium; antero-ventral cirri as long as four-fifths of postero-ventral cirri and extending beyond palpophore; cirrophores of anterior cirri ring-shaped, those in posterior cirri cylindrical, postero-dorsal cirrophores 1.3 times as long as antero-dorsal cirrophores, antero-ventral cirrophores broadest, postero-ventral ones narrowest.
Pharynx not everted, previously dissected. Jaws with three barely developed denticles; pulp cavity with two canals. Pharynx with paragnaths dusky-yellow amber on maxillary ring (Figure 6D) and brownish paragnaths on oral ring (Figure 6D, E), consisting of uniform-base cones, except broad-petite bars on area VI; merged paragnaths and plate-like basements absent. Area I: 2, one longitudinal row of cones with similar size; areas IIa: 18 and IIb: 19, two irregular rows of uneven cones in crescent, markedly curved patch, distal cones curved and longer; area III: 47, four irregular rows of cones with similar size in rectangular patch, with distinct isolated lateral groups; areas IVa: 14 and IVb: 17, two irregular rows of uneven cones in crescent, markedly curved patch, medial cones longer; area V: 3, triangular patch of coarse cones of similar size, two proximal cones in transverse row and single distal cone on same level as paragnaths on area VI; areas VIa: 2 and VIb: 2, one oblique row of uneven broad-petite bars with rounded tip and melted base (Figure 1G–I, 6D, E), separated, inner bar slightly longer; areas VII–VIII: 39, two well-separated bands of coarse cones, with anterior band consisting of two transversely aligned rows (furrow row and ridge row with one cone on each region), and posterior band with two transverse rows slightly displaced from each other (furrow row proximal with one cone on each region, ridge row distal with two cones on region A and one cone in remaining regions). Areas VI–V–VI ridge pattern, λ-shaped. Gap between area VI and areas VII–VIII broad, as wide as palpophore (Figure 6D, E).
Paired oesophageal caeca present.
Parapodia with distinct, whitish glandular notopodial patches.
Body regionalized into two distinct sections (Figures 6A, F, 7A): 19 pre-natatory chaetigers and 93 natatory chaetigers but incomplete, becoming gradually slender towards posterior end.
Pre-natatory chaetigers with notopodia consisting of dorsal cirrus, dorsal ligule (distal and proximal), and median ligule in biramous parapodia; and neuropodia consisting of neuroacicular ligule with superior and inferior lobes, ventral ligule, and ventral cirrus. First eight dorsal and ventral cirri modified: dorsal cirri teardrop-shaped with distinctly convex upper edge, markedly longer than distal dorsal ligule in parapodia 1–2 (Figure 7B), becoming narrower and barely longer than dorsal ligule in parapodia 3–7, dorsal cirri broad and slightly papillated ventrolaterally in parapodia 8 (Figure 7C); ventral cirri tapering, thick, acuminated, subequal to ventral ligule in parapodia 1–2 (Figure 7B), becoming narrower and shorter than ventral ligule in parapodia 3–8 (Figure 7C). Dorsal and ventral cirri of parapodia 9–19 unmodified, cirriform (Figure 7D). Distal dorsal ligules digitiform in parapodia 1–2 (Figure 7B), conical in following parapodia; slightly longer than median ligule throughout.
Natatory chaetigers with notopodia consisting of dorsal cirrus, unilobate distal dorsal ligule, unilobate proximal dorsal ligule, notopodial prechaetal lobe, and unilobate median ligule; neuropodia consisting of neuroacicular ligule with superior and inferior lobes, neuropodial postchaetal lobe, unilobate ventral ligule, ventral cirrus, and tri-lobate cirrophore of ventral cirrus. Dorsal cirri elongated, 1.5 times as long as proximal dorsal ligule in anterior and medial parapodia (Figure 7E, F), subequal to proximal dorsal ligule in following parapodia; dorsal cirri with ventrolateral papillae in parapodia 25–95 (Figure 7F), 3 papillae in first papillated parapodia, up to 8 papillae reached in medial parapodia. Ventral cirri elongated, longer than ventral ligule in anterior parapodia (Figure 7E), slightly shorter in following parapodia; ventral cirri with two sub-distal, barely developed papillae in anterior parapodia, absent in following parapodia. Ventral cirri cirrophore with reniform lower lobe present from parapodia 20 (Figure 7A) but markedly enlarged from parapodia 23 to posterior end, digitiform upper lobe present from parapodia 22 to posterior end, and digitiform upper secondary lobe present from parapodia 23 to posterior end, shorter than upper lobe. Proximal dorsal ligule with reniform upper lobe present from parapodia 20 but markedly enlarged from parapodia 25 to posterior end (Figure 7E–G). Distal dorsal ligule lanceolate, slightly elongated, lamellar, with a basal, knob-shaped upper secondary lobe. Notopodial prechaetal lobe enlarged, short, wider than long. Median ligule sub-oval, slightly elongated, barely lamellar, with a basal lower secondary lobe from parapodia 25 to posterior end, reniform in medial parapodia (Figure 7G), digitiform in anterior and posterior parapodia (Figures 7E, H). Neuroacicular ligule slightly elongated and slender, subequal or barely longer than ventral ligule throughout. Neuropodial postchaetal lobe with upper lamella in parapodia 24, enlarged in parapodia 26 but broad flabellate from about parapodia 28 towards posterior end (Figure 7F, G). Superior lobe rounded, not enlarged. Inferior lobe enlarged, rounded, lamellar. Ventral ligule slightly elongated, barely lamellar, sub-oval, with a bluntly conical and basal upper secondary lobe from parapodia 23 to posterior end, more distinct in medial parapodia (Figure 7F, G). Notoaciculae with expanded basal end (Figure 7I). Atoke chaetae not entirely replaced, only a few remain. Epitoke chaetae paddle-like, present in both notochaetae and neurochaetae from parapodia 25 to posterior end; spinigers and falcigers (Figure 7J) as atoke holotype of N. (Neanthes) linea.
Pygidium missing.
Spermatozoa with spherical head, somewhat-inflated conical acrosome, and long flagellum (ect-aquasperm type) (Figure 6G).
Holotype of Perinereis vancaurica tetradentata Imajima, 1972
Atoke, complete but regenerating posterior end, in good condition, 192 mm LT, 16.5 mm L15, 5 mm W15, with 172 chaetigers. Body colour greyish brown (Figure 8A, B), pigmentation completely faded, except brownish posterior dorsum of prostomium.
Prostomium campanulate (Figure 8A), slightly longer than wide; anterior end broad, distally complete; anterolateral gap aside palpophore broad, twice as wide as basal diameter of antennae.
Palpophores sub-conical, thick (Figure 8A), 1.5 times longer than wide, as long as entire prostomium; sub-distal transverse groove distinct. Palpostyles oval, one-third as wide as diameter of palpophore.
Antennae tapered, thick, short (Figure 8A); extending forwards to tip of palpophore and posteriorly to distal third of length of prostomium; antennae separated with gap as wide as basal diameter of antennae.
Paired eyes blackish, arranged in trapezoid form (Figure 8A); gap between both pairs 2.3 times as wide as diameter of posterior pair of eyes; anterior pair of eyes reniform, two-thirds as wide as basal diameter of antennae, gap between both eyes as wide as 6 times diameter of eyes, with lens distinct, whitish, covering 75% of eye; posterior pair of eyes rounded, two-thirds as wide as basal diameter of antennae, with lens distinct, whitish, placed mid-posteriorly in eyes and covering 60% of it.
Apodous anterior segment (Figure 8A) 6 times wider than long, 1.5 times as long as chaetiger 1, with even anterior margin, dorsum without marked transverse wrinkles.
Tentacular cirri smooth (Figure 8A); postero-dorsal cirri extending posteriorly to chaetiger 6, twice as long as antero-dorsal cirri; antero-dorsal cirri extending posteriorly to chaetiger 2; postero-ventral cirri extended over first quarter of prostomium; antero-ventral cirri as long as one-third of postero-ventral cirri and extending to three-quarters of palpophore; cirrophores cylindrical, except ring-shaped postero-ventral one, postero-dorsal cirrophores 1.5 times as long as antero-dorsal cirrophores, antero-ventral cirrophores broadest, postero-ventral ones narrowest.
Pharynx everted (Figure 8A, B). Jaws with 7 well-developed and blunt denticles; pulp cavity with two canals. Pharynx with paragnaths orange-amber on maxillary and oral rings (Figure 8A, B), consisting of uniform-base cones, except broad-petite bars on area VI; merged paragnaths and plate-like basements absent. Area I: 4, cones of similar size in sub-rhomboidal patch, except proximal cone slightly longer; areas IIa: 19 and IIb: 21, two irregular rows of uneven cones in crescent and markedly curved patch, distal cones curved and longer; area III: 55, four irregular rows of cones with similar size in sub-rectangular patch, without distinct isolated lateral groups; areas IVa: 23 and IVb: 24, three irregular rows of uneven cones in crescent, markedly curved patch, medial cones longer; area V: 3, triangular patch of coarse cones of similar size, two proximal cones in transverse row and single distal cone slightly behind paragnaths on area VI; areas VIa: 2 and VIb: 2, one row of even broad-petite bars with slightly pointed tip, separated; areas VII–VIII: 69, two well-separated bands of cones, with anterior band consisting of two transversely aligned rows (furrow row and ridge row with one cone on each region, former with cones slightly stouter), and posterior band with two transverse rows slightly displaced from each other (furrow row proximal with one cone on each region, ridge row distal with three cones on region A and one or three cones in remaining regions). Areas VI–V–VI ridge pattern, λ-shaped. Gap between area VI and areas VII–VIII broad, as wide as palpophore.
