Introduction
Transgenic cotton (event MON88701), marketed as XtendFlexTM cotton, with resistance to glyphosate, glufosinate, and dicamba is now commercially available in the United States (USDA-APHIS 2015). XtendFlexTM cotton has a pending registration in Australia. Glufosinate-resistant (LibertyLink®) cotton became commercially available in Australia in 2006 (OGTR 2006). However, this trait is no longer available and was underused when available for several reasons. These included no registration for summer grasses on the Liberty® 200 label, poor control of larger weeds, and a higher carrier volume requirement than glyphosate. Weed size limitations have also been reported in the United States with growers struggling to make timely applications to Palmer amaranth (Amaranthus palmeri S. Wats.) due to its rapid growth rate (Culpepper et al. Reference Culpepper, Webster, Sosnoskie and York2010; Vann et al. Reference Vann, York, Cahoon, Buck, Askew and Seagroves2017b). Glufosinate also exhibits variability of control in low humidity (Anderson et al. Reference Anderson, Swanton, Hall and Mersey1993; Coetzer et al. Reference Coetzer, Al-Khatib and Loughin2001; Petersen and Hurle Reference Petersen and Hurle2001) and cooler temperatures. Low humidity is common in the Australian growing season. Glufosinate has generally also been underused in broadacre cropping. As a result, there are currently no cases of weeds resistant to glufosinate in cotton and grain systems in Australia (Heap Reference Heap2020; Preston Reference Preston2020).
Dicamba has been used in winter cereals for several years, and apart from some summer fallow use, most weeds that grow in the cotton season have generally had little previous exposure to this chemical. However, past dicamba use in winter cereals means that common sowthistle (Sonchus oleraceus L.) and flaxleaf fleabane (Conyza bonariensis (L.) Cronquist), which are also present in the cotton growing season, have likely had some previous exposure to this herbicide (Wu et al. Reference Wu, Walker and Robinson2008). Currently, a population of S. oleraceus resistant to dicamba has been found in South Australia in a winter cereal cropping system (Preston Reference Preston2020). This population is also resistant to other Group 4 herbicides 2,4-D and clopyralid. The synthetic auxin dicamba is a benzoic acid that causes growth inhibition, senescence, and tissue decay in sensitive dicots. Its mode of action is complex and has been reviewed by Grossmann (Reference Grossmann2010).
In Australian cotton production, glyphosate-resistant cotton has been used since the 2000–2001 season. Its introduction enabled increased flexibility in weed control in-crop with glyphosate applications over the top of the crop in addition to other weed management tactics that were available (Werth et al. Reference Werth, Thornby and Walker2011). However, the overreliance of glyphosate in crop and fallow has resulted in the evolution and proliferation of glyphosate resistance in five key weeds in cotton growing regions. These weeds are feather fingergrass, windmill grass, flaxleaf fleabane, junglerice, and common sowthistle (Preston Reference Preston2020). As a result, growers have reduced their reliance on glyphosate and reintroduced tactics such as residual herbicides and cultivation.
The introduction of XtendFlexTM cotton with glufosinate and dicamba as in-crop options will provide growers with more flexibility in weed management and potentially aid in the control of glyphosate-resistant species in Australia. In the United States, the addition of these two herbicides has provided benefits for the management of glyphosate-resistant palmer amaranth (Cahoon et al. Reference Cahoon, York, Jordan, Everman, Seagroves, Culpepper and Eure2015; Sosnoskie and Culpepper Reference Sosnoskie and Culpepper2014; Vann et al. Reference Vann, York, Cahoon, Buck, Askew and Seagroves2017a, 2017b; York et al. Reference York, Culpepper, Sosnoskie and Bollman2012).
