Introduction
First commercialised in 1996, transgenic plants expressing insecticidal proteins from the bacterium Bacillus thuringiensis (Bt) have generally been associated with reduced use of broad-spectrum insecticides (Shelton et al., Reference Shelton, Zhao and Roush2002; Mendelsohn et al., Reference Mendelsohn, Kough, Vaituzis and Matthews2003), thereby reducing the risk of these compounds to human health and the environment. Corn (Zea mays L.) is one of the crop plants that has been genetically modified to express Bt genes and provides protection from herbivorous insect pests (Koziel et al., Reference Koziel, Beland, Bowman, Carozzi, Crenshaw, Crossland, Dawson, Desai, Hill, Kadwell, Launis, Lewis, Maddox, Mcpherson, Meghji, Merlin, Rhodes, Warren, Wright and Evola1993; Moellenbeck et al., Reference Moellenbeck, Peters, Bing, Rouse, Higgins, Sims, Nevshemal, Marshall, Ellis, Bystrak, Lang, Stewart, Kouba, Sondag, Gustafson, Nour, Xu, Swenson, Zhang, Czapla, Schwab, Jayne, Stockhoff, Narva, Schnepf, Stelman, Poutre, Koziel and Duck2001).
The European corn borer, Ostrinia nubilalis (Hübner) (Lepidoptera: Crambidae), is one of the most destructive pests of corn in the United States and Europe. In most of the US Corn Belt, O. nubilalis is a bivoltine insect-pest that causes significant economic losses by infesting whorl- and reproductive-stage corn and has been the target of several different management strategies (Hudon et al., Reference Hudon, LeRoux and Harcourt1989; Mason et al., Reference Mason, Rice, Calvin, Van Duyn, Showers, Hutchison, Witkowski, Higgins, Onstad and Dively1996). In 2003, Cry1F-expressing corn hybrids (Chambers et al., Reference Chambers, Jelen, Gilbert, Jany, Johnson and Gawronburke1991), derived from genetic transformation event TC1507 (see profile at http://www.agbios.com), were commercially deployed in the US for control of European corn borer. Cry1F-expressing corn hybrids, as well as other Bt expressing hybrids, represent an important alternative to conventional insecticides in terms of environmental safety and have increasingly been shown to have negligible effects on non-target organisms (Hellmich et al., Reference Hellmich, Siegfried, Sears, Stanley-Horn, Daniels, Mattila, Spencer, Bidne and Lewis2001; Shelton et al., Reference Shelton, Zhao and Roush2002; Naranjo et al., Reference Naranjo, Head and Dively2005).
Although genetically modified plants producing their own protective insecticides provide an important new approach to insect control, there is a concern that a large-scale deployment of transgenic crops expressing insecticidal proteins could rapidly lead to the development of Bt resistance within pest populations (Andow & Hutchison, Reference Andow, Hutchison, Mellon and Rissler1998; ILSI, 1998; Ferré & Van Rie, Reference Ferré and Van Rie2002; Shelton et al., Reference Shelton, Zhao and Roush2002; Glaser & Matten, Reference Glaser and Matten2003). The impact of insects developing resistance to corn hybrids expressing a Bt protein could include reduced yields, a return to reliance on more environmentally disruptive control options and potential resistance to related Bt proteins that comprise active ingredients of sprayable formulations. Reducing the risk of resistance evolution and developing sustainable pest management strategies remains a high priority.
In order to delay the evolution of insect resistance, adoption of appropriate resistance management strategies is necessary. Among the theoretical strategies for resistance management, the high-dose/refuge and pyramiding of more than one toxin with different target sites have been most widely cited (Roush, Reference Roush1997, Reference Roush1998; Gould, Reference Gould1998; Zhao et al., Reference Zhao, Cao, Li, Collins, Roush, Earle and Shelton2003). With the high-dose/refuge approach, a high expression level of the insecticidal protein is intended to reduce the fitness of heterozygotes such that the inheritance of resistance is functionally recessive. This high dose must be combined with a refuge from exposure in order to maintain a pool of susceptible homozygotes that mate with rare individuals that are homozygous for resistance. Assuming that the resistance is functionally recessive, the heterozygous progeny are susceptible to the high-dose expression. A major assumption of the high-dose/refuge approach is that resistance is a recessive trait such that heterozygotes are susceptible to the high doses of toxin delivered by the transgenic plants. Although the high-dose/refuge strategy currently in place to manage resistance in Bt corn has been widely adopted, empirical data that validate this approach are generally lacking.
Well-characterised resistant strains provide a means to validate proposed resistance management strategies (Shelton et al., Reference Shelton, Tang, Roush, Metz and Earle2000; Zhao et al., Reference Zhao, Cao, Li, Collins, Roush, Earle and Shelton2003; Morin et al., Reference Morin, Henderson, Fabrick, Carrière, Dennehy, Brown and Tabashnik2004). Laboratory-selected strains that exhibit high levels of resistance to a toxin are especially useful in validating and improving resistance management strategies when the genetic architecture of resistance in those strains is similar to that expected to evolve under field conditions.
Although high levels of resistance to Cry1Ab and formulated Bt toxins have been selected for, under laboratory conditions in a number of O. nubilalis colonies (Huang et al., Reference Huang, Higgins and Buschman1997; Bolin et al., Reference Bolin, Hutchison and Andow1999; Chaufaux et al., Reference Chaufaux, Seguin, Swanson, Bourguet and Siegfried2001; Siqueira et al., Reference Siqueira, Moellenbeck, Spencer and Siegfried2004) to date there have been no reports that these colonies survive on Cry1Ab-expressing plants. In the present study, we report results of experiments designed to characterise a resistant strain of O. nubilalis that was selected in the laboratory for high levels of resistance to Cry1F (Pereira et al., Reference Pereira, Lang, Storer and Siegfried2008). The inheritance of the resistance and the ability of susceptible, resistant and F1 progeny to survive on transgenic plants was determined. The results of this research have direct implications for resistance management of O. nubilalis to Cry1F corn.
Materials and methods
Insect strains
The Cry1F-selected strain was initially developed by Mycogen Seeds (B. Lang, personal communication) and originated from a population derived from field collections at ten geographically distinct locations within the central US Corn Belt in 1996. A total of 115 females and 135 males were combined into one mating cage. The resulting offspring were used to establish a laboratory strain that was maintained using standard rearing techniques (Lewis & Lynch, Reference Lewis and Lynch1969) with slight modifications (Siqueira et al., Reference Siqueira, Moellenbeck, Spencer and Siegfried2004). Briefly, larvae were reared at 27±0.7°C and 80% RH under a 24-h photophase on a wheat germ-based diet. Pupae were transferred to mating cages and held under 16-h photophase. Egg masses were deposited onto waxed paper lining the top of the cage, gathered daily and incubated in Petri dishes lined with moistened filter paper until hatching.