Notopodia consisting of dorsal cirrus, dorsal ligule (distal and proximal), and median ligule in biramous parapodia; notopodial prechaetal lobe or notoacicular process not developed throughout.
Dorsal cirri from cirriform to conical, thick, short, extending up to three-quarters of distal dorsal ligule throughout; dorsal cirri longer than proximal ligule in anteriormost parapodia, subequal in anterior parapodia, shorter in following parapodia; dorsal cirri inserted basally to dorsal ligules in anteriormost parapodia, one-third in anterior parapodia, medially in medial and posterior parapodia, sub-distally in posteriormost parapodia.
Proximal dorsal ligule even towards posterior end except slightly enlarged in posterior parapodia; shorter than distal dorsal ligule in anterior parapodia, becoming longer than distal dorsal ligule from medial parapodia to end of body.
Distal dorsal ligule extending markedly beyond end of notoaciculae throughout; conical, subequal or slightly smaller than median ligule throughout.
Median ligule conical throughout, becoming slightly shorter and narrower from medial parapodia towards posterior end.
Neuropodia consisting of neuroacicular ligule with superior and inferior lobes, ventral ligule, and ventral cirrus; neuropodial postchaetal lobe reduced throughout.
Neuroacicular ligule shorter than ventral ligule in anterior parapodia, becoming slightly longer in following chaetigers, subequal to ventral ligule in posterior parapodia; neuroacicular ligule twice as wide as ventral ligule throughout.
Superior lobe rounded, slightly shorter than inferior lobe in anterior parapodia, reduced from medial parapodia towards posterior end.
Inferior lobe rounded, longer than neuroacicular ligule in anterior and medial parapodia, becoming shorter and narrower towards posterior end.
Ventral ligule bluntly conical, thick and as long as three-quarters of median ligule in anterior parapodia, becoming conical and shorter in following parapodia.
Ventral cirri conical, thick and as long as one half of ventral ligule in anterior parapodia, becoming narrower and distinctly shorter, barely reaching base of ventral ligule in medial parapodia, shorter in posterior parapodia.
Pygidium regenerating, anal cirri missing.
Aciculae black, with basal end uncoloured. Notoaciculae absent in chaetigers 1 and 2. Neuroaciculae extending beyond distal end of notoaciculae throughout body.
Notochaetae all homogomph spinigers, present throughout. Supracicular neurochaetae consisting of homogomph spinigers and heterogomph falcigers, both present throughout. Subacicular neurochaetae consisting of heterogomph spinigers and heterogomph falcigers, both present throughout.
Blades of both homogomph and heterogomph spinigers finely serrated towards toothed edge, evenly spaced, long with high b/a ratio. Blades of heterogomph falcigers long with low b/a ratio, slender, straight, distal end club-shaped with incurved terminal tooth forming very long tendon (equalling about one-third of total blade length); blades of falcigers partially serrated, with serrations capilliform, curved, looking upwards, present in about two-fifths of total blade length (Figure 8C); vertex between distal and basal end on serrated edge markedly prominent, sub-conical. Shaft of falcigers camerated, with cavity divided sub-distally into three longitudinal partitions (Figure 8C).
Non-type material from Korea
Atoke, complete, in good condition (NIBRIV0000783811), 154 mm LT, 21.8 mm L15, 5.2 mm W15, with 148 chaetigers. Body colour cream in preserved specimens, living individuals with dark green dorsum. Prostomium campanulate (Figure 9A), as long as wide; anterior end broad, distally complete; anterolateral gap aside palpophore broad. Palpophores sub-conical, thick (Figure 9A), longer than wide; sub-distal transverse groove distinct. Palpostyles oval. Antennae tapered, thick, short (Figure 9A), distinctly separated from each other. Paired eyes blackish, arranged in trapezoid form, medium sized; anterior pair of eyes reniform, posterior pair rounded, both pairs well-separated from each other (Figure 9A), with lens distinct, whitish, large (Figure 9A), placed mid-posteriorly in posterior pair of eyes. Apodous anterior segment longer than chaetiger 1 (Figure 9A). Tentacular cirri smooth, medium-sized (Figure 9A); postero-dorsal cirri extending posteriorly to chaetiger 6, twice as long as antero-dorsal cirri; antero-dorsal cirri extending posteriorly to chaetiger 4; postero-ventral cirri extended over two-thirds of prostomium antero-ventral cirri as long as three-fourths of postero-ventral cirri and extending to three-fourths of palpophore; cirrophores of anterior cirri ring-shaped, those in posterior cirri cylindrical, postero-dorsal cirrophores 1.5 times as long as antero-dorsal cirrophores, antero-ventral cirrophores broadest, postero-ventral ones narrowest.
Pharynx with dark brown jaws, 9 denticles; pulp cavity with two canals. Paragnaths on maxillary and oral rings (Figures 9A–C) consisting of uniform-base cones, except broad-petite bars on area VI (Figure 9A); merged paragnaths and plate-like basements absent. Area I: 5 (2–8) in rhombus patch; areas IIa: 23 (8–25) and IIb: 20 (10–25), 2–3 rows of uneven cones in crescent patch, distal cones curved and longer; area III: 48 (32–70), 4–5 rows of cones with similar size in sub-rectangular patch, without lateral isolated groups; areas IVa: 23 (11–26) and IVb: 21 (13–31), three rows of uneven cones in crescent patch, medial cones longer; area V: 2 (2–5), coarse cones in triangular patch; areas VIa: 2 (seldom 1–3) and VIb: 2 (seldom 1–3), one row of broad-petite bars with tip slightly pointed or rounded (seldom one conical paragnath instead of a bar), separated, inner bars sometimes slightly longer; areas VII–VIII: 58 (43–81), two well-separated bands of cones, with anterior band consisting of two transversely aligned rows (furrow row and ridge row with one cone on each region, former with cones slightly stouter), and posterior band with two transverse rows slightly displaced from each other (furrow row proximal with one stout cone on each region, ridge row distal with 1–2 cones on region A and 1–3 cones in remaining regions). Areas VI–V–VI ridge pattern, λ-shaped. Gap between area VI and areas VII–VIII subequal or as broad as three-quarters of palpophore.
Dorsal cirri from cirriform to conical, thick, short, extending up to three-quarters of distal dorsal ligule throughout, sub-distally inserted to dorsal ligule in posterior parapodia. Proximal dorsal ligule slightly enlarged in posterior parapodia; distal dorsal ligule conical, subequal to median ligule throughout. Notopodial prechaetal lobe reduced, sometimes notoacicular papillae in anterior parapodia of large specimens. Median ligule conical. Neuroacicular ligule shorter than ventral ligule in anterior parapodia, becoming longer towards posterior end. Superior and inferior lobes present in anterior and medial parapodia, subequal in length. Neuropodial postchaetal lobe reduced. Ventral ligule conical, shorter than median ligule throughout, as long as one-half of median ligule in posterior parapodia. Ventral cirri conical, thick, distinctly shorter than ventral ligule.
Pygidium with tapering cylindrical anal cirri.
Aciculae black, with basal end uncoloured. Notoaciculae absent in chaetigers 1 and 2. Neuroaciculae extending beyond distal end of notoaciculae throughout. Notochaetae all homogomph spinigers, present throughout. Supracicular neurochaetae consisting of homogomph spinigers and heterogomph falcigers, both present throughout. Subacicular neurochaetae consisting of heterogomph spinigers and heterogomph falcigers, both present throughout. Blades of both homogomph (Figure 9K) and heterogomph spinigers (Figure 9J) finely serrated, long. Blades of heterogomph falcigers long, slender, straight, distal end club-shaped with incurved terminal tooth very long forming distinct tendon (equalling about one-third of total blade length); blades of falcigers partially serrated, with serrations capilliform, curved, looking upwards, present in about one-third to two-fifths of total blade length (Figure 9I); vertex markedly prominent, sub-conical. Shaft of falcigers camerated, with cavity divided sub-distally into three longitudinal partitions (Figure 9I).
DNA sequence data
Locality: Several localities in Korea and another possibly from China (NIBRIV0000810291, NIBRIV0000810299; Table 1). GenBank accession numbers: MT511711–MT511715 (COI), MT540476–MT540480 (16S rDNA) (Table 1). Aligned length of sequences: 622 bp fragment from COI obtained with primer pair polyLCO/polyHCO (Carr et al., Reference Carr, Hardy, Brown, Macdonald and Hebert2011; Table 2), 479 bp fragment from 16S rDNA obtained with primer pair 16SarL/16SbrH (Palumbi, Reference Palumbi, Hillis, Moritz and Mable1996). Intraspecific genetic distances did not exceed 1% for both genes (Table 3).