In Australia, the double-knock technique has been widely adopted in cotton and grains systems for control of glyphosate-resistant and difficult-to-control weeds in fallow situations (WeedSmart 2020). This technique can best be described as a sequential application of postemergent herbicides, with differing modes of action, to kill any survivors of the first application in order to prevent potential seed production (Werth et al. Reference Werth, Walker, Boucher and Robinson2010a). The prevention of seed production is critical for resistance prevention and weed management in general (McGillion and Storrie Reference McGillion and Storrie2006). This technique has been used successfully to delay the evolution of glyphosate-resistant rigid ryegrass (Lolium rigidum Gaud.) (Borger and Hashem Reference Borger and Hashem2007). Treatments with paraquat or paraquat+diquat as the follow-up herbicide have proven successful in the control of several glyphosate-resistant populations of flaxleaf fleabane, junglerice, feather fingergrass, and windmill grass (Werth et al. Reference Werth, Walker, Boucher and Robinson2010a, Widderick and McLean Reference Widderick and McLean2018). While glufosinate alone is expected to contribute little to the control of summer grasses, we propose that it may prove effective when used as the follow-up herbicide in a double-knock situation. XtendFlexTM cotton would allow this type of use pattern in crop. In addition, it is important to determine the most effective timeframe for the follow-up application of glufosinate to maximize control. This study was designed to investigate how the introduction of these herbicides is likely to affect the management of these five key weeds in cotton growing regions that have existing glyphosate-resistant populations.
Materials and Methods
Source of Seeds
Seeds used in the experiments are described in Table 1. Both populations of feather fingergrass had some tolerance to glyphosate. When previously sprayed at 330 g ae ha−1 glyphosate, the glyphosate-susceptible (GS) population biomass was reduced by 78% compared with the untreated control, whereas the glyphosate-resistant (GR) population had a 6% reduction (Walker et al. unpublished data). Other windmill grass, populations collected in New South Wales have had an EPSPS gene amplification conferring resistance to glyphosate (Ngo et al. Reference Ngo, Malone, Boutsalis, Gill and Preston2017). However, the mechanism of resistance in this GR population has not been confirmed.
Table 1. Source of glyphosate-resistant and glyphosate-susceptible seed for each species used in both experiments. a

a Abbreviations: GR, glyphosate-resistant; GS, glyphosate-susceptible; NSW DPE, New South Wales Department of Primary Industries; QDAF, Queensland Department of Agriculture and Fisheries
Experimental Procedure
Experiments with each weed species were repeated once (Experiment 1 and Experiment 2). All experiments were conducted from December 2014 through until January 2017.
Experiments were conducted in a shade house covered in shade cloth that provided 10% shade under ambient conditions at the Leslie Research Facility Toowoomba (27.53°S; 151.99°E). Each experiment was conducted using the same methodology. Seeds were sown onto the surface of pots with a diameter of 17 cm containing potting mix (J. C. and A. T. Searle, unpublished observations). The pots were watered regularly to promote germination, and after emergence seedlings were thinned to four plants per pot. Herbicides were initially applied when plants had reached large rosettes with initiation of stem elongation for flaxleaf fleabane (8 to 10 cm diameter) and common sowthistle (10 to 15 cm diameter), and mid-tillering for the grasses (15 to 20 cm diameter). Plants were sprayed in a research track sprayer at 93 L ha−1 water with DG95015EVS nozzles at 2 bar (TeeJet Australasia Pty Ltd, Newtown, VIC, Australia). Plants were harvested 28 d after the last herbicide application by collecting all the green material, which was then dried and weighed (Koger et al. Reference Koger, Poston, Hayes and Montgomery2004). Plant material from the four plants in the pot were combined to produce one dry weight for the pot.
