Introduction
Larval cestodes of the family Gryporhynchidae (Cestoda: Cyclophyllidea) infect the internal organs of both brackish and freshwater fish and mature in piscivorous birds (Jarecka, Reference Jarecka1970a, Reference Jareckab; Kozicka, Reference Kozicka1971; Murai et al., Reference Murai, Molnár and Gubanyi1997). Although these parasites have been recorded from much of Europe, Asia, Africa and America, published information from many geographical localities, including Britain, remains sparse (Khalil & Thurston, Reference Khalil and Thurston1973; Hoffman, Reference Hoffman1999; Natural History Museum Host Parasite Database (http://www.nhm.ac.uk/research-curation/research/projects/host-parasites/database/); Scholz et al., Reference Scholz, Steele, Beckham and Bray2002, Reference Scholz, Bray, Kuchta and Řepová2004, Reference Scholz, Boane and Saraiva2008). A recent review of larval gryporhynchid cestodes by Scholz et al. (Reference Scholz, Bray, Kuchta and Řepová2004), which included data on 21 species worldwide, confirmed a paucity of information on these parasites from the British Isles. This work highlighted the difficulties associated with the detection and identification of these tapeworms and the need to improve understanding of their geographical distribution, host ranges and pathological effects. Molnár (Reference Molnár2005) supported this, suggesting that these larval tapeworms can be pathologically important parasites warranting further study.
Compared with other tapeworms of British freshwater fish, members of the Gryporhynchidae have received very little attention. Khatun (Reference Khatun1973) provided light micrographs of Paradilepis scolecina (Rudolphi, 1819) in a PhD thesis on stickleback parasites, suggesting that this might represent a new species record for Britain. Only Valipora campylancristrota (Wedl, 1855), however, was detailed in a subsequent key to the tapeworms of British freshwater fish (Chubb et al., Reference Chubb, Pool and Veltkamp1987). Kirk (Reference Kirk2000) included three species of gryporhynchid tapeworm in a checklist of British fish parasites, but highlighted that these records lacked detail and required confirmation. This scarce and fragmented information has led to uncertainty over current species records and confusion with identification of these tapeworms during parasitological examinations. Chubb et al. (Reference Chubb, Pool and Veltkamp1987) emphasized the need for up-to-date species descriptions to promote the rapid and accurate identification of fish parasites and to raise awareness of those species not yet encountered.
This paper provides the first detailed account of larval gryporhynchid cestodes from British freshwater fish. Tissue digestion methodologies are explored as a technique to aid parasite identification. Pathological changes associated with infections of P. scolecina in the liver of wild tench, Tinca tinca (L.), are described for the first time.
Materials and methods
Sample collection
Records of larval gryporhynchid tapeworms from freshwater fisheries in Britain were collated from both published and grey literature, including unpublished parasitological surveys. Archived material held by the lead author was used to support species identification. A sample of T. tinca and rudd, Scardinius erythrophthalmus (L.), was obtained from a stillwater fishery in Berkshire, southern England to provide additional material for parasite identification and histopathological study. Fish were killed by an overdose of the anaesthetic benzocaine (Sigma-Aldrich, Poole, Dorset, UK). Parasitological examinations included low-power examinations of the liver, gall bladder and intestinal tract and organ squashes using a compound microscope at 100–400 × magnification.
Morphology and morphometry
Morphometrics of the rostellar hooks were recorded according to Bona (Reference Bona1975). Measurements of the hook handle and blade were made using the web-based software ‘ImageJ’ (Abramoff et al., Reference Abramoff, Magelhaes and Ram2004). Parasites were either examined live, or fixed in hot saline and transferred to 80% ethanol for long-term storage. Berlese fluid and glycerine–ammonium picrate were used to aid clarification of fixed specimens, as described by Ergens (Reference Ergens1969) and Baccarani et al. (Reference Baccarani, Bona, Buriola and Canestri-Trotti1998). Additional ethanol-fixed specimens of P. scolecina were subjected to complete proteolytic digestion in efforts to gain clear rostellar hook preparations. Parasites were rinsed for 20 min in nanopure water and digested with 4.5 μl of a proteinase K-based solution (100 μg/ml proteinase K (Sigma–Aldrich®); 50 mm Tris-HCl; 7 mm EDTA; 3.5% SDS). The state of digestion was monitored by visual observations of specimens at × 20 magnification. Digestion was arrested by the addition of 3.5 μl of a 50:50 formaldehyde:glycerine solution. Preparations were cover slipped (18 × 18 ‘0’ thickness coverslip (VWR International, Lutterworth, Leicestershire, UK)) and sealed in nail varnish. Specimens of P. scolecina and V. campylancristrota were deposited in the Natural History Museum, London (accession numbers 2009.9.29.1 and 2009.9.29.2, respectively).