Selection for Cry1F resistance was initiated in 1998 after the colony had been reared for seven generations under laboratory conditions. The conditions for rearing and selection methods are described in Pereira et al. (Reference Pereira, Lang, Storer and Siegfried2008). Briefly, the Cry1F toxin used for selection was produced through fermentation of recombinant Pseudomonas fluorescens, strain MR872, and consisted of a proteolytically activated and chromatographically purified toxin (provided by Dow AgroSciences LLC, Indianapolis, IN). The O. nubilalis strain was selected with increasing concentrations of Cry1F incorporated into the rearing diet for 30 generations and then maintained at 35 μg ml−1 for ten generations. During 2001 and 2002, the colony was transferred to the University of Nebraska, where further selection was conducted using Cry1F from the same source used from 1998–2001 but applied to the surface of artificial diet with exposure of neonates for seven days and then transferring surviving larvae to untreated diet (Pereira et al., Reference Pereira, Lang, Storer and Siegfried2008). After generation 53, maintenance of the Cry1F-selected strain was achieved by exposing neonates to 60 ng cm−2, which corresponds to the upper limit of the 95% confidence interval of the concentration that kills 99% of the individuals (LC99) from susceptible populations (B. Siegfried, personal communication). This maintenance selection was applied every three generations to eliminate any susceptible individuals remaining in the population.
The susceptible strain used for genetic crosses and survival experiments was derived from the Cry1F-selected strain after the first 30 generations of selection before resistance was fixed. Neonates were exposed to a low concentration of Cry1F on the diet (2 ng cm−2, equivalent to half of the LC50 for susceptible populations) for seven days. Individual larvae that had not initiated feeding and had not grown beyond first instar were transferred to rearing diet in the absence of toxin and used to initiate the susceptible colony. Subsequently, three more rounds of this process of selection for susceptibility were conducted. This method proved to increase susceptibility and decrease genetic variance of the strain, as indicated by lower lethal concentrations and steeper slopes in the probit regression of concentration-mortality curves compared with other susceptible strains (B.D.S., unpublished data).
Analysis of inheritance
Mass reciprocal crosses between selected and control strains were conducted, and the response of F1 and backcross progeny to the Cry1F toxin was evaluated. The reciprocal crosses were repeated twice in two different generations. Backcross progeny were produced by mass crossing of F1 obtained from reciprocal crosses to the selected strain. To generate virgin males and females for the reciprocal crosses, corn borer pupae were separated by gender, and 150 Cry1F-selected females were pooled with 150 control males, and 150 control females were pooled with 150 Cry1F-selected males in mating cages. These crosses provided enough offspring for bioassays and backcross with the selected strain. To obtain the backcross generation, the two F1 progenies (from mass crosses between selected and control strains) were combined, reared, sexed and crossed with the selected strain using the same procedure for reciprocal crosses.
F1 and backcross progenies were tested for susceptibility to Cry1F using the standard bioassays developed in our laboratory (Marçon et al., Reference Marçon, Young, Steffey and Siegfried1999). The Cry1F toxin used for bioassays was the same used in the selection experiments. Briefly, the susceptibility of neonates to Cry1F was determined by exposure to varying concentrations of toxin applied on the surface of artificial diet. A single neonate (<24-h after eclosing) was placed in each well of a 128-well tray (CD International, Pitman, NJ) and held for seven days at 27°C, 24-h scotophase, and 80% RH until larval mortality was recorded (Marçon et al., Reference Marçon, Young, Steffey and Siegfried1999). Dilutions were prepared in 0.1% Triton-X 100; bioassays were conducted in duplicate on two dates and included at least seven concentrations of purified toxin plus a control (0.1% Triton-X 100 applied to the diet surface). Larval mortality was recorded after seven days of exposure.
Concentration-mortality data were analyzed by probit regression using POLO-PC (LeOra Software, 1987) to generate lethal concentrations and to determine significance of differences among strains and generations. A likelihood ratio test was conducted at α=0.05 to determine the significance of resistance ratios and compare dose-response curves from the reciprocal crosses (Russell et al., Reference Russell, Robertson and Savin1977; Preisler et al., Reference Preisler, Hoy and Robertson1990). Dominance of resistance was calculated using the formula D X=(XRS–XSS)/(XRR–XSS), where XRR, XRS and XSS are the quantitative values for a trait X (i.e. logLC50) for resistant homozygotes, heterozygotes and susceptible homozygotes, respectively (Bourguet et al., Reference Bourguet, Genissel and Raymond2000). However, because the resistant strain did not exhibit significant mortality at the highest Cry1F concentration tested, a low-end estimate of the LC50 was used for dominance calculations based on the highest concentration tested. The monogenic inheritance model was tested directly by using the Chi-square test to compare observed and expected mortality of the backcross progeny at different Cry1F concentrations (Preisler et al., Reference Preisler, Hoy and Robertson1990; Tabashnik, Reference Tabashnik1991; Tabashnik et al., Reference Tabashnik, Schwartz, Finson and Johnson1992). If the resistance is monogenic, a backcross of F1 (SS×RR)×RR will produce progeny that are 50% RS and 50% RR. To test this hypothesis, the expected mortality in the backcross progeny at toxin concentration x was calculated using the formula Y X=0.50(MRS+MRR), where MRS and MRR are the mortalities of the presumed RS (F1) and RR (Cry1F-sel. parental strain) genotypes at concentration x, respectively.
On-plant assays
Greenhouse experiments were carried out with a commercially available Cry1F-expressing hybrid (Event TC1507) and its near isoline in the vegetative (V6-9) and reproductive (R1-3) stages. These stages were chosen to represent those that coincide with infestations of the 1st and 2nd O. nubilalis generations common to bivoltine populations that occur throughout most of the US Corn Belt (Hudon et al., Reference Hudon, LeRoux and Harcourt1989; Mason et al., Reference Mason, Rice, Calvin, Van Duyn, Showers, Hutchison, Witkowski, Higgins, Onstad and Dively1996). Six overall treatment combinations were tested in a factorial design that involved two corn hybrids (Event TC1507 that expresses Cry1F and a near isoline) and three O. nubilalis genotypes (the Cry1F-selected and control strains assumed to be homozygous for resistance and susceptibility, respectively, and the hybrid F1 progeny derived from reciprocal crosses between susceptible and resistant adults). To generate the F1 progeny, corn borer pupae were separated by gender, and 100 Cry1F-selected females were pooled with 100 control males, and 100 control females were pooled with 100 Cry1F-selected males in mating cages. Because concentration-mortality data from the inheritance analysis indicated that resistance is autosomal, the progeny of the reciprocal crosses were pooled by combining the egg masses produced in each mating cage.