Treatments were combinations of glyphosate with or without dicamba or clethodim with glufosinate applied as a double knock partner at set intervals of 1, 3, 7, and 10 d after the first spray (Tables 2 to 5). Dicamba has little activity on grasses; therefore, clethodim was substituted for dicamba for windmill grass, feather fingergrass, and junglerice experiments. Clethodim is registered for control of junglerice and feather fingergrass in cotton (PestGenie 2020a). The commercial dicamba herbicide (Xtendimax®) that will be used in Xtendflex® cotton was not registered at the time of the experiment. Clarity®, with the same diglycolamine salt, was used in its place. Glyphosate was sprayed at 1,035 g ae ha−1 (Roundup Ready® Herbicide; Monsanto, Melbourne, VIC, Australia); dicamba was sprayed at 528 g ae ha−1 (Clarity®; Monsanto); clethodim was sprayed at 240 g ai ha−1 + 471 g L−1 paraffin oil (Sequence® + BonzaTM at 1 L per 100 L−1 water; Nufarm Australia Ltd., Laverton North, VIC, Australia); and glufosinate was sprayed at 750 g ai ha−1 (Basta®; Bayer Crop Science Australia Pty. Ltd., Hawthorne, VIC, Australia).
Table 2. Percent control of glyphosate-resistant and glyphosate-susceptible populations of flaxleaf fleabane. a,b
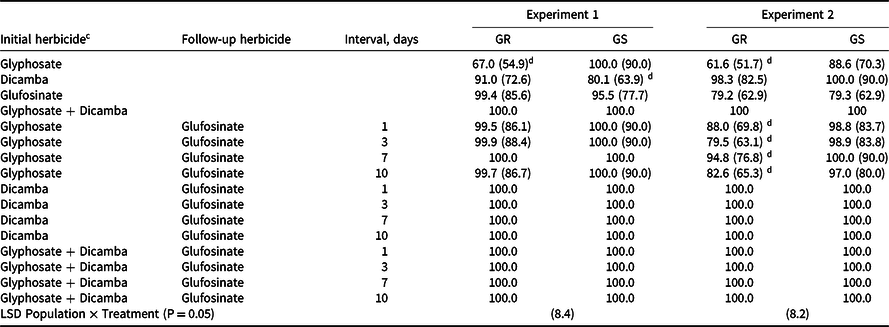
a Abbreviations: GR, glyphosate-resistant; GS, glyphosate-susceptible.
b Numbers in parentheses are angular transformed with the means being back transformed. Means without transformed numbers in parentheses were removed from analysis as they did not fit the normality assumption required for analysis of variance. Analysis compared resistant to susceptible population for each year separately.
c Herbicide rates used are as follows: glyphosate at 1,035 g ae ha−1, dicamba at 528 g ae ha−1, and glufosinate at 750 g ai ha−1.
d Indicates control of the resistant population significantly differed from the susceptible population.
Statistical Analysis
The experiment was designed as a randomized complete block with four replicates. Percent control compared to untreated pots was calculated using the following formula:
For flaxleaf fleabane and windmill grass in both experiments and common sowthistle and feather fingergrass in Experiment 2, treatments that had 100% control (i.e., no green material) in both glyphosate-susceptible and -resistant populations in all replications were removed because they violated the homogeneity and normality required for the analyses. For flaxleaf fleabane the effect of control between each experiment was significant (P < 0.001), and for windmill grass treatments that were removed differed in each experiment. Due to the high levels of control for common sowthistle and feather fingergrass in Experiment 1, and junglerice in both experiments, the data did not fit the assumptions required for ANOVA and were not analyzed. Therefore, each experiment was analyzed independently. Remaining data was angular transformed, and then analyzed by ANOVA using GenStat® software (16th edition; VSN International, Hemel Hampstead, United Kingdom). Angular transformation in GenStat® has the following formula:
All treatments controlled both GR and GS junglerice at least 93%, except glyphosate, which had reduced control on the GR population (47% in Experiment 1 and 60% in Experiment 2).
Results and Discussion
Flaxleaf Fleabane
Control of flaxleaf fleabane ranged from 61% to 100% across both experiments. However, 100% control was observed with dicamba alone or glyphosate plus dicamba was followed by (fb) glufosinate in a double knock (Table 2). In Experiment 1, dicamba alone had significantly less control on the glyphosate-susceptible (80%) compared to the glyphosate-resistant population (91%); reasons for this are unknown. In contrast, other studies have shown some slight reductions in control of glyphosate-resistant compared to susceptible flaxleaf fleabane populations with dicamba (Flessner et al. Reference Flessner, McElroy, McCurdy, Toombs, Wehtje, Burmester, Price and Ducar2015; Kruger et al. Reference Kruger, Davis, Weller and Johnson2010). Control with dicamba alone was greater in both populations in Experiment 2. Treatments in Experiment 1 were applied in May 2015, and November 2016 for Experiment 2. Warmer conditions at the time of application may have contributed to improved control in Experiment 2. Each herbicide when used individually gave inconsistent control; however, when used in combinations, control was improved.