Histopathology
Samples of liver from T. tinca infected with P. scolecina were fixed in Bouin's fixative and processed for histopathological examination. Five-micrometre-thick sections were stained using Mayer's haematoxylin and eosin (H&E) and examined microscopically for pathological changes.
Results
Occurrence and host records of parasites from British freshwater fish
Three species of larval gryporhynchid tapeworm have been recorded from freshwater fish in Britain, namely P. scolecina, Neogryporhynchus cheilancristrotus (Wedl, 1855) and V. campylancristrota. The morphology (fig. 1a) and measurements of the large and small rostellar hooks (table 1) are summarized from specimens recovered from freshwater fisheries in England.
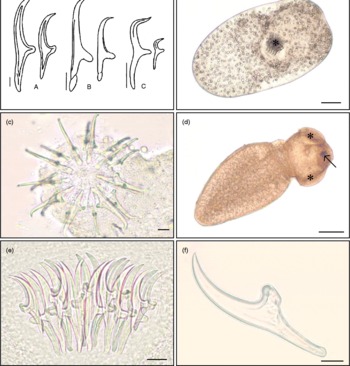
Fig. 1 (a) Morphology of rostellar hooks of Paradilepis scolecina (A), Neogryporhynchus cheilancristrotus (B) and Valipora campylancristrota (C) from freshwater fish in England (scale bar 10 μm). (b) Paradilepis scolecina following removal from its thin-walled cyst, showing the fully inverted rostellum (*) (scale bar 100 μm). (c) Extended rostellum of N. cheilancristrotus (apical view) following removal from the intestinal tract of Carassius carassius, showing concentric rings of ten large and ten small rostellar hooks. (scale bar 10 μm). (d) Valipora campylancristrota from the gall bladder of Cyprinus carpio, showing suckers (*) and rostellum (arrow) emerging from the body of the parasite (scale bar 100 μm). (e) Squash preparation of P. scolecina, showing the overlying hooks of the inverted rostellum (specimen cleared with Berlese fluid) (scale bar 20 μm). (f) Individual small hook of P. scolecina following proteolytic digestion (scale bar 10 μm). (A colour version of this figure can be found online at journals.cambridge.org/jhl)
Table 1 Measurements of rostellar hooks of larval gryporhynchid tapeworms from British freshwater fish (measurements taken from a minimum of three large and three small hooks of each specimen examined).
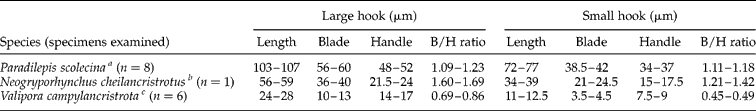
Hook measurements were based on specimens obtained from aScardinius erythrophthalmus, bCyprinus carpio and cTinca tinca. B/H ratio, blade/handle ratio.
Records of gryporhynchid tapeworms from unpublished parasitological examinations were sparse and included a number of unclear and unconfirmed species identifications. Despite this confusion, records confirm that all three species have long been established in Britain, but differ markedly in distribution.
Adults of P. scolecina were first recorded in North Wales from the intestines of cormorants in 1960 (J. Chubb, UK, pers. comm.). Larval parasites were first reported in Britain in 1973, following the examination of three-spine sticklebacks, Gasterosteus aculeatus (L.), from a brook in Surrey, south-east England (Khatun, Reference Khatun1973). In the past decade, P. scolecina has been confirmed from 16 fisheries in England and Wales, with ten of these observations made in the past 5 years. These records suggest that P. scolecina is the most common and widely distributed species of gryporhynchid tapeworm in England and Wales. This parasite has so far been recovered from G. aculeatus, S. erythrophthalmus, T. tinca and crucian carp, Carassius carassius (L.).