The experiments were carried out in a greenhouse at the University of Nebraska, Lincoln, NE, in 2003 and 2004. Seeds of TC1507 and its isoline were provided by Dow AgroSciences LLC, Indianapolis, IN. The corn hybrids were planted in a soil mixture composed of 50% black soil, 30% peat and 20% sand in 18.9-l pots using three seeds per pot. Two weeks later, the plants were thinned to a single plant per pot, which were then watered daily and fertilised as needed until reaching the appropriate phenological stage for infestation. The vegetative- and reproductive-stage experiments were conducted separately. In each experiment, the six treatments were randomly assigned to six areas in the greenhouse, and 30 plants of each hybrid were utilised for corn borer infestations (ten plants per treatment). The greenhouse was divided into six areas with a 2-m aisle separating the areas to reduce the risk of larvae moving among treatments. The TC1507 plants were tested for toxin expression with TraitCheckTM Bt1F Lateral Flow Kit (Strategic Diagnostics Inc., Newark, DE). Plants were infested (40 neonates per plant) by placing a 1.5-ml microfuge tube containing the neonates one leaf below either the whorl (V8 stage) or the primary ear (R1 stage) (Ritchie et al., Reference Ritchie, Hanway and Benson1992; Mason et al., Reference Mason, Rice, Calvin, Van Duyn, Showers, Hutchison, Witkowski, Higgins, Onstad and Dively1996). All plants were dissected 15 days after infestations to record survival and determine larval weight.
The data were analyzed as a randomised complete block design with years 2003 and 2004 as block factors using a two-way analysis of variance (ANOVA), with corn borer genotypes (RR, RS and SS) and corn hybrids (Herculex and Isoline) as the main factors. Percent survival was arcsine transformed and weight of survivors were log transformed before each ANOVA was performed to meet the assumptions of normality and homogeneity of variance (PROC UNIVARIATE, PROC GPLOT) (SAS Institute, 2002). Differences among means in each corn stage were tested using Fisher's protected least significant difference (PROC MIXED) (SAS Institute, 2002).
Dominance of Cry1F resistance at the toxin concentration expressed by Cry1F corn (D X) was determined using formulas described by Bourguet et al. (Reference Bourguet, Genissel and Raymond2000) and methods adapted from Liu & Tabashnik (Reference Liu and Tabashnik1997). Values of D X range from zero (completely recessive resistance) to one (completely dominant resistance). When D X is 0.5, resistance is referred to as codominant or additive. The traits used in the calculation of dominance were larval survival, percentage of weight gain 15 days after infestation and the combination of the two traits. The fitness of resistant homozygotes on Cry1F-expressing corn was defined as one. Fitness of susceptible homozygotes was estimated as the phenotypic value of a trait for Cry1F-selected larvae divided by the phenotypic value of the trait for control larvae. Likewise, fitness of heterozygotes was estimated as the phenotypic value of a trait for F1 progeny divided by the phenotypic value of the trait for Cry1F-selected larvae. For each corn borer genotype, survival on Cry1F corn was estimated by adjusting for mortality on non-expressing plants using Abbott's correction, and percentage of weight gain was calculated relative to the larval weight of each genotype on control corn. To take into account the fact that Cry1F corn may affect the survival and weight gain of the larvae, we combined these two components of fitness by multiplying their genotypic values and proceeded as above for calculation of dominance.
Results
Inheritance of Cry1F resistance
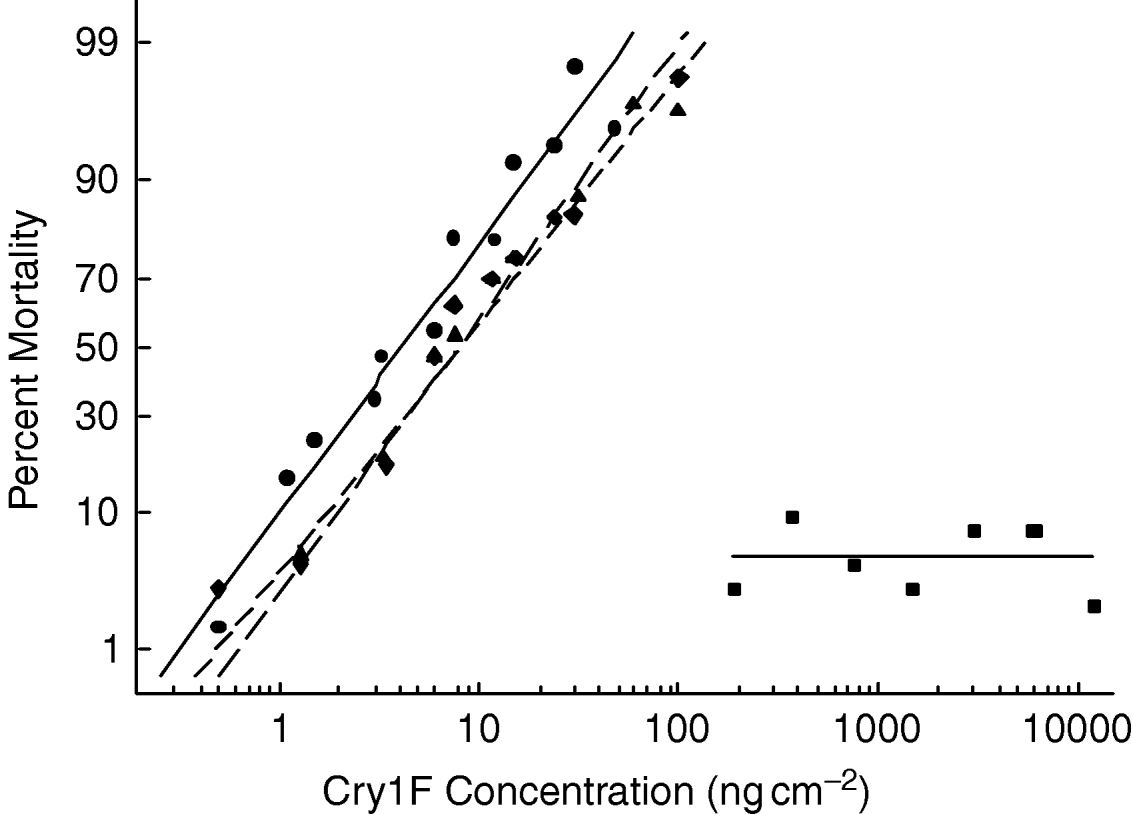
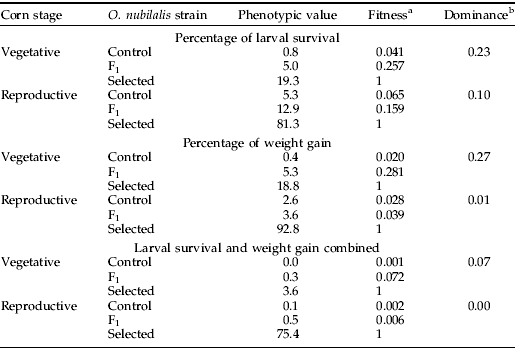
The progeny of resistant females crossed with susceptible males displayed response curves nearly identical to those of the progeny of susceptible females crossed with resistant males (fig. 1). When tested for equality of slopes and intercepts, the concentration-mortality curves for the two reciprocal crosses were not significantly different (χ12=1.60, P=0.450), indicating that resistance was autosomally inherited with no maternal effects. The response of the F1 generation was similar to that of the control strain, indicating recessive resistance (fig. 1). The regression lines of the F1 and control strain were parallel (χ12=1.72, P=0.423), indicating that the phenotypic distance between heterozygous and homozygous susceptible individuals was constant over the range of Cry1F concentrations tested. Although we could only determine a lower limit for the LC50 of the selected strain, calculated dominance based on the methods proposed by Bourguet et al. (Reference Bourguet, Genissel and Raymond2000) indicates a dominance level (DLC) less than 0.11, which is consistent with recessive inheritance.