The glyphosate fb glufosinate double-knock treatment gave inconsistent control, with less control on the glyphosate-resistant population in the second experiment particularly at 3-d and 10-d intervals between double knock timings (80% and 83% control, respectively). Reasons for this result are unknown; however, it indicates the importance of not relying on glyphosate alone for flaxleaf fleabane control, even when following up with glufosinate. The most effective timing of the follow-up glufosinate was 7 d after glyphosate application with at least 95% flaxleaf fleabane control (Table 2). Control ranged from 80% to 100% following all other glyphosate fb glufosinate timings, a similar result to that reported by Werth et al. (Reference Werth, Walker, Boucher and Robinson2010a) who observed more consistent control using paraquat as the follow-up herbicide. Following dicamba alone or glyphosate plus dicamba, the timing of the follow-up glufosinate application did not affect control in either experiment.
Common sowthistle
Treatments applied in Experiment 1 resulted in almost total (>94%) control of common sowthistle (Table 3). The high level of control (97%) of the resistant population may be attributed to the rate of glyphosate used. The label rate for control of common sowthistle in fallow is 1.3 L ha−1 of Roundup Ultramax® (570 g ae L−1), which is 742 g ae ha−1. The rate of glyphosate used in this experiment was 1.5 kg ha−1 of Roundup Ready Herbicide® (690 g ae ha−1) or 1,035 g ae ha−1. Control with glyphosate and dicamba was lower in Experiment 2. This reduction may have been due to cooler temperatures during growth. Experiment 2 was planted approximately 1 mo later in April 2016, compared to March 2015 for Experiment 1. In terms of thermal time, Experiment 1 was sprayed 41 d (871 growing degree days; GDD) after emergence, and Experiment 2 was sprayed 49 d (906 GDD) after emergence. This may indicate that plants in Experiment 2 were slower growing, and had reduced uptake, and translocation of the herbicides, contributing to poorer control (Ganie et al. Reference Ganie, Jugulam and Jhala2017). The poor result of dicamba in this experiment may also be attributed to weed size. Cadence® (a sodium salt form of dicamba) is registered for control of common sowthistle in sorghum in Australia up to the rosette stage from 161 to 280 g ae ha−1 (PestGenie 2020b). Plants sprayed in this experiment were at large rosettes with some initiating stem elongation. It is unknown why control with dicamba was further reduced in the glyphosate-resistant population in Experiment 2.
Table 3. Percent control of glyphosate-resistant and glyphosate-susceptible populations of common sowthistle. a,b

a Abbreviations: GR, glyphosate-resistant; GS, glyphosate-susceptible.
b Numbers in parentheses are angular transformed with the means being back transformed. Means without transformed numbers in parentheses were removed from analysis as they did not fit the normality assumption required for analysis of variance. Analysis compared resistant to susceptible population for each year separately.
c Herbicide rates used are as follows: glyphosate at 1,035 g ae ha−1, dicamba at 528 g ae ha−1, and glufosinate at 750 g ai ha−1.
d Indicates control of the resistant population significantly differed from the susceptible population.
Application of glufosinate alone provided greater than 99% control of glyphosate-resistant and -susceptible populations in both experiments. In Experiment 2, the double knock treatments with glufosinate also provided effective (92% to 100%) control regardless of resistance to glyphosate. The timing of the second glufosinate knock was not significant in either experiment, with the exception of glyphosate fb glufosinate 1 d later in Experiment 2 (although above 90% control was still observed).