Neogryporhynchus cheilancristrotus was first recorded from a stillwater fishery in south-eastern England in 1997 (Environment Agency, unpublished). In contrast to P. scolecina, this tapeworm has been recorded on only one occasion since, also from a stillwater fishery in southern England. To date, only very small numbers of this parasite ( < 5) have been recovered from the intestinal tract of common carp, Cyprinus carpio L., and C. carassius.
Valipora campylancristrota was first reported in England in 1979 (Dearsley et al., Reference Dearsley, Moore and Thomas1982), later published in Chubb et al. (Reference Chubb, Pool and Veltkamp1987). The parasite has since been confirmed from approximately 14 freshwater fisheries in England and Wales. Host records of this parasite in Britain include T. tinca, C. carassius, S. erythrophthalmus, C. carpio and roach, Rutilus rutilus (L.). Historical records of this parasite from the liver and intestinal tract of T. tinca and C. carassius require confirmation as these represent atypical locations for this species, and possible misidentifications of other species cannot be ruled out. There are currently no published records of gryporhynchid cestodes from Scotland or Northern Ireland (D. Evans, UK, pers. comm.).
Detection and attachment characteristics
Paradilepis scolecina were found exclusively on the external surfaces of the liver and gall bladder of the tench and rudd. Heavy infections, comprising up to 200 parasites per host, were conspicuous during low-power microscopic examinations. Light infections dispersed throughout the liver and connective tissues surrounding the gall bladder were less obvious and required thorough microscopic examinations. Paradilepis scolecina were recorded in oval cysts, measuring approximately 1 mm in length. The thickness of the cyst wall varied considerably between parasites, presumably related to longevity of infection. Parasites within these cysts varied between 400 and 900 μm in length and usually possessed an invaginated rostellum (fig. 1b). Activity of these tapeworms often increased markedly following removal from the host. For parasites in thin-walled cysts, this movement occasionally led to their unaided emergence, with elongation of the body and either partial or full extension of the suckers and hooks. However, parasites in thicker cysts, maintaining an inverted rostellum, were also recovered. Detailed examinations of heavy P. scolecina infections in T. tinca revealed both live and degenerate parasites, the latter characterized by greater opacity and thickness of the cyst wall. Microscopic examination of these cysts revealed only remnants of recognizable hooks.
Detection of N. cheilancristrotus within the intestine required microscopic examination due to the near transparency of these very small tapeworms and their attachment between the intestinal folds. Detection was aided by the slow movement of these parasites and the presence of small, raised nodules which surrounded them. Specimens of N. cheilancristrotus recovered from the intestine of C. carassius measured approximately 350 μm in length. Most of the parasites found within the intestine possessed an inverted rostellum, although a small number of specimens with extended hooks (fig. 1c) were also recovered.
Detection of V. campylancristrota was relatively straightforward due to the localization of tapeworms within the gall bladder. Parasites were usually found attached by their suckers to the internal wall of the gall bladder and bile duct. Specimens removed from the gall bladder measured between 450 and 900 μm in length, depending on extension or retraction of the scolex. Parasites usually became increasingly active following removal from the host, with gradual emergence of suckers and occasionally the rostellum (fig. 1d). A number of inactive parasites with permanently inverted hooks were also recovered.
Parasite handling and identification
Gaining clear preparations of the rostellar hooks for measurement was dependent upon the size and position of the rostellum. The circular arrangement of these overlaying structures occasionally impeded clear and consistent hook preparations. The addition of Berlese fluid aided clarity of these structures (fig. 1e) and was considered essential for the examination of fixed parasites, especially those with inverted hooks.
Tissue digestion provided clear and consistent hook preparations (fig. 1f) irrespective of parasite condition and rostellum position. Full parasite digestion took approximately 2–3 min and resulted in the disconnection of hooks from their normal circular arrangement. This reduced the risk of hook damage through excessive flattening, producing clear hook preparations in a single plane. Maintaining parasites on a microscope slide during the process of digestion eliminated risks of hook damage or loss.