Remarks
Perinereis linea (Treadwell, Reference Treadwell1936) belongs to the P. aibuhitensis species group featured by having short dorsal cirri throughout the body and heterogomph falcigers' blades straight with incurved terminal tooth markedly elongated forming a distinct tendon. Perinereis linea resembles P. babuzai comb. nov. and P. singaporiensis because they share the areas VI–V–VI ridge pattern λ-shaped (Figures 5D, 6E, 8A, 9A), contrary to the π-shaped pattern present in the remaining species of the group (Figure 4F), including P. aibuhitensis (Figure 3A, B). Nonetheless, in P. linea, area VI has broad-petite bars (Figures 5D, 6E, 8A, 9A), whereas P. singaporiensis has long smooth-bars in area VI. In P. linea, the distal dorsal ligules are conical in anterior parapodia (Figures 5G, 6D, 9E), whereas in P. singaporiensis they are bluntly rounded in the same parapodia. In P. linea, the distal dorsal ligule is subequal to the median ligule in posterior parapodia (Figures 5I, J, 9G, H), whereas in P. singaporiensis they project beyond median ligule in the same parapodia. In P. linea, the area III lacks laterally isolated paragnaths (Figures 5D, 8B, 9B), present in P. singaporiensis. Finally, in P. linea the area V has usually three (seldom 4–5) paragnaths, whereas P. singaporiensis usually has one paragnath (rarely none) in the same area. On the contrary, P. linea is readily distinguished from P. babuzai comb. nov. by the distal dorsal ligule of medium size and projecting markedly beyond the notoaciculae (short, barely projected in P. babuzai).
Treadwell (Reference Treadwell1936) described Nereis (Neanthes) linea and N. (Neanthes) orientalis as new species from Amoy, China, based upon single atoke and epitoke specimens, respectively. Hartman (Reference Hartman1938) re-examined the type materials and regarded both species as identical to P. aibuhitensis (Grube, Reference Grube1878) from Palau, no further details on the synonymies were given although both species were treated as synonyms of P. aibuhitensis in further studies (Hartman, Reference Hartman1956, Reference Hartman1959). Later, Wu (Reference Wu1967) reviewed some nereidid species from Taiwan, including N. (Neanthes) linea which was considered valid in Perinereis; however, it was distinguished from P. aibuhitensis by having area I with four paragnaths in a quadrate arrangement (area I with 1–2 in P. aibuhitensis) and postero-dorsal tentacular cirri extending to chaetiger 6 (chaetigers 2–5 in P. aibuhitensis). It is noteworthy that Olga Hartman read the first draft of Wu's study (Wu, Reference Wu1967: 74) and likely recognized the treatment of P. linea as a species different from P. aibuhitensis, although any argumentations were given by Wu (Reference Wu1967).
Imajima (Reference Imajima1972) described P. vancaurica tetradentata from Tokyo (Japan) based upon a single specimen and distinguished from the stem species by having area I with four cones in a cross (two in P. vancaurica) and from P. linea by presenting area V with cones in a triangle (longitudinal row in P. linea); likewise, the single record of P. linea from Taiwan (Wu, Reference Wu1967) was included in the subspecies. Subsequently, Paik (Reference Paik1975, Reference Paik1977, Reference Paik1989), following Imajima's (Reference Imajima1972) study, repeatedly recorded P. vancaurica tetradentata from several localities in the south and west of Korea.
Wu et al. (Reference Wu, Sun and Yang1985) synonymized Treadwell's species N. (Neanthes) linea and N. (Neanthes) orientalis with P. aibuhitensis based upon the original descriptions and atoke and epitoke individuals from China, although no argumentations on the proposal of synonymies were provided. They recorded the species from the Yellow Sea's subtropical waters until Xiamen and Taiwan, but it was also recorded in Hainan's tropical island. On the other hand, P. vancaurica tetradentata and its records from Korea (e.g. Paik, Reference Paik1975, Reference Paik1977, Reference Paik1989), and P. linea from Taiwan (Wu, Reference Wu1967), were not included in the monography. Hutchings et al. (Reference Hutchings, Reid and Wilson1991) reviewed Perinereis species recorded from Australia, including the type material of P. aibuhitensis. They maintained N. (Neanthes) linea and N. (Neanthes) orientalis as junior synonyms of P. aibuhitensis based upon the literature; however, after examining the type material of P. vancaurica and compared with the original description of P. vancaurica tetradentata, they considered the latter as a synonym of the stem species.
Lee et al. (Reference Lee, Je and Choi1992) studied P. aibuhitensis from Korea's western coast, using both atoke and epitoke specimens. They stated that the variation on the number, arrangement and shape of paragnaths has led to confusion of P. aibuhitensis with other species such as N. (Neanthes) linea, N. (Neanthes) orientalis, P. vancaurica tetradentata, and even Neanthes virens Sars, Reference Sars1835 (= Alitta sensu Bakken & Wilson, Reference Bakken and Wilson2005). They also maintained both Treadwell's species as P. aibuhitensis, but proposed P. vancaurica tetradentata as a junior synonym of P. aibuhitensis. Later, Imajima (Reference Imajima1996) recognized the synonymy of his subspecies. Khlebovich (Reference Khlebovich1996) and Sun & Yang (Reference Sun and Yang2004) examined specimens of P. aibuhitensis from Korea and China. They continued considering N. (Neanthes) linea and N. (Neanthes) orientalis synonyms mostly based upon Wu et al. (Reference Wu, Sun and Yang1985), although P. vancaurica tetradentata was not mentioned either.
More recently, Arias et al. (Reference Arias, Richter, Anadón and Glasby2013) reinstated and featured P. linea by combining specimens from Spain, Korea, and China. They presented it as a redescription even though the type material was not examined. Their available specimens of P. linea were compared to and distinguished from Australian specimens of P. aibuhitensis by having area III without lateral groups of paragnaths (present in P. aibuhitensis), area II with crescentic rows of paragnaths (short and straight rows in P. aibuhitensis), and epitokes with 28–29 pre-natatory chaetigers (21–23 chaetigers in P. aibuhitensis). Likewise, they deemed P. linea as an alien species in the Mar Menor lagoon, Spain (Western Mediterranean), after determining fish bait importation as the possible vector of introduction and studying some reproductive and ecological traits from the population. Moreover, they suggested that P. linea may be similar to P. vancaurica tetradentata, and this was later supported by Sato (Reference Sato, Motokawa and Kajihara2017) after examining the subspecies type specimen.
In the present study, we confirm P. linea as a senior synonym of P. vancaurica tetradentata and propose the former species as a senior synonym of N. (Neanthes) orientalis based on the examination and redescription of type materials and comparison with one type specimen and topotypes of P. aibuhitensis.
The redescription of the type material of N. (Neanthes) linea matches consistently with the original description except that the paragnaths on area V are arranged in a triangle instead of the longitudinal row reported by Treadwell (Reference Treadwell1936). The morphological characters of atoke holotype of N. (Neanthes) linea and the non-modified features of epitokal type specimen of N. (Neanthes) orientalis are identical to each other, such as the areas VI–V–VI ridge pattern, the shape, and arrangement of paragnaths, the type of falcigers, among others. These two nominal species were described in the same publication as new species (Treadwell, Reference Treadwell1936). However, since N. (Neanthes) linea is valid nowadays in Perinereis and the type specimen of N. (Neanthes) orientalis is in a reproductive stage, we consider P. linea has priority over N. (Neanthes) orientalis (ICZN, 1999, Arts 24.2.1, 24.2.2.).
The atoke morphology of P. linea's specimens and that of the holotype of P. vancaurica tetradentata is similar. Some slight differences between both species can be found, such as the slightly narrower, shorter and paler paragnaths in P. vancaurica tetradentata; however, the single Japanese specimen revised renders difficult its recognition as a distinct species based solely in such slight differences. Additional Japanese material was not available in this study. No further taxonomic records of P. linea, either as P. vancaurica tetradentata or P. aibuhitensis, have been reported in Japan since the original description. The populations have likely been extirpated by habitat loss due to anthropological developments in estuarine mudflats (Sato, Reference Sato, Motokawa and Kajihara2017). The species has been reported in Japan (as P. vancaurica tetradentata) in biochemical studies, but these are doubtful since the means of identification are unknown (e.g. Ina & Matsui, Reference Ina and Matsui1980; Kobayashi et al., Reference Kobayashi, Takasaki, Takagi and Konishi1984; Yuasa & Takagi, Reference Yuasa and Takagi2001).