No differences in efficacy between glyphosate-resistant and susceptible populations were measured in Experiment 1. However, the reduced control from glyphosate alone on the glyphosate-resistant population was significant in Experiment 2. Also, in Experiment 2, dicamba alone was significantly less effective on the glyphosate-resistant population (76% control of susceptible vs 53% control of resistant). Reasons for this are unknown; however, it is not thought this is linked to glyphosate resistance.
Feather fingergrass
Consistent control of feather fingergrass was achieved only when the herbicides were used in double-knock treatments (Table 4). In both experiments, 99% to 100% control was achieved with 7-d and 10-d intervals between initial herbicides and glufosinate. This result was independent of the initial herbicides used. Control with the follow-up glufosinate was significantly lower at 1- and 3-d intervals in Experiment 2. The optimal timing of the follow-up glufosinate in these experiments was comparable with the optimal timing of paraquat identified by Widderick and McLean (Reference Widderick and McLean2018), when 7 to 14 d was optimal for glyphosate fb paraquat and 1 to 4 d optimal for haloxyfop fb paraquat.
Table 4. Percent control of glyphosate-tolerant and less tolerant populations of feather fingergrass. a,b

a Abbreviations: GR, glyphosate-resistant; GS, glyphosate-susceptible.
b Numbers in parentheses are angular transformed with the means being back transformed. Means without transformed numbers in parentheses were removed from analysis as they did not fit the normality assumption required for analysis of variance. Analysis compared resistant to susceptible population for each year separately.
c Herbicide rates used are as follows: glyphosate at 1,035 g ae ha−1, dicamba at 528 g ae ha−1, and glufosinate at 750 g ai ha−1.
d Indicates control of the resistant population significantly differed from the susceptible population.
Clethodim alone provided greater than 90% control of both glyphosate-resistant and susceptible populations, which was slightly better than when clethodim and glyphosate were combined in a tank mix. The effect of clethodim alone being more effective than glyphosate plus clethodim was less noticeable when the double knock intervals were 1 and 3 d for the follow-up glufosinate.
Control with glufosinate alone was lower in Experiment 2 with a significant difference between the glyphosate-susceptible (57%) compared to the glyphosate-resistant population (78%). There was also significantly reduced control of the glyphosate-resistant compared to the susceptible populations at the 1- and 3-d timings between glyphosate and glufosinate double knocks. Reasons for the overall reduced control of glufosinate on feather fingergrass are unknown because plants were grown and sprayed under similar conditions in both experiments. This result highlights the overall variability of control with glufosinate on this species.
Windmill grass
Control of windmill grass was generally less than other species, even in a glasshouse environment, as can be seen by the results in Table 5. No treatment provided total control of glyphosate-resistant windmill grass in both years, although the clethodim fb glufosinate 10 d later and the glyphosate plus clethodim fb glufosinate 7 and 10 d later gave at least 99% control. The treatments with a glufosinate application 10 d after the initial herbicides appeared to be generally more effective across both glyphosate-resistant and susceptible populations.
Table 5. Percent control of glyphosate-resistant and glyphosate-susceptible populations of windmill grass. a,b

a Abbreviations: GR, glyphosate-resistant; GS, glyphosate-susceptible.
b Numbers in parentheses are angular transformed with the means being back transformed. Means without transformed numbers in parentheses were removed from analysis as they did not fit the normality assumption required for analysis of variance. Analysis compared resistant to susceptible population for each year separately.
c Herbicide rates used are as follows: glyphosate at 1,035 g ae ha−1, dicamba at 528 g ae ha−1, and glufosinate at 750 g ai ha−1.
d Indicates control of the resistant population significantly differed from the susceptible population.