Histopathological changes associated with P. scolecina
Paradilepis scolecina was recorded in all of the T. tinca examined for histopathological study (n = 30), with a maximum intensity of approximately 120 parasites per host. Tapeworms were recorded primarily on the surface of the liver, but also throughout the hepatic parenchyma and occasionally within the exocrine pancreatic tissues. Live parasites were encapsulated within a thin wall of connective tissue (fig. 2a). A small number of lymphocytes were recorded in the immediate proximity of encysted P. scolecina. The inflammatory changes associated with these parasites, however, were generally mild. The use of Bouin's fixative resulted in the uptake of picric acid by the rostellar hooks, promoting detection of these tapeworms within tissue sections. Depending upon the orientation of sectioning, both morphology and rostellar hook number (fig. 2b) were occasionally distinguishable.
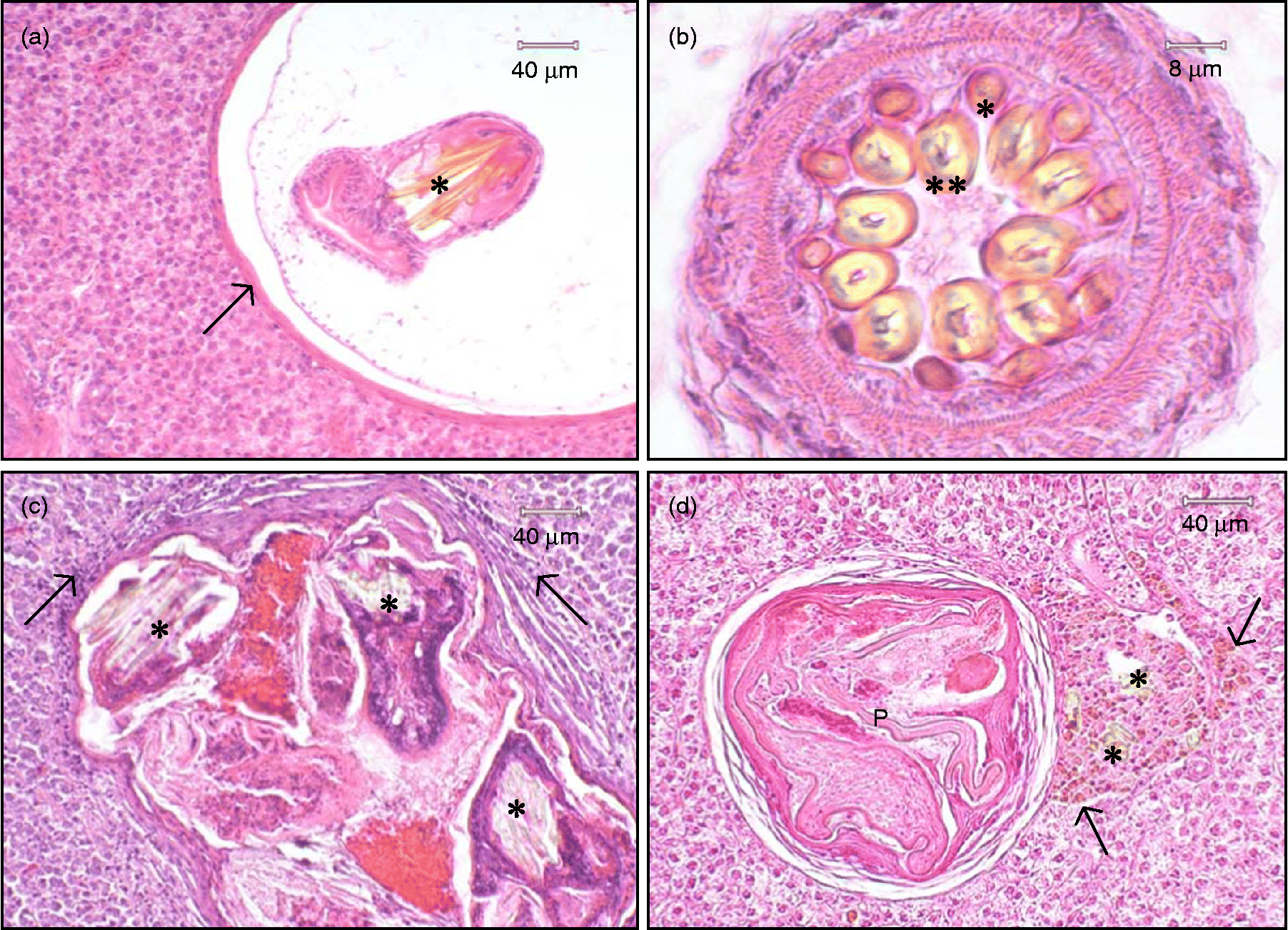
Fig. 2 (a) Histopathological section of Paradilepis scolecina encysted within the liver of Tinca tinca. The parasite, exhibiting stained hooks (*), is separated from the hepatic parenchyma by a thin wall of connective tissue (arrow). (b) Transverse section through the rostellum of P. scolecina showing concentric rings of ten small (*) and ten large (**), hollow rostellar hooks. (c) Aggregation of four degenerate P. scolecina encapsulated in a fibrous cyst within the liver of T. tinca. Remnants of the rostellar hooks (*) can be seen along with lymphocytes (arrows) within the surrounding hepatic tissue. (d) Hook fragments (*) discharged from an encysted, degenerate P. scolecina (P) located within the hepatic parenchyma of T. tinca. These structures are surrounded by a focus of macrophages engorged with light brown pigment (arrows).
Heavy infections of P. scolecina within the liver of infected T. tinca comprised both live tapeworms as well as parasites in varying states of degeneration. This included aggregations of degenerate tapeworms encapsulated within thickened layers of connective tissue (fig. 2c). Hook fragments discharged from a small number of degenerate parasites were occasionally found lodged within the hepatic parenchyma. These were surrounded by a focus of macrophages engorged with light brown pigment (fig. 2d). In all of the tench examined, the hepatic cells throughout the liver were considered normal, even during infections comprising over 100 parasites.
Discussion