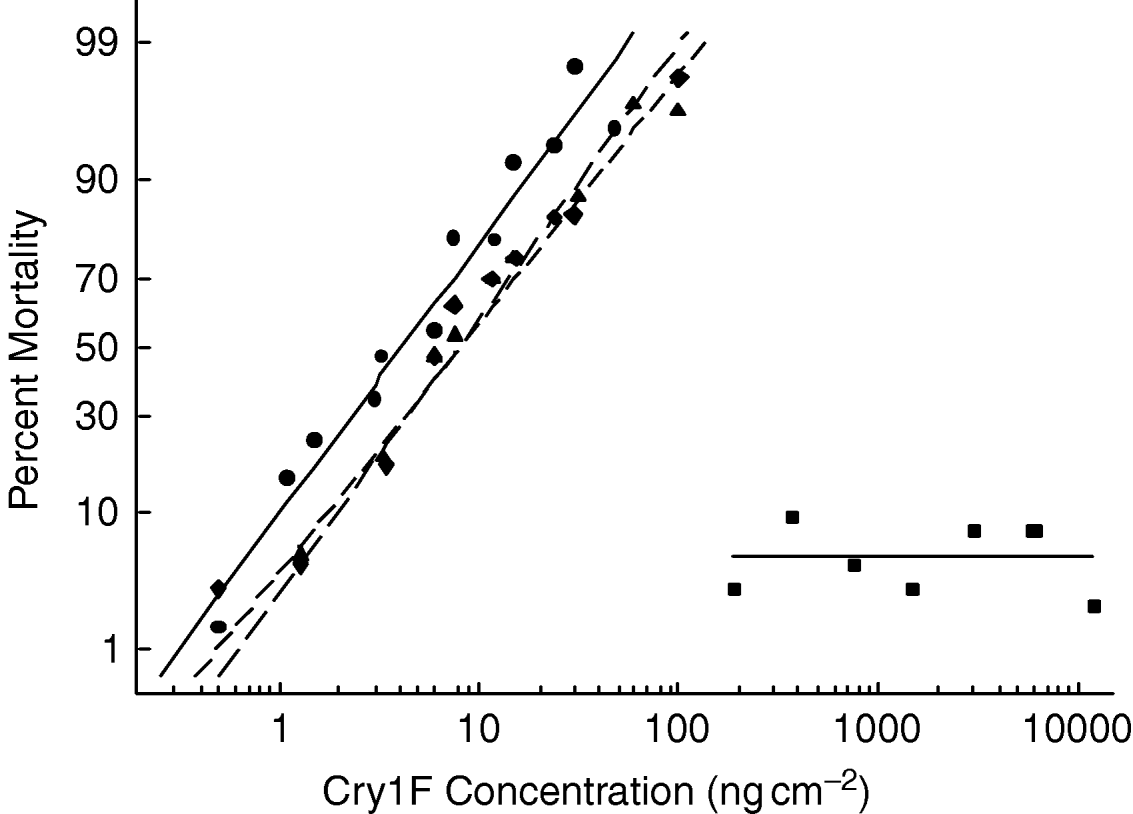
Fig. 1. Concentration-mortality curves of the progeny derived from reciprocal crosses of the Cry1F-selected and control O. nubilalis strains compared with the parental strains (–•–, SS parents; - -◆- -, SS Male×RR Female; - -▲- -, SS Female×RR Male; –■–, RR Parents).
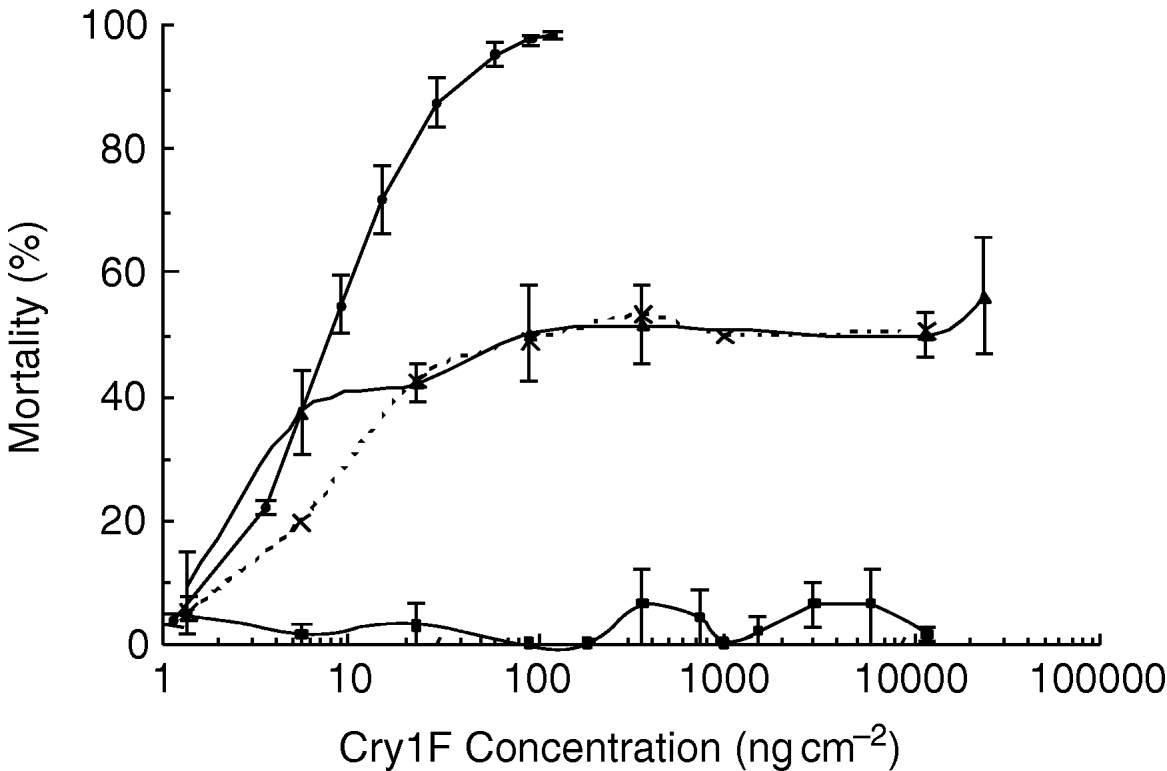
Monogenic versus the polygenic inheritance models were tested by backcrossing the F1 generation with the selected strain and comparing the progeny's response with that of the parents. If resistance is conferred by a single locus or set of tightly linked loci, when the heterozygotes F1 (RS) are backcrossed to the resistant strain (RR), the resulting progeny will consist of a 1:1 ratio of RS:RR genotypes. Since there was no mortality of the resistant parental strain at the highest concentrations used, a plateau at 50% mortality of the backcross progeny is expected if the resistance is conferred by a single genetic factor. Such a plateau was apparent in the backcross generation, suggesting that half of backcross progeny responded to concentrations of Cry1F that kill heterozygotes while the other half did not (fig. 2). Additionally, the Chi-square test showed no significant departure from the expected ratio for a single factor inheritance (χ62=8.39, P=0.211). Therefore, the pattern of response obtained in the backcross is consistent with simple monogenic inheritance of resistance.