It is interesting to note that in the susceptible population in Experiment 2 the control achieved by glyphosate alone was higher than glyphosate fb glufosinate 1, 3, and 7 d later, when control was not only lower but variable. This may indicate that in some circumstances the action of glufosinate could interfere with that of glyphosate if applied too soon. Previous studies have reported antagonism between glufosinate and glyphosate when applied together in goosegrass [Eleusine indica (L.) Gaertn; Chuah et al. Reference Chuah, Teh, Cha and Ismail2008), wild mustard (Sinapis arvensis L.), white mustard (Sinapis alba L.; Kudsk and Mathiassen Reference Kudsk and Mathiassen2004), giant foxtail (Setaria faberi Herrm.) and velvetleaf (Abutilon theophrasti Medik). (Besançon et al. Reference Besançon, Penner and Everman2018). Reduced translocation of glyphosate by glufosinate in giant foxtail and to a lesser extent velvetleaf was reported by Besançon et al. (Reference Besançon, Penner and Everman2018). Although in these experiments glufosinate was applied separately to glyphosate, early application of glufosinate (particularly 1 d later) may have restricted translocation of glyphosate. This effect was not observed for the other species tested.
The double-knock strategy with paraquat as the follow-up herbicide has been a successful strategy for the control of a number of problem weed species in fallows. These include rigid ryegrass. (Borger and Hashem Reference Borger and Hashem2007; Neve et al. Reference Neve, Diggle, Smith and Powles2003; Thornby et al. Reference Thornby, Werth and Walker2013), flaxleaf fleabane (Werth et al. Reference Werth, Walker, Boucher and Robinson2010a; Wu et al. Reference Wu, Walker and Robinson2008), junglerice, feather fingergrass, and windmill grass (Widderick and McLean Reference Widderick and McLean2018). Although paraquat and glufosinate have different modes of action, they share some similarities in terms of being largely contact herbicides with limited translocation (Coetzer et al. Reference Coetzer, Al-Khatib and Loughin2001; Hawkes Reference Hawkes2014; Pline et al. Reference Pline, Wu and Hatzios1999; Slade and Bell Reference Slade and Bell1966). Both herbicides have similar parameters for application, needing good coverage with higher carrier volumes than required for systemic herbicides such as glyphosate. Using glufosinate as the follow-up herbicide has proven successful in these experiments. Timings for the follow-up glufosinate are also similar to paraquat, even though paraquat acts faster than glufosinate.
The addition of dicamba to existing weed programs to control palmer amaranth was effective when applied in succession (Cahoon et al. Reference Cahoon, York, Jordan, Everman, Seagroves, Culpepper and Eure2015). Vann et al. (Reference Vann, York, Cahoon, Buck, Askew and Seagroves2017a) also found that mixing glufosinate and dicamba gave effective control of palmer amaranth when applied at the right growth stage. When the initial application was followed by another application of glufosinate and dicamba, control improved even when the initial application was delayed. Although this is useful in salvage situations, this approach was not recommended for resistance management (Vann et al. Reference Vann, York, Cahoon, Buck, Askew and Seagroves2017a).
The combination of glyphosate, glufosinate, and dicamba has generally resulted in effective control of both glyphosate-resistant and -susceptible populations of the weeds tested in this experiment. The most consistent results have come from using glufosinate as a double-knock partner, particularly when the timing of the follow-up application is 7 and 10 d. There were also no consistent results to determine that control of either dicamba, glufosinate, or clethodim alone were reduced on glyphosate-resistant compared to -susceptible populations. This shows that resistance to glyphosate in these species is unlikely to negatively affect the performance of the other herbicides.
The results of this study indicate that the ability to use dicamba and glufosinate in XtendFlexTM cotton should be beneficial to weed management in-crop. However, these herbicides should be used in addition to existing weed control tactics such as preemergence herbicides and nonchemical tactics to ensure the weed seed bank is kept low and to minimize the likelihood of resistance evolution to these herbicides (Thornby et al. Reference Thornby, Werth, Hereward, Keenan and Chauhan2018; Werth et al. Reference Werth, Preston, Taylor, Charles, Roberts and Baker2008). Future research should test the effectiveness of using glufosinate as a double-knock partner on these and other species in the field.
Acknowledgments
This research was funded by the Cotton Research and Development Corporation. No conflicts of interest have been declared.