This paper provides the first descriptions of P. scolecina, V. campylancristrota and N. cheilancristrotus from British freshwater fish. The rostellar hooks of these tapeworms conform to previous morphological descriptions (Priemer & Scholz, Reference Priemer and Scholz1989; Scholz, Reference Scholz1989a, Reference Scholzb; Baccarani et al., Reference Baccarani, Bona, Buriola and Canestri-Trotti1998; Scholz & Salgado-Maldonado, Reference Scholz and Salgado-Maldonado2001; Scholz et al., Reference Scholz, Bray, Kuchta and Řepová2004). It is hoped that this paper will complement existing keys of fish tapeworms, aiding the accurate identification of these small and inconspicuous parasites (Chubb et al., Reference Chubb, Pool and Veltkamp1987).
Records suggest that gryporhynchid tapeworms are infrequently encountered during parasitological examinations and that N. cheilancristrotus may have a particularly sparse distribution in England. This is perhaps surprising in view of the wide fish-host ranges of these parasites, the role of common piscivorous birds as definitive hosts, the number and frequency of fish movements in England and Wales further facilitating spread, and the large numbers of parasitological examinations conducted annually on susceptible fish species from both wild and farmed fish populations (Jarecka, Reference Jarecka1970a, Reference Jareckab; Kennedy, Reference Kennedy1974; Kirk, Reference Kirk2000; Scholz et al., Reference Scholz, Bray, Kuchta and Řepová2004; Environment Agency and Cefas Live Fish Movement Database 2009). In contrast, the larval tapeworms Ligula intestinalis (L.), Schistocephalus solidus (Müller, 1776), Triaenophorus nodulosus (Pallas, 1781) and Diphyllobothrium spp. have been documented throughout Britain and have received considerable attention (Fraser, Reference Fraser1960; Arme & Kennedy, Reference Arme and Kennedy1968; Kennedy, Reference Kennedy1974; Chubb et al., Reference Chubb, Pool and Veltkamp1987; Hoole, Reference Hoole, Pike and Lewis1994; Barber et al., Reference Barber, Hoare and Krause2000; Dezfuli et al., Reference Dezfuli, Pironi, Simoni, Shinn and Giari2007). This interest has undoubtedly been stimulated by the pronounced clinical abnormalities caused by these large plerocercoid infections and the pathogenic importance of these parasites to fish populations. In contrast, the small size and relatively benign appearance of gryporhynchid cestode infections, as well as the lack of comprehensive literature detailing their identification and impact, may have limited awareness, understanding and interest in these parasites in Britain (Kennedy, Reference Kennedy1974).