The atoke differences between P. linea and P. aibuhitensis stated by Arias et al. (Reference Arias, Richter, Anadón and Glasby2013) regarding the presence of lateral groups of paragnaths on area III and the arrangement of paragnaths on area II are here confirmed as useful to distinguish the species. Also, we found that two other diagnostic features on the pharynx can readily distinguish both species. In P. linea, the areas VI–V–VI ridge pattern is λ-shaped (Figures 5D, 6E, 8A, 9A), whereas in P. aibuhitensis it is π-shaped (Figure 3A, B). Likewise, in P. linea the anterior band of areas VII–VIII has two rows with single paragnath on both furrows and ridges (Figures 5D, 8B, 9B), whereas in P. aibuhitensis the anterior band has one row with single paragnath only on furrows (Figure 3C, D). This distinction is also supported by the DNA sequence comparison for both COI and 16S rDNA between P. linea from North-east Asia and P. aibuhitensis from Palau (type locality) (Figure 2). Pairwise distance between P. linea and P. aibuhitensis was ~25% for COI and 10% for 16S rDNA (Table 3).
Furthermore, the single epitoke male of P. linea (type of N. (Neanthes) orientalis) from Xiamen, China (type locality) was here examined and described. Epitoke specimens of P. aibuhitensis were not available in this study. A description of the species' reproductive morphology using individuals from Palau (type locality) has not been performed yet. Nonetheless, Horst (Reference Horst1924) reported the species using epitokes from West Java (Indonesia), and Arias et al. (Reference Arias, Richter, Anadón and Glasby2013) mentioned a few epitokal details of the species based on Australian material. No other differences between both species were detected following Horst (Reference Horst1924) and Arias et al. (Reference Arias, Richter, Anadón and Glasby2013) other than the starting of the natatory parapodia. In P. linea from China, the natatory parapodia start from chaetiger 20 (Figures 6F, 7A; Monro, Reference Monro1934), whereas in the Indonesian and Australian populations of P. aibuhitensis they start from chaetiger 21 and 23, respectively.
Habitat
Euryhaline species distributed in estuarine areas. It dwells in intertidal mudflats and common cordgrass (Spartina anglica) areas (Wu et al., Reference Wu, Sun and Yang1985; Sun & Yang, Reference Sun and Yang2004), and dominates in the uppermost part of the littoral zone with gravels or mud mixed with stones in Changkou, Qingdao (China), among communities of the brachyuran crabs Helice sp. and Scopimera sp. in high densities (100 inds m−2) (Wu et al., Reference Wu, Sun and Yang1985). On the western coast of Korea, the species also reaches high densities (130 inds m−2) at the upper littoral zone, which plays an essential role in recycling intertidal mudflat sediment (Choi & Lee, Reference Choi and Lee1997).
Reproduction
Breeding season in Xiamen (Fujian, China) is from February to May with reproductive peaks from late March to early April, at water temperatures above 17 °C; the ratio of males to females is 1:1.7; the diameter of zygotes is 186–214 μm (Chen et al., Reference Chen, Ye, Chai and Zheng1992; Sun & Yang, Reference Sun and Yang2004). In Mayidao Island, Zhoushan (Zhejiang, China), the swarming is from May to June with water temperatures around 20 °C (Zheng & Fan, Reference Zheng and Fan1986); whereas in the western coast of Korea, it occurs continuously from early spring to autumn (Choi & Lee, Reference Choi and Lee1997).
Type locality
Amoy (Xiamen), Fujian, China.
Distribution
East China Sea, Yellow Sea, Sumida-gawa River (Tokyo, Japan). The records in the Mediterranean Sea (Arias et al., Reference Arias, Richter, Anadón and Glasby2013) are questionable (see Discussion).
Perinereis shigungensis (Hsueh, Reference Hsueh2019) comb. nov.
Neanthes shigungensis Hsueh, Reference Hsueh2019: 190–191, figs 15, 16, table 2.
Diagnosis (based upon Hsueh, 2019)
Species of subgroup 2A belonging to ‘P. aibuhitensis’ species group. Specimens with broad-petite bars on area VI; areas VI–V–VI ridge pattern π-shaped; area III with laterally isolated paragnaths; areas VII–VIII with anterior band consisting of two rows; distal dorsal ligule anteriorly conical, subequal in size throughout; neuroacicular ligule posteriorly shorter than median ligule; falcigers with camerated shaft divided into two partitions; postero-dorsal tentacular cirri extending to chaetiger 3.
Remarks
Neanthes shigungensis Hsueh, Reference Hsueh2019 is here transferred to Perinereis based on having bar-shaped paragnaths on area VI. The species resembles P. rookeri by having broad-petite bars on area VI, distal dorsal ligule subequal to median ligule, areas VI–V–VI ridge pattern π-shaped, areas VII–VIII with the anterior band having two rows, and area III with distinct laterally isolated paragnaths. Nonetheless, both species can be distinguished by the number of paragnaths on several pharyngeal areas, the tendon's length formed by the incurved terminal tooth of falcigers' blade, and the extension of postero-dorsal tentacular cirri. In P. shigungensis comb. nov., the number of paragnaths on some areas is much higher (area I: 7–8; area III: 48–65; area VII–VIII: 57–58) than that in P. rookery (area I: 1–3; area III: 18; area VII–VIII: 33). In P. shigungensis comb. nov., the tendon of falcigers' blade equals half of the total blade length, whereas in P. rookery it equals one-third to two-fifths of the blade length. Finally, in P. shigungensis comb. nov. the postero-dorsal tentacular cirri extend posteriorly to chaetiger 3, whereas in P. rookery they extend posteriorly to chaetiger 1.
Habitat
Intertidal mud bottom.
Reproduction
Unknown.
Type locality
Shigung, Changhua County, Taiwan.
Distribution
The species is known only from the type locality, Shigung (Taiwan).
Perinereis vitabunda (Pflugfelder, Reference Pflugfelder1933) comb. nov.
(Figures 1D–F, 10)
Nereis vitabunda Pflugfelder, Reference Pflugfelder1933: 71–72, fig. 12A–D; Harms, Reference Harms1934: 29–30 (habitat); Wesenberg-Lund, Reference Wesenberg-Lund1958: 29 (species list); Salazar-Vallejo et al., Reference Salazar-Vallejo, Carrera-Parra, Muir, de León-González, Piotrowski and Sato2014: 23 (species list).
Neanthes vitabunda: Hartman, Reference Hartman1959: 251 (source of synonymy); Fauchald, Reference Fauchald1972: 409 (species list); Wilson, Reference Wilson1984: 226 (species list); Glasby et al., Reference Glasby, Timm, Muir and Gil2009: 14 (species list).
Type material
Lectotype (designated here): PMJ Ann-167, Belawan, Sumatra, Indonesia, coll. J.W. Harms, 1927 or 1929.
Diagnosis
Species of subgroup 2A belonging to ‘P. aibuhitensis’ species group. Specimens with transversally arranged broad-petite bars on area VI; areas VI–V–VI ridge pattern π-shaped; area III with laterally isolated paragnaths; areas VII–VIII with anterior band consisting of one row; distal dorsal ligule distinctly short throughout; neuroacicular ligule markedly projected; falcigers with camerated shaft divided into three partitions; postero-dorsal tentacular cirri extending to chaetiger 1.
Description
Lectotype atoke, incomplete, in good condition except already cut off into two parts at fourth and fifth chaetigers, 63 mm LT, 13 mm L15, 3.8 mm W15, with 83 chaetigers. Body colour brownish (Figure 10A), with three darkish longitudinal lines of tegument on dorsum of segments: one mid-dorsal line (maybe due to dark dorsal vessel) and two dorsolateral lines present throughout; body intensively covered by salt granules in dorsum.

Fig. 10. Perinereis vitabunda (Pflugfelder, Reference Pflugfelder1933) comb. nov. Holotype (PMJ Ann-167), Belawan, Sumatra, atoke: (A) anterior region in dorsal view; (B) anterior region in lateral view; (C) non-everted pharynx in ventral view (roman numerals referring to areas); (D–H) parapodia, numbers refer to the chaetiger; (I) homogomph spiniger from neuropodial supracicular fascicle (chaetiger 98); (J) heterogomph falciger from neuropodial supracicular fascicle (chaetiger 98); (K) heterogomph falciger from neuropodial subacicular fascicle (chaetiger 37). Scale bars: A, B, 2 mm; C, 0.5 mm; D–H, 0.2 mm; I–K, 20 μm.
Prostomium campanulate, as long as wide; anterior end broad, distally complete; anterolateral gap aside palpophore broad, twice as wide as basal diameter of antennae (Figure 10A). Nuchal organs deeply embedded, small, as wide as one-third of diameter of posterior pair of eyes.
Palpophores sub-conical, thick, as long as wide, as long as half of entire prostomium; sub-distal transverse groove distinct, deeply embedded (Figures 10A, B). Palpostyles oval, half as wide as diameter of palpophore.
Antennae tapered, thick, short; extending forwards to three-fifths of palpophore and posteriorly to distal quarter of length of prostomium; antennae separated with gap 1.6 times as wide as basal diameter of antennae (Figure 10A).