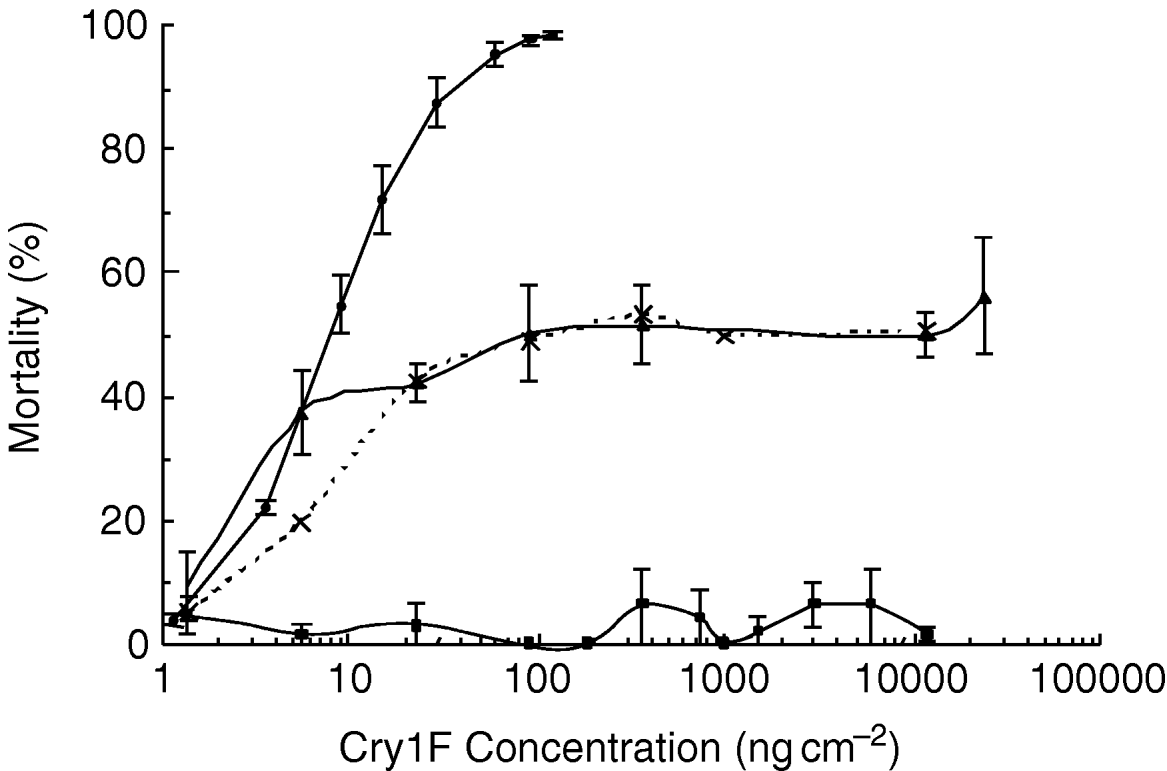
Fig. 2. Response curves of the backcross progeny compared with those of the F1 (RS) and resistant (RR) parents. Error bars represent standard errors of the mean mortality attained at each concentration of Cry1F applied (–•–, RS parents; –▲–, RS×RR progeny; –■–, RR parents; - - -×- - -, expected RS×RR).
Survival on Cry1F corn
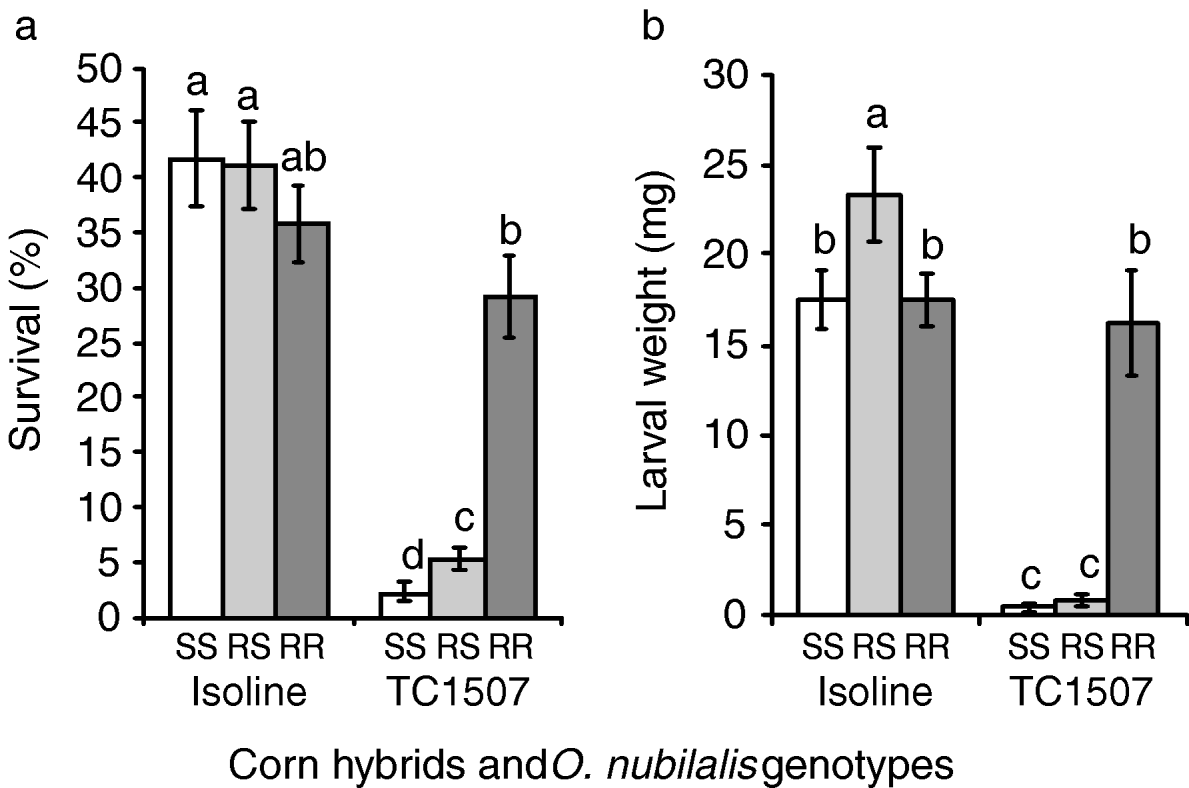
Results of on-plant assays conducted with greenhouse-grown vegetative and reproductive stage plants are presented in figs 3 and 4, respectively. Throughout these tests, the Cry1F-selected and control O. nubilalis strains as well as the progeny of crosses between the two strains effectively colonised non-Bt corn, as indicated by relatively high survival rates and larval weight of survivors 15 days after infestation (figs 3 and 4).