The detection of tapeworms in fish is generally a straightforward process (Chubb et al., Reference Chubb, Pool and Veltkamp1987; Hoole et al., Reference Hoole, Bucke, Burgess and Wellby2001). Although the detection and identification of larval gryporhynchid tapeworms is not necessarily difficult, it is more problematic than for most fish tapeworms. Protocols that omit organ squashes and gall bladder examinations (Hoole et al., Reference Hoole, Bucke, Burgess and Wellby2001) risk failed detection. Even when encountered, the tiny size and appearance of these parasites differ markedly from that of other adult and larval cestodes, providing the potential for taxonomic uncertainty. This is particularly evident for species that encyst within host tissues or possess an inverted scolex. The absence of N. cheilancristrotus and P. scolecina from existing keys of British tapeworms (Chubb et al., Reference Chubb, Pool and Veltkamp1987) and limitations of alternative texts prior to the comprehensive review of Scholz et al. (Reference Scholz, Bray, Kuchta and Řepová2004) may have impeded accurate identification of these tapeworms in Britain.
Records of tapeworms from historical parasitological examinations highlight two notable observations, namely a scarcity of records of the intestinal parasite N. cheilancristrotus and, in comparison with other species, increasing reports of P. scolecina within the past decade. It is proposed that this may reflect differences in the detection of these parasites, but also the abundance of their definitive hosts.
The primary definitive hosts of V. campylancristrota and N. cheilancristrotus are herons, Ardea spp., whereas P. scolecina matures in the cormorant, Phalacrocorax carbo (L.) (Jarecka, Reference Jarecka1970a, Reference Jareckab; Pietrock & Scholz, Reference Pietrock and Scholz2000; Scholz et al., Reference Scholz, Bray, Kuchta and Řepová2004). Although these birds are common residents of freshwater fisheries, cormorant populations have undergone a rapid increase in the past decade (Mitchell et al., Reference Mitchell, Newton, Ratcliffe and Dunn2004; Mavor et al., Reference Mavor, Parsons, Heubeck and Schmitt2006; Newson et al., Reference Newson, Marchant, Ekins and Sellers2007). Until the 1980s, the cormorant was almost exclusively a coastal breeding bird, but has since established inland colonies throughout England and Wales. Although adult P. scolecina have been recorded in cormorants in Britain since 1960, it is likely to be the rapid increase in abundance of these birds that has led to more frequent findings of their larvae in freshwater fish. The potential for very large numbers of cormorants to frequent individual fisheries may, in turn, increase infections within resident fish populations, thus increasing the likelihood of successful detection. In view of the continued rise in the number of cormorants breeding inland, it is likely that P. scolecina will become an increasingly common parasite of freshwater fisheries.
The scarcity of records for N. cheilancristrotus is not so easily explained. Herons are common piscivorous birds in Britain (Sterry, Reference Sterry1997) and the intestines of cyprinid fish have been subjected to large numbers of parasitological examinations spanning many decades. Pietrock & Scholz (Reference Pietrock and Scholz2000) detailed a number of factors that may dictate the prevalence of gryporhynchid larvae in fisheries, including seasonality, copepod abundance, and the distribution and feeding behaviour of both intermediate and definitive hosts. Current observations, however, suggest that the scarcity of records for N. cheilancristrotus may also be due to the inconspicuous appearance of this parasite within the fish host.
The relatively large size of encysted P. scolecina and their attachment to the external surfaces of the liver aid detection during parasitological examinations. Although slightly smaller in size, the mobility of V. campylancristrota and their localized attachment within the gall bladder also makes detection a relatively straightforward process during low-power microscopic examinations.