Paired eyes blackish, arranged in trapezoid form; gap between both pairs twice as wide as diameter of posterior pair of eyes (Figure 10A); anterior pair of eyes sub-oval, twice as wide as basal diameter of antennae, gap between both eyes as wide as 3 times diameter of eyes, with lens distinct, whitish, covering 80% of eye; posterior pair of eyes sub-rounded, 1.5 times as wide as basal diameter of antennae, with lens distinct, whitish, placed in middle of eye and covering 70% of it.
Apodous anterior segment 2.5 times wider than long, 1.5 times as long as chaetiger 1 (Figure 10A, B), with even anterior margin, dorsum without marked transverse wrinkles.
Tentacular cirri slender, smooth (Figures 10A, B); postero-dorsal cirri extending posteriorly to chaetiger 1, 1.5 times as long as antero-dorsal cirri; antero-dorsal cirri extending posteriorly to half of apodous segment; postero-ventral cirri extended over first quarter of prostomium; antero-ventral cirri as long as postero-ventral cirri and extending to half of palpophore; cirrophores cylindrical, postero-dorsal cirrophores as long as antero-dorsal cirrophores, antero-ventral cirrophores broadest, postero-ventral cirrophores narrowest.
Pharynx not everted, previously dissected with pharyngeal bulb and its surrounding muscle removed from body, separated in vial. Jaws with eight slightly developed and blunt denticles; pulp cavity as long as one-half of jaw, with two thick canals. Pharynx with paragnaths brownish on maxillary ring and reddish paragnaths on oral ring (Figure 10C), consisting of uniform-base cones, except broad-petite bars on area VI; merged paragnaths and plate-like basements absent. Area I: 6, cones of similar size in pentagon-shaped patch, one central cone; areas IIa: 12 and IIb: 11, three irregular rows of uneven cones in slightly curved, conical patch, distal cones smaller; area III: 36, four slightly regular rows of cones with similar size in sub-rounded patch, with distinct lateral isolated groups; areas IVa: 31 and IVb: 31, four regular transverse rows of uneven cones in slightly curved, sub-oval patch, distal-most cones shorter; area V: 3, triangular patch of coarse cones of similar size, two proximal cones in transverse row and single distal cone on same level as distal-most paragnath on area VI; areas VIa: 2, VIb: 2, one transverse row of even, coarse broad-petite bars with rounded tip and slightly melted base (Figures 1D–F, 10C), coalesced; areas VII–VIII: 39, cones only, coarse, even; paragnaths disposed of in two well-separated bands of coarse and similar-sized cones, with anterior band consisting of one furrow row (one stout paragnath on each region), and posterior band with two transverse rows displaced from each other (furrow row proximal with one cone on each region, ridge row distal with 2–3 cones on each region). Areas VI–V–VI ridge pattern, π-shaped. Gap between area VI and areas VII–VIII broad, as wide as palpophore.
Paired oesophageal caeca present.
Parapodia with blackish, glandular, notopodial patches, more distinct in posterior chaetigers.
Notopodia consisting of dorsal cirrus, dorsal ligule (distal and proximal), and median ligule in biramous parapodia; notopodial prechaetal lobe or notoacicular process not developed throughout.
Dorsal cirri conical, thick, short (Figure 10D–H), extending up to two-thirds of distal dorsal ligule throughout; dorsal cirri subequal to proximal ligule throughout (Figure 10D–H), inserted medially to dorsal ligules in all parapodia.
Proximal dorsal ligule even towards posterior end; 1.3 times as long as distal dorsal ligule throughout; one small, sub-oval glandular patch throughout.
Distal dorsal ligule becoming gradually shorter towards posterior end (Figure 10E–H), extending beyond end of notoaciculae in anterior parapodia (Figure 10E), subequal in medial parapodia (Figure 10F, G), shorter in posterior parapodia (Figure 10H); bluntly conical in anterior parapodia (Figure 10E), conical in following ones (Figure 10F–H); markedly shorter than median ligule throughout; one small, sub-oval glandular patch, smaller than that in proximal dorsal ligule throughout.
Median ligule bluntly rounded in anteriormost and anterior parapodia (Figure 10D, E), bluntly conical and becoming slightly shorter and narrower in following parapodia (Figure 10F–H).
Neuropodia consisting of neuroacicular ligule with superior and inferior lobes, ventral ligule, and ventral cirrus; neuropodial postchaetal lobe reduced throughout.
Neuroacicular ligule subequal to ventral ligule in anteriormost parapodia (Figure 10G), slightly longer in anterior parapodia, distinctly longer in following parapodia (Figure 10I–K); neuroacicular ligule 1.7 times as wide as ventral ligule in anteriormost and anterior parapodia, 2.3 times as wide as ventral ligule in medial and posterior parapodia.
Superior lobe rounded, subequal to inferior lobe and neuroacicular ligule throughout (Figure 10D–F).
Inferior lobe rounded, slightly longer than neuroacicular ligule in first 27 parapodia (Figure 10D, E), becoming narrower in following parapodia.
Ventral ligule digitiform, thick and subequal to median ligule in anteriormost parapodia (Figure 10D), becoming narrower and shorter in following parapodia (Figure 10E–H).
Ventral cirri conical, thick and markedly short in anteriormost and anterior parapodia (Figure 10D–F), becoming slightly longer and narrower in following parapodia; ventral cirri as long as one-half of ventral ligule in anteriormost parapodia, one-quarter in anterior parapodia, extending to base of ventral ligule in following parapodia.
Pygidium missing.
Aciculae black, with basal end uncoloured. Notoaciculae absent in chaetigers 1 and 2 (Figure 10D). Neuroaciculae markedly extending beyond distal end of notoaciculae throughout and from distal end of median ligule in medial and posterior parapodia (Figure 10G, H).
Notochaetae all homogomph spinigers; 5–7 spinigers present in anterior parapodia, 8–9 spinigers in medial parapodia, 10–12 spinigers in posterior parapodia.
Supracicular neurochaetae consisting of homogomph spinigers and heterogomph falcigers, both present throughout; 4–7 spinigers present in anteriormost and anterior parapodia, 3–4 spinigers in medial parapodia, 5–6 spinigers in posterior parapodia; 12–14 falcigers in anteriormost parapodia, 6–8 falcigers in anterior parapodia, 5–6 falcigers in medial parapodia, 3–4 falcigers in posterior parapodia.
Subacicular neurochaetae consisting of heterogomph spinigers and heterogomph falcigers, both present throughout; two spinigers present in anteriormost and anterior parapodia, 1–2 spinigers in medial and posterior parapodia; 16–20 falcigers in anteriormost and anterior parapodia, 14–15 falcigers in medial parapodia, 8–10 falcigers in posterior parapodia.
Blades of both homogomph (Figure 10I) and heterogomph spinigers finely serrated towards toothed edge, evenly spaced, long with high b/a ratio (10–14.5). Blades of heterogomph falcigers long with low b/a ratio (b/a: 2–2.3), slender, straight, distal end digitiform with incurved terminal tooth very long forming distinct tendon (equalling about two-fifths of total blade length: 0.39–0.43); blades of falcigers partially serrated, with serrations capilliform, curved, looking upwards, present in about three-fifths (0.6–0.61) of total blade length (Figure 10J, K); vertex between distal and basal end on serrated edge markedly prominent, sub-conical. Shaft of falcigers camerated, with cavity divided sub-distally into three longitudinal partitions (Figure 10J).
Remarks
Perinereis vitabunda (Pflugfelder, Reference Pflugfelder1933) comb. nov. belongs to the P. aibuhitensis species group based on having short dorsal cirri throughout the body, and blade of heterogomph falcigers straight with incurved terminal tooth markedly elongated forming a distinct tendon. Perinereis vitabunda comb. nov. resembles P. aibuhitensis, P. kinmenensis comb. nov., P. rookeri and P. belawanensis comb. nov. These species share areas VI–V–VI ridge pattern π-shaped, area VI with broad-petite bars, and area III with distinct laterally isolated paragnaths. Nonetheless, P. vitabunda comb. nov. is different from P. rookeri and P. belawanensis comb. nov. by having the anterior band of areas VII–VIII with one transverse row present on furrows (two rows on furrows and ridges of the latter two species). Likewise, P. vitabunda comb. nov. can be distinguished from P. aibuhitensis, P. kinmenensis comb. nov. and P. rookeri because the neuroacicular ligule is projecting markedly beyond median ligule in posterior parapodia (Figure 10G, H), and the distal dorsal ligule does not project beyond notoaciculae in medial and posterior parapodia (Figure 10G, H); whereas in P. aibuhitensis, P. kinmenensis comb. nov. and P. rookeri the neuroacicular ligule is subequal to or slightly shorter than the median ligule, and the distal dorsal ligule is markedly projecting beyond notoaciculae in medial and posterior parapodia. Perinereis vitabunda comb. nov. is more related to P. belawanensis comb. nov. in terms of habitat and locality; however, they are different in several aspects (see remarks of the latter species). The combination of both the markedly short tentacular cirri and distal and proximal dorsal ligule regions is unique in P. vitabunda comb. nov., rendering it easy to recognize among the Perinereis G2.