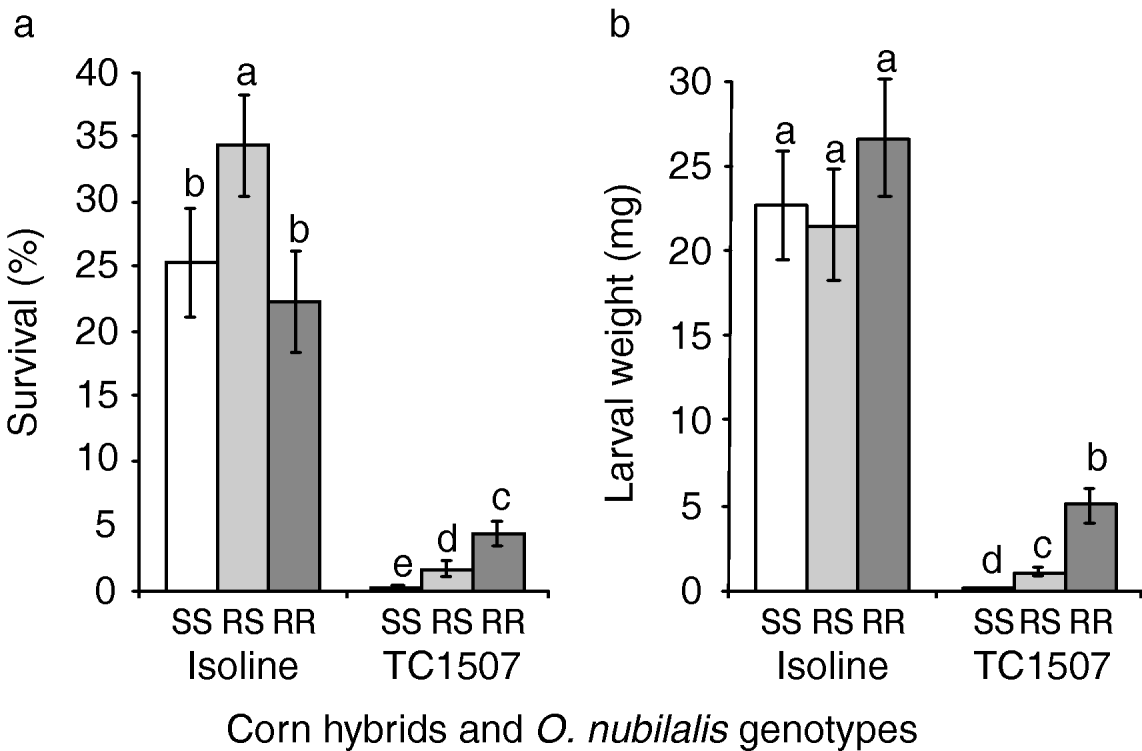
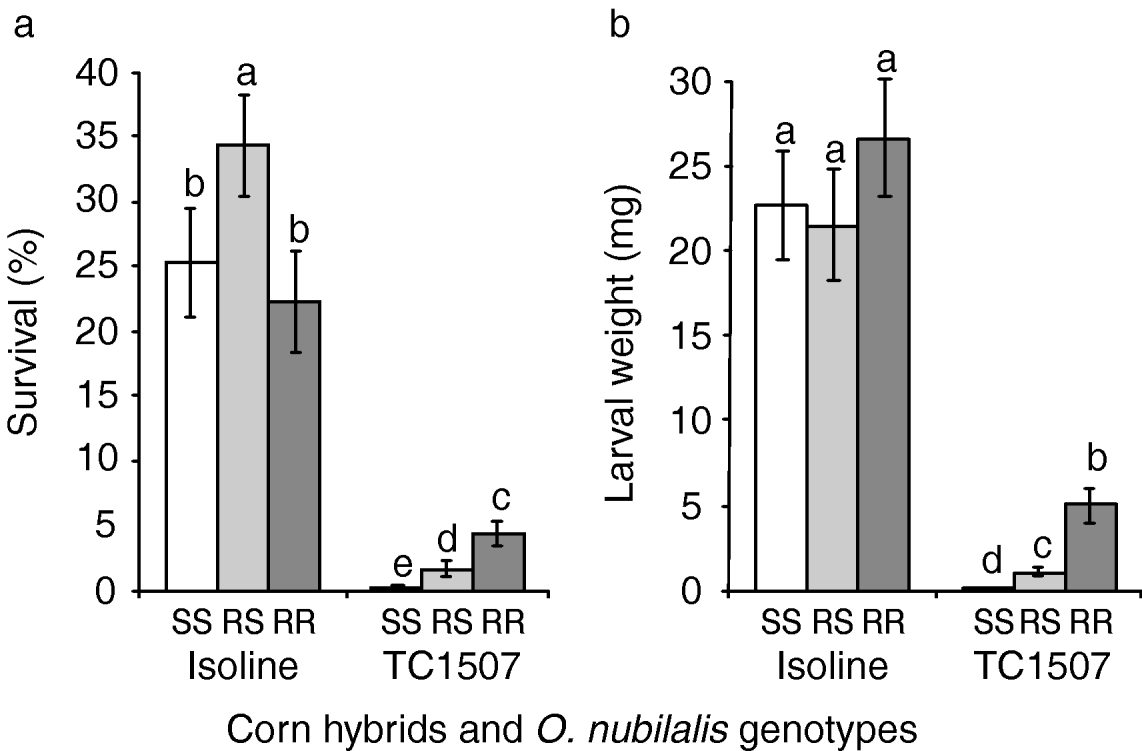
Fig. 3. Greenhouse tests with corn plants in the vegetative stage. (a) Percentage of survival and (b) larval weight of European corn borers homozygous for susceptibility to Cry1F (SS), heterozygous for Cry1F resistance (RS) and homozygous for Cry1F resistance (RR) after 15 days feeding on vegetative stages of Cry1F-expressing corn plants (event TC1507) and their non-Cry1F, isoline counterparts. Error bars represent standard errors. Bars in a chart followed by the same letter are statistically similar (Fisher's protected LSD, P>0.05).