In contrast, N. cheilancristrotus recovered from the intestines of cyprinid fish were much smaller and harder to detect. The literature highlights considerable variation in the size of N. cheilancristrotus from fish, with specimens measuring as little as 180 μm reported from the intestines of cyprinid fish in Europe (Scholz, Reference Scholz1989a; Pietrock & Scholz, Reference Pietrock and Scholz2000). This factor, combined with the large surface area of the intestinal tract, near transparency of these parasites and attachment between the intestinal folds, can make detection problematic, especially during light infections. Records of V. campylancristrota from the intestines of cyprinid fish (Environment Agency, unpublished) require confirmation as it is feasible that these represent misidentifications for N. cheilancristrotus (Scholz et al., Reference Scholz, Bray, Kuchta and Řepová2004).
Despite these difficulties, it is clear that P. scolecina, N. cheilancristrotus and V. campylancristrota exhibit different site preferences within the fish host and possess hooks of markedly different sizes. This simplifies the identification of the species present in Britain, even allowing tentative species identification from straightforward post-mortem observations. However, due to the morphological similarity of other gryporhynchid species (Scholz et al., Reference Scholz, Bray, Kuchta and Řepová2004, Reference Scholz, Boane and Saraiva2008) and potential for the introduction of tapeworms to new localities with fish movements or bird migrations (Chubb & Yeomans, Reference Chubb and Yeomans1995), it is vital that the identification of these tapeworms is based on clear rostellar hook measurements (Bona, Reference Bona1975; Scholz et al., Reference Scholz, Boane and Saraiva2008).
The fastest and most simple approach for the identification of these parasites is to flatten live specimens on a microscope slide, followed by the subsequent measurement of hooks. The examination of fixed specimens benefits from clearing techniques that clarify individual hooks in squash preparations. This may be achieved through use of glycerine–ammonium picrate, which provides semi-permanent mounts for medium-term storage (Ergens, Reference Ergens1969). However, this process can be time-consuming and preparations are susceptible to crystallization, requiring re-mounting after 2–3 years (Ergens, Reference Ergens1969). Clarification of parasites with Berlese fluid was found to be very quick and overcomes many of these problems. This is considered the most desirable method for the routine examination of these larval tapeworms, providing clear preparations that can be sealed if permanent mounts are required.
Tissue digestion methodologies, identical to those employed for the identification of monogenean parasites (Shinn et al., Reference Shinn, Gibson and Sommerville2001), provided an alternative approach to aid tapeworm identification. Although not always necessary, this technique was simple and rapid, providing a consistent and reliable way of obtaining very clear hook preparations, irrespective of rostellum position, hook size or quality of specimen fixation. This makes it a suitable technique if there is limited material available for study, or if subsequent examinations of the rostellar hooks are needed (e.g. scanning electron microscopy). Tissue digestion is also considered to be a valuable and reliable method for the differentiation of morphologically similar species and deposition of reference material. This is important in view of the lack of good-quality reference material that exists for these parasites, a factor that has contributed to considerable taxonomic confusion and persistent nomenclatural problems (Bona, Reference Bona1975; Scholz, Reference Scholz1989a; Scholz et al., Reference Scholz, Bray, Kuchta and Řepová2004).
Literature on the pathology of gryporhynchid cestodes is largely confined to the species N. cheilancristrotus and V. campylancristrota (Bauer et al., Reference Bauer, Musselius and Strelkov1969; Körting, Reference Körting1984; Molnár, Reference Molnár2005). Reports suggest that these parasites can be pathogenic to both fish and bird hosts (Karstad et al., Reference Karstad, Sileo, Okech and Khalil1982; Molnár, Reference Molnár2005). Valipora campylancristrota, the cause of valiporosis, is considered of pathogenic importance to common carp, C. carpio (Bauer et al., Reference Bauer, Musselius and Strelkov1969; Sapozhnikov et al., Reference Sapozhnikov, Skvortsova and Ladukhin1974; Schäperclaus et al., Reference Schäperclaus, Kulow and Schreckenbach1991). According to these latter authors, carp fry lost condition and even died as a consequence of heavy parasite infections. Jara & Orech (Reference Jara and Orech1964) described marked congestion and mechanical damage to intestinal blood vessels as a result of V. campylancristrota infections in common carp (recorded as Cysticercus dilepis-campylancistrotae Aubert), although no adverse influences on the weight gain of infected fry were found.