Perinereis vitabunda comb. nov. is a semi-terrestrial species from Sumatra described in Nereis by Pflugfelder (Reference Pflugfelder1933). The type material was never redescribed since the original description. The species was transferred to Neanthes by Hartman (Reference Hartman1959) and recognized in that genus ever since (Fauchald, Reference Fauchald1972; Wilson, Reference Wilson1984; Glasby et al., Reference Glasby, Timm, Muir and Gil2009). Nonetheless, after revising the type specimens, the species is here transferred to Perinereis. Perinereis vitabunda comb. nov. has not been recorded since the original description. The specimens used by Pflugfelder (Reference Pflugfelder1933) were collected by Jürgen W. Harms in 1927 or 1929, who provided a detailed description of the habitat of the species (Harms, Reference Harms1934).
The lot with the syntypes of P. vitabunda comb. nov. (PMJ 167) consisted of two specimens. The shape of the prostomium, the paragnaths' arrangement on the pharynx, the longest tentacular cirri, and the shape of the 20th dissected chaetiger of one of the specimens match the description and illustrations by Pflugfelder (Reference Pflugfelder1933). Consequently, that specimen has above been redescribed, newly illustrated, and it is here designated as lectotype (ICZN, 1999, Art. 74.7). The second syntype shared the diagnostic features of P. belawanensis comb. nov., and it was removed and separated (PMJ Ann-167a).
Habitat
Semi-terrestrial. Living in burrows dug within low-humidity sandy-clay soil covered with grass turf or within the chimney-like mud mounds constructed by the mud lobster Thalassina sp.; inland dispersal limited by the level of highest tide, which is usually only reached once a month during the full moon (Harms, Reference Harms1934). Living specimens are very agile; they escape at high speed into their burrows, usually found along with its congener P. belawanensis comb. nov. (Pflugfelder, Reference Pflugfelder1933).
Reproduction
Unknown.
Type locality
Belawan, Sumatra, Indonesia.
Distribution
The species is known only from the type locality, Belawan, and Perbaoengan (= Perbaungan), both in Sumatra (Indonesia).
Discussion
Species groupings in Perinereis
Current Perinereis species groupings (sensu Hutchings et al., Reference Hutchings, Reid and Wilson1991) based on the number of paragnaths on area VI are troublesome since some species can overlap in two or even three groups. The number of transverse bars on area VI can vary on some Perinereis species typically regarded with one transverse bar (Horst, Reference Horst1889; de Saint-Joseph, Reference de Saint-Joseph1898; Fauvel, Reference Fauvel1914), although this is more frequent in species having typically two (Wu et al., Reference Wu, Sun and Yang1985; Lee et al., Reference Lee, Je and Choi1992; Khlebovich, Reference Khlebovich1996; Arias et al., Reference Arias, Richter, Anadón and Glasby2013; this study) or more transverse bars (Wilson & Glasby, Reference Wilson and Glasby1993; Glasby & Hsieh, Reference Glasby and Hsieh2006; Park & Kim, Reference Park and Kim2007; Tosuji et al., Reference Tosuji, Nishinosono, Hiseh, Glasby, Sakaguchi and Sato2019; Villalobos-Guerrero, Reference Villalobos-Guerrero2019). A more reliable solution could be based primarily on the simultaneous usage of this and other two or three less variable features, such as the enlargement of proximal dorsal ligule throughout the body (suggested secondarily by Hutchings et al., Reference Hutchings, Reid and Wilson1991), the relative length of dorsal cirri, the areas VI–V–VI ridge patterns, the type of transverse bars on area VI, the number of anterior bands of paragnaths on areas VII–VIII, or the type of heterogomph falcigers' blade. However, a better understanding of the genus is still needed to select the appropriate characters.
Notwithstanding, the subgroup 2A (hereafter S2A) seems to construct a distinct, independent clade (Bakken & Wilson, Reference Bakken and Wilson2005) and probably deserves a separate genus. Perinereis variodentata was the single species of this subgroup included by Bakken & Wilson (Reference Bakken and Wilson2005) in a phylogenetic analysis of Nereidinae sensu Fitzhugh (Reference Fitzhugh1987). It was nested in a different clade from the type species P. amblyodonta and separated from a sibling clade with two ‘P. nuntia’ species and two ‘Neanthes’ species. The S2A members' morphology is markedly different from that of the type species based upon Hutchings et al. (Reference Hutchings, Reid and Wilson1991) and our observations of some Australian P. amblyodonta specimens (ZMB 5274), but similar to ‘P. nuntia’ species group sensu Wilson & Glasby, Reference Wilson and Glasby1993 also belonging to different genera (Glasby & Hsieh, Reference Glasby and Hsieh2006; Villalobos-Guerrero, Reference Villalobos-Guerrero2019). The members of these two (S2A and ‘P. nuntia’ group) can only be distinguished by the number of bars on area VI following Hutchings et al. (Reference Hutchings, Reid and Wilson1991), but this character used alone is not reliable. We have noticed that S2A is mostly represented by members with short dorsal cirri and blade of heterogomph falcigers straight with markedly elongated terminal tooth forming distinct tendon, and thus here consistently arranged into the ‘P. aibuhitensis’ species group. None of the members within the ‘P. nuntia’ species group present these characters simultaneously; whereas the remaining species of S2A have elongated dorsal cirri and blade of heterogomph falcigers convex with short terminal tooth and inconspicuous tendon. A more comprehensive revision of the species within S2A is imperative to delimit their morphology and set the proper foundations to establish or reinstate a genus.
Current species in Perinereis G2
According to Hutchings et al. (Reference Hutchings, Reid and Wilson1991), Perinereis G2 was represented only by the species of the S2A featured by having area VI with two bar-shaped paragnaths and dorsal ligules (= proximal dorsal ligules, this study) not greatly expanded in posterior parapodia. This group encompassed eight species (type localities in brackets): Perinereis aibuhitensis (Grube, Reference Grube1878) [Palau], P. brevicirrata (Treadwell, Reference Treadwell1920) [Brazil], P. camiguinoides (Augener, Reference Augener and Skottsberg1922) [Juan Fernandez Islands], P. jascooki Gibbs, Reference Gibbs1972 [Cook Islands], P. kuwaitensis Mohammad, Reference Mohammad1970 [Kuwait], P. singaporiensis (Grube, Reference Grube1878) [Singapore], P. vancaurica (Ehlers, Reference Ehlers1868) [Nicobar Islands] and P. variodentata (Augener, Reference Augener, Michaelsen and Hartmeyer1913) [Western Australia]. They considered that the subgroup 2B (hereafter S2B) species were not yet recorded; however, they overlooked P. mochimaensis Liñero-Arana, Reference Liñero-Arana1983 from Venezuela, which presented two bars on area VI and enlarged proximal dorsal ligules in posterior parapodia. Later, de León-González & Solís-Weiss (Reference de León-González and Solís-Weiss1998) described two species with proximal dorsal ligules becoming enlarged towards the posterior end and included into S2B: Perinereis cariboea from the Mexican coast of the Caribbean Sea, and P. osoriotaffali from the Gulf of California. More recently, de León-González & Goethel (Reference de León-González and Goethel2013) described P. rookeri from the northern Gulf of Mexico and incorporated it into the Perinereis S2A.
Two additional species need to be included in Perinereis S2A due to having this subgroup's characteristic features: Perinereis horsti Gravier, Reference Gravier1902 from Djibouti was previously considered a junior synonym of P. vancaurica, but it was re-established and treated as a valid species (Yousefi et al., Reference Yousefi, Rahimian, Nabavi and Glasby2011); whereas P. linea (Treadwell, Reference Treadwell1936) from China was recognized as different to his previous senior synonym P. aibuhitensis (Arias et al., Reference Arias, Richter, Anadón and Glasby2013) and is confirmed as a valid species in this study. The five species formerly considered in Neanthes but here transferred to Perinereis also have characteristic features of G2 species: Perinereis babuzai comb. nov., P. belawanensis comb. nov., P. kinmenensis comb. nov., P. shigungensis comb. nov. and P. vitabunda comb. nov. In this regard, a total of 19 Perinereis G2 species are currently valid. Sixteen species belong to S2A (65% are members of ‘P. aibuhitensis’ group), and the remaining three species to S2B (Table 4).