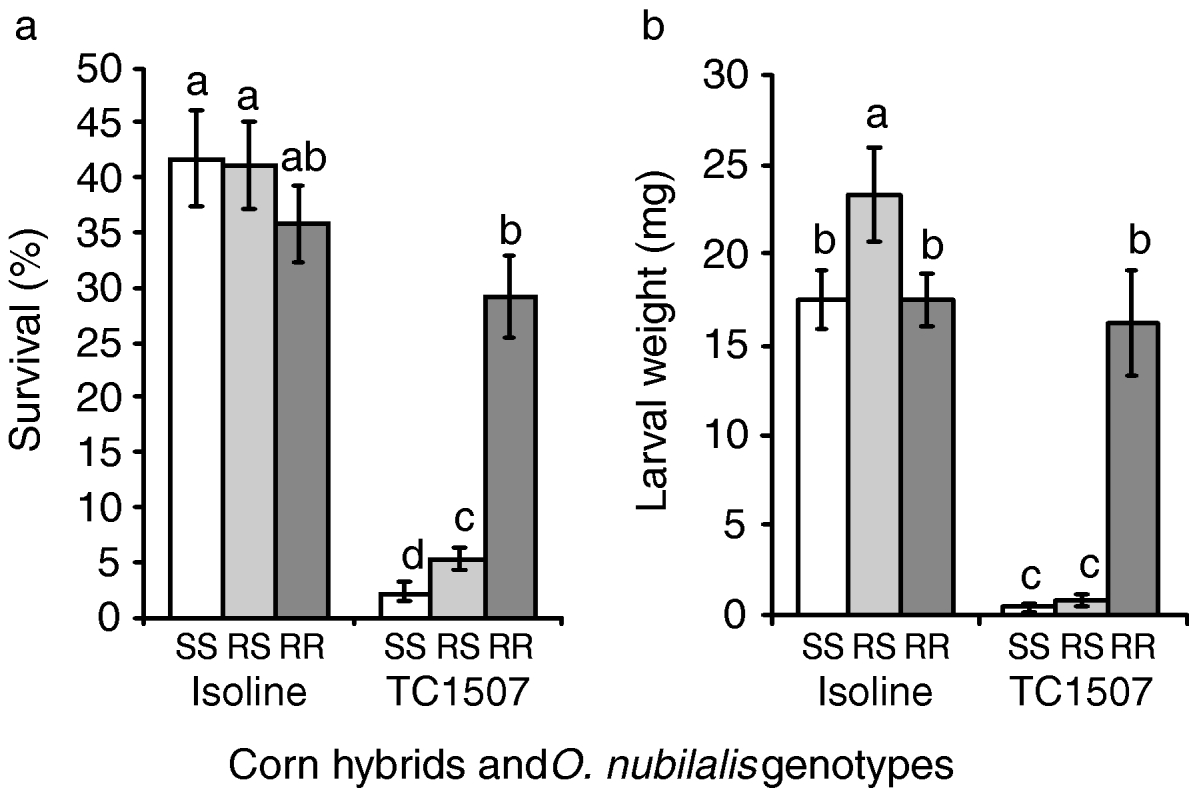
Fig. 4. Greenhouse tests with corn plants in the reproductive stage. (a) Percentage of survival and (b) larval weight of European corn borers homozygous for susceptibility to Cry1F (SS), heterozygous for Cry1F resistance (RS) and homozygous for Cry1F resistance (RR) after 15 days feeding on reproductive stages of Cry1F-expressing corn plants (event TC1507) and their non-Cry1F, isoline counterparts. Error bars represent standard errors. Bars in a chart followed by the same letter are statistically similar (Fisher's protected LSD, P>0.05).
Vegetative stages
Comparisons of O. nubilalis larval survival and weight 15 days after infestation of whorl-stage corn are presented in fig. 3. Despite the almost complete insensitivity to Cry1F displayed by the selected strain in laboratory bioassays, survivorship of the selected strain on Cry1F-expressing corn plants (mean±SEM=4.3±0.9%) was only 19% of that obtained on control plants (22.3±4.0%). Survival rates of susceptible homozygotes (i.e. control larvae) were not statistically different from zero on Cry1F-expressing plants (t=1.0, n=20, P=0.165). On Cry1F corn, significantly more F1 progeny (i.e. heterozygotes) survived relative to control larvae, although their weight was significantly reduced relative to resistant larvae on Cry1F-expressing plants (fig. 3b).
Reproductive stages
Figure 4a, b depicts survival rates and weights of larvae surviving on control and Cry1F corn infested during the reproductive stage. In contrast to results from vegetative-stage plants, larvae of the selected strain released on Cry1F-expressing plants displayed survival rates and larval weights similar to those released on isoline plants. Susceptible and F1 progeny larvae, however, were highly affected by the high dose of toxin delivered by Cry1F corn, as indicated by their relatively low survival rates and larval weights on transgenic plants. On Cry1F corn, larvae heterozygous for resistance survived slightly more than susceptible homozygotes despite the similarity of larval weight of the two genotypes.
Dominance
Assessment of dominance at the toxin concentration delivered by Cry1F corn showed that the resistance was functionally recessive as the estimates were less than 0.5 (table 1). Based solely on larval survival or weight gain as fitness components, resistance was more recessive in the reproductive stage than in the vegetative stage of Cry1F corn (table 1). However, when survival and weight gain were jointly considered, dominance values approached zero for both stages of plant development.
Table 1. Dominance of Cry1F resistance in O. nubilalis on transgenic corn expressing Cry1F.
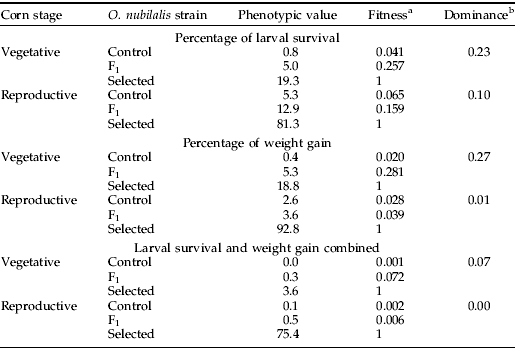
F1 is the hybrid progeny pooled from the two reciprocal mass crosses between the two parental strains, control and Cry1F selected.
For each corn borer genotype, survival on Cry1F corn was estimated by adjusting for mortality on non-Cry1F, control corn using Abbott's correction; percentage of weight gain was calculated relative to the larval weight of each genotype on control corn.
a Fitness is the phenotypic value of a trait (survival, weight gain, or both) for the larvae divided by the phenotypic value of the trait for Cry1F-selected larvae (see Materials and Methods).
b Dominance can vary from zero (completely recessive resistance) to one (completely dominant resistance) (see Materials and Methods).
Discussion
Results of this research confirm that the high level of resistance to Cry1F present in our laboratory-selected strain of O. nubilalis (Pereira et al., Reference Pereira, Lang, Storer and Siegfried2008), considered the highest level of resistance to a Bt toxin ever reported for O. nubilalis (Huang et al., Reference Huang, Higgins and Buschman1997; Bolin et al., Reference Bolin, Hutchison and Andow1999; Chaufaux et al., Reference Chaufaux, Seguin, Swanson, Bourguet and Siegfried2001; Siqueira et al., Reference Siqueira, Moellenbeck, Spencer and Siegfried2004), enabled our Cry1F-selected strain to feed on tissues of reproductive-stage Cry1F corn and to develop to late-instars displaying body mass similar to European corn borers feeding on non-Bt corn (see fig. 4). These results suggest that the laboratory-selected strain may be able to complete development on late-stage Cry1F corn, which makes it particularly relevant for refining resistance management recommendations for O. nubilalis to Cry1F corn.