Körting (Reference Körting1984) described focal necrosis, haemorrhage and signs of enteritis resulting from the attachment of N. cheilancristrotus in the intestines of carp. Molnár (Reference Molnár2005) supported these findings with detailed observations of intestinal pathology caused by N. cheilancristrotus. This latter author proposed that this species, as well as other gryporhynchid cestodes, may be considered pathogenic parasites of fish. It is not known, however, whether this statement is consistent for other gryporhynchid species, in particular P. scolecina (Scholz, Reference Scholz1989b; Molnár, Reference Molnár2005). Karstad et al. (Reference Karstad, Sileo, Okech and Khalil1982) detailed the pathology of adult P. scolecina in the intestines of wild cormorants, P. carbo, from Kenya. Parasite attachment involved near complete penetration of the scolex through the gut, accompanied by fibrosis and pronounced inflammatory responses. Murai et al. (Reference Murai, Molnár and Gubanyi1997) described the component layers of the cysts surrounding P. scolecina metacestodes on the gall bladder of common bream. However, these authors highlighted that the pathological significance of larval P. scolecina in the fish host remains unclear.
During the current study, T. tinca heavily infected with P. scolecina exhibited no gross abnormalities or clinical signs of disease. The pathological changes caused by these infections to the liver, pancreas and surface of the gall bladder were generally localized and mild. The hepatocytes of heavily infected individuals showed no obvious signs of abnormality and were interspersed with only mild inflammatory responses. These changes were not considered of pathological importance to the fish examined. In contrast, Cone & Anderson (Reference Cone and Anderson1977) provided some evidence that heavy infections of larval gryporhynchid parasites in the livers of pumpkinseed, Lepomis gibbosus L., were capable of mortality. Furthermore, it is well documented that heavy infections of other larval tapeworms can cause significant liver pathology, leading to host debilitation and even death (Williams & Jones, Reference Williams and Jones1994).
Heavy infections of Triaenophorus nodulosus plerocercoids in the livers of perch, Perca fluviatilis L., can cause serious pathological alterations leading to reduced growth (Brinker & Hamers, Reference Brinker and Hamers2007). Plerocercoids of Diphyllobothrium ditremum (Creplin, 1825) are also known to cause severe pathological lesions in the livers of salmonid fish, resulting in pronounced liver dysfunction and mortality during heavy infections (Weiland & Meyers, Reference Weiland and Meyers1989). Current observations suggest that this is an unlikely consequence of P. scolecina infection. This is consistent with observations of Saraiva et al. (Reference Saraiva, Boane and Cruz2009), who detailed chronic inflammatory reactions in common carp from infections of Parvitaenia samfyia (Mettrick, 1967) and an unidentified species of Cyclustera, but no obvious detrimental impacts on heavily infected hosts. It is proposed that the very small size of these tapeworms and their encapsulation within host tissues limit damage to infected fish. These observations contrast with species that use their rostellum to penetrate host tissues during attachment (Karstad et al., Reference Karstad, Sileo, Okech and Khalil1982; Molnár, Reference Molnár2005). It is likely that both depth of attachment and insertion of hooks into host tissues are responsible for the greatest organ damage and host responses to infection. Observations during the current study of inflammatory reactions, including macrophage accumulations around fragments of rostellar hooks discharged from degenerate P. scolecina, provide support for this.
Acknowledgements
The authors wish to thank staff of the Environment Agency for assistance with fish sampling. We give particular recognition to Jody Armitage for assistance with fish examinations and line drawings, and David Bucke for assistance with interpretation of histopathology. We would also like to thank Bernice Brewster (Aquatic Consultancy Ltd), Shaun Leonard (Sparsholt College) and Ian Wellby (Blue Roof Aquatic Consultancy) for supplying parasitological data and Adriana García Vásquez (Institute of Aquaculture, University of Stirling) for assistance with the proteolytic digestion of specimens. We are also indebted to Dr James C. Chubb for providing valuable information on historical observations of V. campylancristrota and P. scolecina from England and Wales. Financial support of the Institute of Parasitology (project nos. Z60220518 and LC522) to T.S. is also acknowledged.