Table 4. Valid Perinereis species currently in species Group 2 sensu Hutchings et al. (Reference Hutchings, Reid and Wilson1991)
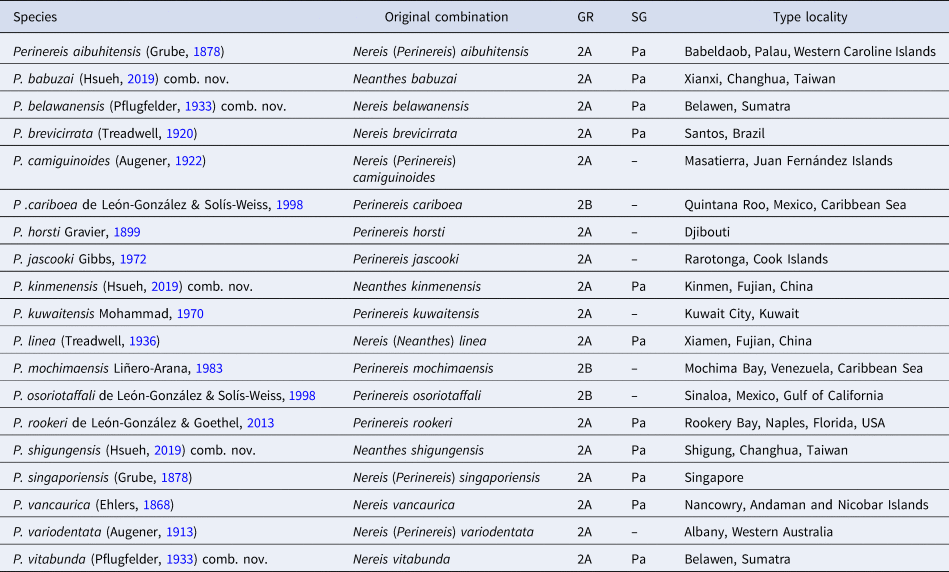
GR, Perinereis G2 species groupings sensu Hutchings et al. (Reference Hutchings, Reid and Wilson1991); SG, Perinereis subgroups within Group 2A established in this study; Pa, species belonging to the ‘Perinereis aibuhitensis’ species group.
Perinereis is well-represented in the Eastern and South-eastern Asian seas, although before this study only four species of G2A there were known: Perinereis aibuhitensis, P. linea, P. singaporiensis and P. vancaurica. Another five species are here recognized; thus, nine species of G2 are now recorded from these regions.
Perinereis linea: species complex or alien species?
Perinereis linea is here regarded as a senior synonym of N. (Neanthes) orientalis and P. vancaurica tetradentata, with distribution restricted to the East China Sea, Yellow Sea and a single freshwater locality in Tokyo (Japan). However, the status of a population reported from the Mediterranean Sea remains to be resolved.
Arias et al. (Reference Arias, Richter, Anadón and Glasby2013) reported an alien population of P. linea in the Mediterranean coast of Spain apparently imported by anglers as live bait for recreational fishing in the Mar Menor lagoon. They studied atoke and epitoke specimens of that population, compared them with specimens from South-western Korea (obtained from a fishing-bait supplier), and assigned all material to a single species. The epitoke males and females were characterized by having 28–29 pre-natatory chaetigers, fertilization occurring internally in the female coelom, zygotes and larvae released through openings in the body wall and incubated in gelatinous masses attached to the female parapodia. Additionally, Mediterranean Sea males have mature sperm with a long and cylindrical head, being of the ent-aquasperm type sensu Jamieson & Rouse (Reference Jamieson and Rouse1989). However, the epitoke morphology, the reproductive mode, and the sperm anatomy from those populations are different from specimens of P. linea from the type locality (Xiamen, China). According to Chen et al. (Reference Chen, Ye, Chai and Zheng1992), in populations from Xiamen fertilization occurs in the water column where both males and females swarm to discharge gametes and larval development occurs. Also, the epitoke male holotype of N. (Neanthes) orientalis (= P. linea, this study) from Xiamen has 19 pre-natatory chaetigers, and mature sperms have a shorter and spherical head (Figure 6G), being of the ect-aquasperm type sensu Jamieson & Rouse (Reference Jamieson and Rouse1989).
Setting apart epitokal modifications, differences in the reproductive modes and gamete morphology also reveal nereidid sibling species (Smith, Reference Smith1958; Clark, Reference Clark1961, Reference Clark, Reish and Fauchald1977; Sato, Reference Sato1999; Sato & Nakashima, Reference Sato and Nakashima2003). Several groups with similar morphology have diverged into distinct species based on differences in breeding biology: Platynereis dumerilii/massiliensis (Hauenschild, Reference Hauenschild1951; Clark, Reference Clark1961, Reference Clark, Reish and Fauchald1977; Pfannenstiel et al., Reference Pfannenstiel, Grünig and Lücht1987), Hediste diversicolor/japonica/limnicola (Smith, Reference Smith1958; Clark, Reference Clark, Reish and Fauchald1977), H. japonica/atoka/diadroma (Sato, Reference Sato1999; Sato & Nakashima, Reference Sato and Nakashima2003), Alitta virens/grandis (Khlebovich et al., Reference Khlebovich, Komendantov and Shklyarevich1980; Khlebovich, Reference Khlebovich1996), among others. Several species have shown different reproductive patterns, and tentatively suggested as belonging to separate species: Perinereis cultrifera (Grube, Reference Grube1840) (Durchon, Reference Durchon1955, Reference Durchon1957, Reference Durchon1965) and Composetia costae (Grube, Reference Grube1840) (Durchon, Reference Durchon1956, Reference Durchon1957, Reference Durchon1965). In this regard, the Xiamen population of P. linea is different from that studied by Arias et al. (Reference Arias, Richter, Anadón and Glasby2013) from the Mediterranean and (an uncertain locality from) South-western Korea by the different epitoke morphology, the reproductive mechanisms, and the shape of spermatozoa.
Arias et al. (Reference Arias, Richter, Anadón and Glasby2013) indicated that the species imported to Europe is commercially referred to as the ‘Korean ragworm’ (also ‘Korean blue ragworm’ or ‘Korean jumbo’). However, the precise origin of the Mediterranean population was not addressed. The ‘Korean ragworm’ were collected in South Korea and exported by wholesalers to France, Italy and Spain from the 1980s to 2000s; however, after the government prohibition of indiscriminate collection of baitworms in the early 2000s, South Korea imports the baitworms from China, North Korea and other Asian regions (T. Park personal observation). Arias et al. (Reference Arias, Richter, Anadón and Glasby2013) also obtained some ‘Korean ragworms’ apparently from South Korea through a fishing-bait supplier. Thus, the precise origin of their population is uncertain. They may come somewhere from the East China Sea or the Yellow Sea, as occurs in the Japanese trade (Saito et al., Reference Saito, Kawai, Umino and Imabayashi2014). In this study, we analysed western Korean specimens of P. linea collected in nature and purchased at Korean and Japanese fishing-bait shops. We demonstrated that all specimens corresponded to the species based upon the atoke morphology and the molecular data (Figure 2) even though no epitoke material was available for a detailed study of reproductive features. As shown in this study, another species closely related to P. linea from the East China Sea is P. babuzai comb. nov. We have not examined specimens of that species, but it is readily distinguished from P. linea by the development of distal dorsal ligule in posterior parapodia. The parapodia of the specimens reviewed by Arias et al. (Reference Arias, Richter, Anadón and Glasby2013) seems otherwise more similar to P. linea; thereby, we cannot disregard the possibility that additional distinct species also occur in the Yellow Sea.
The exotic status of P. linea in the Mediterranean Sea is questioned until further detailed studies on epitokal morphology and molecular analyses using material from both the Yellow and Mediterranean Seas are carried out to clarify its taxonomy.
Key to species of Perinereis Kinberg, Reference Kinberg1865 belonging to group 2
This key includes all species now regarded as Perinereis G2 sensu Hutchings et al. (Reference Hutchings, Reid and Wilson1991). However, some excluded species deserve discussion.
Perinereis brevicirrata (Treadwell, Reference Treadwell1920) from Santos (Brazil) apparently belongs to the ‘P. aibuhitensis’ species group but it is excluded from the key because its morphology is incompletely known, the description is brief and poorly illustrated, and there have been no further redescriptions of the species. de León-González & Goethel (Reference de León-González and Goethel2013) pointed out that P. brevicirrata and P. rookeri differed in the presence of notopodial prechaetal lobes, the number of transverse rows on areas VII–VIII, and the number of paragnaths on area V. However, we noticed based on the original descriptions that both species lack notopodial prechaetal lobes and the number of rows on areas VII–VIII is unclear in P. brevicirrata.
Neanthes multidentata Fassari & Mòllica, Reference Fassari and Mollica2000 from Sicily (Italy) may also belong to the ‘P. aibuhitensis’ species group, and seems more closely allied to P. linea; however, it differs from P. linea by having only conical paragnaths on area VI and spinigers all homogomph in neurochaetae. The bars on area VI of Perinereis G2 species are typically regarded as cones due to its small size and pointed shape, and the same misperception could have occurred in N. multidentata; also, the spinigers all homogomph in neuropodia is an uncommon condition in Perinereis. Neanthes multidentata may belong to the ‘P. aibuhitensis’ species group although a re-examination of the type material needs to be performed to re-assess those two features.
Finally, N. jihueiensis Hsueh, Reference Hsueh2019 and N. sanguensis Hsueh, Reference Hsueh2019 may belong to Perinereis G2. They were originally described with conical paragnaths on area VI, which are seemingly bar-shaped, but the pictures in ventral view lack sufficient detail (Hsueh, Reference Hsueh2019: 180, 188, Figs 5C, 13C). An examination of the type materials is needed before judging these species in Perinereis.