When feeding on a Cry1F-expressing corn hybrid, O. nubilalis larvae heterozygous for Cry1F resistance exhibited survival rates and larval weights more similar to the susceptible homozygotes than the resistant homozygotes, especially on reproductive stage corn plants (see figs 3 and 4, table1). Assessment of dominance levels at the toxin concentration expressed by Cry1F corn revealed that resistance was indeed functionally recessive as estimates were less than 0.5 (table 1). Based solely on survival or weight gain as fitness components, the resistance expressed by the selected strain was more recessive for the reproductive stage than for the vegetative stage of Cry1F corn plants (figs 3 and 4, table 1). This result must be interpreted with caution since the survival and weight of resistant larvae on vegetative-stage, Cry1F corn were quite low (fig. 3), which may have affected the phenotypic distances among the three genotypes (Bourguet et al., Reference Bourguet, Genissel and Raymond2000). When larval survival and weight gain were jointly considered, dominance values and the relative fitnesses of the heterozygotes were almost zero for both corn stages tested. This result suggests that the resistance is functionally recessive or, more specifically, that heterozygotes have fitness very close to zero at the high dose of Cry1F delivered by transgenic corn plants. This functionally-recessive pattern of resistance supports the high-dose/refuge approach currently in place to manage resistance to Cry1F-expressing corn (Roush, Reference Roush1997; Bates et al., Reference Bates, Zhao, Roush and Shelton2005).
Despite the high level of resistance exhibited by the selected strain, as demonstrated by its virtual insensitivity to Cry1F in laboratory bioassays, larval survival on vegetative stage plants was only 20% of that on non-expressing Bt plants (see fig. 3), suggesting that Cry1F expression in green tissues is high enough to render the resistance functionally recessive (Carrière & Tabashnik, Reference Carrière and Tabashnik2001). In terms of resistance management recommendations for Cry1F corn, this result suggests that even if survivors from the previous season mate with each other, producing resistant progeny, fitness will be reduced by the high dose of toxin delivered by vegetative-stage plants, thereby slowing resistance evolution. This finding, along with the evidence of recessive resistance demonstrated in our survivorship tests, indicate that the high-dose/refuge strategy is likely to function effectively to delay the evolution of O. nubilalis resistance to Cry1F corn, provided that compliance with the refuge requirements exists, and the frequency of the resistance allele is low in the field.
Survival of heterozygotes on transgenic plants has been measured with Plutella xylostella in transgenic broccoli, expressing either Cry1Ac (Metz et al., Reference Metz, Roush, Tang, Shelton and Earle1995; Tang et al., Reference Tang, Collins, Metz, Earle, Zhao, Roush and Shelton2001) or Cry1Ca (Zhao et al., Reference Zhao, Collins, Tang, Cao, Earle, Roush, Herrero, Escriche, Ferre and Shelton2000), and with Pectinophora gossypiella in transgenic cotton, expressing Cry1Ac (Liu et al., Reference Liu, Tabashnik, Dennehy, Patin and Bartlett1999). In all three cases, the effective or functional dominance of resistance was zero, which is consistent with the results of the present study. Huang et al. (Reference Huang, Buschman, Higgins and Li2002) conducted tests with the Dipel-selected strain of O. nubilalis and concluded that resistant larvae were unable to establish reproducing populations on corn hybrids expressing Cry1Ab. Also, there was no evidence that the Cry1Ab-selected strains (Chaufaux et al., Reference Chaufaux, Seguin, Swanson, Bourguet and Siegfried2001; Siqueira et al., Reference Siqueira, Moellenbeck, Spencer and Siegfried2004) had enough resistance to survive on tissues of commercial Bt corn (B. Siegfried, personal communication). Therefore, to our knowledge, this study is the first to show the potential of O. nubilalis to develop levels of resistance high enough to survive on Bt plants and the recessive nature of the resistance on Bt corn.
The inheritance of resistance to Cry1F indicates that it is governed by a single, recessive, autosomal gene. This mode of inheritance is common in instances of ‘Mode 1’ (Van Rie et al., Reference Van Rie, Mcgaughey, Johnson, Barnett and Vanmellaert1990; Tabashnik et al., Reference Tabashnik, Liu, Finson, Masson and Heckel1997, Reference Tabashnik, Liu, Malvar, Heckel, Masson and Ferre1998; Gahan et al., Reference Gahan, Gould and Heckel2001; Morin et al., Reference Morin, Biggs, Sisterson, Shriver, Ellers-Kirk, Higginson, Holley, Gahan, Heckel, Carriere, Dennehy, Brown and Tabashnik2003) resistance to B. thuringiensis toxins (Ferré & Van Rie, Reference Ferré and Van Rie2002), but it contrasts with the intermediate resistance of Cry1Ab-selected O. nubilalis (Alves et al., Reference Alves, Spencer, Tabashnik and Siegfried2006) and the dominant resistance observed in another strain of O. nubilalis resistant to Dipel, a multi-toxin formulation of B. thuringiensis (Huang et al., Reference Huang, Buschman, Higgins and McGaughey1999). The genetic dominance observed in the Dipel-resistant strain, however, was expressed over a relatively low range of toxin concentrations; at high doses all individuals are expected to be susceptible. In our bioassays, heterozygotes for Cry1F resistance responded in a manner similar to the susceptible homozygotes, and this result is supported by the functionally recessive resistance observed in survivorship tests conducted with greenhouse grown plants.
Although no indication of reduced growth and asynchronous development of resistant homozygotes and heterozygotes relative to susceptible homozygotes were observed in control corn (as indicated by their similar if not greater larval weight in figs 3 and 4), it is important to investigate the existence of fitness costs associated with resistance. Comparison of fitness traits, such as development time, fecundity and longevity (Siegfried et al., Reference Siegfried, Zoerb and Spencer2001) in the susceptible and resistant strains, as well as the F1 hybrid progeny, should provide valuable information for resistance management. If Cry1F resistance involves alteration in target sites of the toxin, as has been reported in the majority of the cases of Bt resistance (Ferré & Van Rie, Reference Ferré and Van Rie2002), such alterations can lead to disruptive effects on behaviors and traits related to phenology (Carrière et al., Reference Carrière, Deland, Roff and Vincent1994).
In summary, this research shows that our Cry1F-selected strain developed a high level of resistance and survived on commercially available transgenic corn expressing the Cry1F toxin. However, progeny resulting from crosses of resistant and susceptible individuals had fitness close to zero on these plants. This result provides evidence that the resistance is functionally recessive and indicates that the high-dose/refuge strategy in place to manage O. nubilalis resistance to Cry1F corn is appropriate. The availability of this selected strain will provide an opportunity to determine the biochemical and molecular base of the resistance, which will provide further information to assist in the development of resistance management of O. nubilalis to Cry1F corn.
Acknowledgements
We thank Terence Spencer for assisting with surface selection, insect rearing and bioassays. We also thank Prof. Lance Meinke for the greenhouse space used for the on-plant survivorship tests. Funding for this research was provided by Dow AgroSciences LLC. A fellowship for E. Pereira was provided by the Brazilian Ministry of Education through the CAPES Foundation.