1. Proximal dorsal ligule barely or not enlarged in posterior parapodia Subgroup 2A…2
Proximal dorsal ligule markedly enlarged in posterior parapodia Subgroup 2B16
2. Dorsal cirri short, not projecting beyond distal dorsal ligule in medial parapodia; blades of heterogomph falcigers straight with markedly elongated incurved terminal tooth‘Perinereis aibuhitensis’ species group3
Dorsal cirri long, projecting distinctly beyond distal dorsal ligule in medial parapodia; blades of heterogomph falcigers distinctly convex with short incurved terminal tooth12
3. Ridges of area VI distally and sub-medially coalesced (areas VI–V–VI ridge pattern λ-shaped)4
Ridges of area VI distally separated from each other (areas VI–V–VI ridge pattern π-shaped) 6
4. Distal dorsal ligule distinctly short in posterior parapodia, projecting barely beyond notoaciculae P. babuzai (Hsueh, Reference Hsueh2019) comb. nov. (Taiwan)
Distal dorsal ligule of medium length in posterior parapodia, projecting distinctly beyond notoaciculae 5
5. Area VI with long bars (smooth-bars); distal dorsal ligule bluntly rounded in anterior parapodia; distal dorsal ligule projecting beyond median ligule in posterior parapodia; area II with paragnaths arranged in oval patch; area III with laterally isolated paragnaths; area V with usually one paragnath (rarely none) P. singaporiensis (Grube, Reference Grube1878) (Singapore)
Area VI with short bars (broad-petite bars); distal dorsal ligule conical in anterior parapodia; distal dorsal ligule subequal to median ligule in posterior parapodia; area II with paragnaths arranged in distinct crescentic rows; area III without laterally isolated paragnaths; area V with usually three paragnaths (seldom 4–5) P. linea (Treadwell, Reference Treadwell1936) (Xiamen, China)
6. Area VI with long bars (smooth-bars); area III without laterally isolated paragnaths; areas VII–VIII with anterior band having a medial patch of many tiny paragnaths P. vancaurica (Ehlers, Reference Ehlers1868) (Nicobar Islands, Andaman Sea)
Area VI with short bars (broad-petite bars); area III with distinct laterally isolated paragnaths; areas VII–VIII with anterior band lacking medial patch of tiny paragnaths 7
7. Areas VII–VIII with anterior band having only one furrow row 8
Areas VII–VIII with anterior band having two rows (one on furrows and one on ridges)10
8. Distal dorsal ligule not projecting beyond notoaciculae in medial and posterior parapodia; neuroacicular ligule extending markedly beyond median ligule in posterior parapodia; area III with 36 paragnaths P. vitabunda (Pflugfelder, Reference Pflugfelder1933) (Sumatra, Indonesia)
Distal dorsal ligule markedly extending beyond end of notoaciculae throughout; neuroacicular ligule subequal to or slightly shorter than median ligule in posterior parapodia; area III with up to 31 paragnaths 9
9. Ligules in anterior parapodia slender, acuminate; postero-dorsal tentacular cirri reaching chaetiger 2 P. kinmenensis (Hsueh, Reference Hsueh2019) comb. nov. (Kinmen, China)
Ligules in anterior parapodia thickened with blunt tip; postero-dorsal tentacular cirri reaching chaetiger 4–5 P. aibuhitensis (Grube, Reference Grube1878) (Palau)
10. Neuroacicular ligule projecting markedly beyond ventral ligule in posterior parapodia; distal dorsal ligule shorter than median ligule throughout body; proximal dorsal ligule longer than distal dorsal ligule P. belawanensis (Pflugfelder, Reference Pflugfelder1933) (Sumatra, Indonesia)
Neuroacicular ligule subequal to or slightly shorter than ventral ligule in posterior parapodia; distal dorsal ligule subequal to or barely shorter than median ligule throughout body; proximal dorsal ligule subequal or shorter than distal dorsal ligule 11
11. Area I: 7–8; area III: 48–65; area VII–VIII: 57–58; blade of heterogomph falcigers with incurved terminal tooth equaling half of total blade length; postero-dorsal tentacular cirri reaching chaetiger 3 P. shigungensis (Hsueh, Reference Hsueh2019) comb. nov. (Shigung, Taiwan)
Area I: 1–3; area III: 18; area VII–VIII: 33; blade of heterogomph falcigers with incurved terminal tooth equaling one-third to two-fifths of total blade length; postero-dorsal tentacular cirri reaching chaetiger 1 P. rookeri de León-González & Goethel, Reference de León-González and Goethel2013 (Florida, northern Gulf of Mexico)2
12. Area III with up to six paragnaths 13
Area III with more than 10 paragnaths 14
13. Ridges of area VI distally separated from each other (areas VI–V–VI ridge pattern π-shaped); area VI with 2–4 cones in addition to bars; area I with 4–15 paragnaths P. variodentata (Augener, Reference Augener, Michaelsen and Hartmeyer1913) (Western Australia)
Ridges of area VI distally and sub-medially coalesced (areas VI–V–VI ridge pattern λ-shaped); area VI without additional cones; area I with single paragnath P. camiguinoides (Augener, Reference Augener and Skottsberg1922) (Juan Fernández Islands, South Pacific Ocean)
14. Area III with laterally isolated paragnaths; areas VII–VIII with anterior band having only one furrow row; area I with 1–2 paragnaths; areas VII–VIII with 22–30 paragnaths P. horsti Gravier, Reference Gravier1899 (Djibouti)
Area III without laterally isolated paragnaths; areas VII–VIII with anterior band having two rows (one on furrows and one ridges); area I with four or more paragnaths; areas VII–VIII with 33–49 paragnaths 15
15. Distal dorsal ligules bluntly rounded in anterior parapodia; area V with single paragnath; area VI with one lateral cone in addition to bars; postero-dorsal tentacular cirri reaching chaetiger 7 P. kuwaitensis Mohammad, Reference Mohammad1970 (Kuwait, Persian Gulf)
Distal dorsal ligules bluntly conical in anterior parapodia; area V with three paragnaths; area VI without additional cones; postero-dorsal tentacular cirri reaching chaetiger 15 P. jascooki Gibbs, Reference Gibbs1972 (Cook Islands, South Pacific Ocean)
16. Areas VII–VIII with more than 30; area I with 11 paragnaths P. mochimaensis Liñero-Arana, Reference Liñero-Arana1983 (Venezuela)
Areas VII–VIII with fewer than 15 paragnaths; area I with fewer than 5 paragnaths 17
17. Proximal dorsal ligule as long as median ligule in posterior parapodia; distal dorsal ligule bluntly conical in anterior parapodia; distal dorsal ligule; area III with 7 paragnaths P. cariboea de León-González & Solís-Weiss, Reference de León-González and Solís-Weiss1998 (Quintana Roo, Mexican Caribbean Sea)
Proximal dorsal ligule extending markedly beyond median ligule in posterior parapodia; distal dorsal ligule bluntly rounded; area III with 17 paragnaths P. osoriotaffali de León-González & Solís-Weiss, Reference de León-González and Solís-Weiss1998 (Sinaloa, Gulf of California)
Acknowledgements
The kind support of many curators and collection managers was hugely significant for this study. We are grateful to Karen Osborn, Kathryn Ahlfeld, Geoff Keel, Linda Ward and the late Kristian Fauchald (USNM) for receiving TFVG and TP in their research laboratories. TFVG is also immensely grateful to Birger Neuhaus (ZMB), Tarik Meziane (MNHN) and Luis F. Carrera-Parra (ECOSUR) for providing many facilities in their laboratories to examine the annelid collections in charge, other gathered materials, or both. We much appreciate the kindness of Birger Neuhaus (ZMB), Rolf Beutel (PMJ), Ko Tomikawa (Hiroshima University) and Hironori Komatsu (NSMT), who kindly sent specimens for revision. TP is indebted to Hyun Soo Rho (KIOST) for developing early works of P. linea. TP would like to also thank Yun Kyoung Kim for her valuable assistance with the line drawing. We acknowledge Xuwen Wu (Chinese Academy of Sciences), Jun-Hui Lin (Third Institute of Oceanography China), Shi-Chun Sun (Ocean University of China), and Takeru Sakaguchi (Kagoshima City Aquarium) for providing valuable references of difficult access. We also appreciate the support of Isabel C. Molina-Acevedo (UMT) for assisting throughout the vectorial drawing and Alexandra Pardo (Universidad de Antioquia) for running the identification key. Finally, the careful reading by Anja Schulze, Torkild Bakken and two anonymous reviewers greatly improved the final version of the manuscript.
Financial support
This work was supported by the DAAD Short-Term Grants (TFVG, grant number 91673478); CONACYT (TFVG, grant numbers 291062, 291212); National Institute of Biological Resources, Korea (NIBR) research grant (TP, grant numbers NIBR2013-01-030, NIBR202006101); and ECOSUR (TFVG).
















