Double-cropping soft red winter wheat with summer annual crops is a common practice in the southeastern U.S. states of Georgia, North Carolina, and South Carolina (Chandi et al. Reference Chandi, York, Jordan and Beam2011; Grey Reference Grey2007; Grey et al. Reference Grey, Braxton and Richberg2012). In soft red winter wheat production, acetolactate synthase (ALS)-inhibiting herbicides including chlorosulfuron plus metsulfuron, mesosulfuron, and pyroxsulam applications used in weed control throughout the region. The most common and troublesome weeds are henbit (Lamium amplexicaule L.), wild radish (Raphanus raphanistrum L.), and Italian ryegrass [Lolium perenne L. ssp. multiflorum (Lam.) Husnot] (Webster Reference Webster2012). Biotypes of ALS-resistant henbit and Italian ryegrass have been reported in multiple locations: Italian ryegrass in Arkansas and Mississippi since 1995 and henbit in Kansas in 2014 (Heap Reference Heap2017). While not reported in the United States, ALS-resistant wild radish has been documented in Australia, South Africa, and Brazil (Heap Reference Heap2017). Herbicide resistance presents a worldwide challenge for weed control, because such biotypes possess the genetic material to survive and reproduce when exposed to previously lethal doses of ALS-inhibiting herbicides (Vencill et al. Reference Vencill, Nichols, Webster, Soteres, Mallory-Smith, Burgos, Johnson and McCelland2012). Repeated applications of the same site of action (SOA) (i.e., continued and repeated uses of ALS herbicides), further complicate herbicide-resistance issues (Lindell et al. 2016; Senseman Reference Senseman2016; Vencill et al. Reference Vencill, Nichols, Webster, Soteres, Mallory-Smith, Burgos, Johnson and McCelland2012).
One practice often prescribed to mitigate selection pressure for herbicide resistance is rotating multiple SOAs to target different biological pathways in the same weed species (Lindell et al. Reference Lindell, Rosinger, Schmitt, Strek, van Almsick and Williams2015; Vencill et al. Reference Vencill, Nichols, Webster, Soteres, Mallory-Smith, Burgos, Johnson and McCelland2012). In soft red winter wheat production in Georgia, where ALS inhibitors are currently recommended for weed control (Horton Reference Horton2016), alternative SOAs are needed to expand weed control options for growers. Pyrasulfotole is a selective, POST herbicide labeled for broadleaf weed control in small grains, including winter wheat (Anonymous 2016), that also has soil residual activity (Kaune et al. Reference Kaune, Sliege, Lenz and Riggerger2008). Pyrasulfotole is a benzoylpyrazole within a class of WSSA Group 27 herbicides that inhibit the biochemical pathway of 4-hydroxyphenylpyruvate dioxygenase enzyme (Lindell et al. Reference Lindell, Rosinger, Schmitt, Strek, van Almsick and Williams2015; Moran Reference Moran2014; Schmitte et al. Reference Schmitte, van Almsick and Williams2008). After POST application, sensitive foliage exhibits yellowing and chlorosis or bleaching, before necrosis (Fromme et al. Reference Fromme, Dotray, Grichar and Fernandez2012; Reddy et al. Reference Reddy, Stahlman, Geier, Thompson, Currie, Schlegel, Olson and Lally2013; Schulte and Köcher Reference Schulte and Köcher2008). Pyrasulfotole is marketed as a combination with the crop safener mefenpyr-diethyl to enhance crop tolerance and with bromoxynil to improve weed control efficacy by targeting other sites of action (Reddy et al. Reference Reddy, Stahlman, Geier and Peterson2012; Schmitte et al. Reference Schmitte, van Almsick and Williams2008). The labeled field rate for pyrasulfotole in small grains is 41 g ai ha−1 for a single application, with up to 81 g ai ha−1 during the season (Anonymous 2016). Pyrasulfotole has a pK a of 4.2 and is stable to hydrolysis and photolysis processes in soil (U.S. Environmental Protection Agency [USEPA] 2007), with water solubility of 69.1 g L−1 at pH 7 and 20 C (Braga and Whall Reference Braga and Whall2006). Its soil adsorption coefficient with respect to the organic matter content (KOC) increases with decreasing soil pH (Kaune et al. Reference Kaune, Sliege, Lenz and Riggerger2008). This indicates that pyrasulfotole is adsorbed by soil organic matter and also clay minerals (Kaune et al. Reference Kaune, Sliege, Lenz and Riggerger2008). Under aerobic conditions, pyrasulfotole is moderately susceptible to microbial degradation, with reported half-lives of 6 to 18 d (US EPA 2007), 5 to 31 d in field trials (Kaune et al. Reference Kaune, Sliege, Lenz and Riggerger2008), and 9 d in cropped test plots (Braga and Whall Reference Braga and Whall2006). Overall soil research data indicate pyrasulfotole will dissipate over time, allowing for limited carryover potential.
Past research has reported successful broadleaf weed control in soft red winter wheat production with pyrasulfotole (Fromme et al. Reference Fromme, Dotray, Grichar and Fernandez2012; Reddy et al. Reference Reddy, Stahlman, Geier and Peterson2012, Reference Reddy, Stahlman, Geier, Thompson, Currie, Schlegel, Olson and Lally2013). There are 4- to 9-mo rotational restrictions on some legume and broadleaf crops and irrigation and rainfall total requirements before planting (Anonymous 2016). But there is limited information about the impact of soil residual pyrasulfotole effects on peanut and tobacco planted in rotation. The label registration states that nonspecified crops must have field bioassays completed. Thus, research was conducted to evaluate the response of peanut and tobacco grown in rotation when pyrasulfotole was applied to wheat in Georgia.
Materials and Methods
Field trials were conducted between November 2014 and October 2015 at five locations in Georgia. Carryover experiments for peanut were located at the Ponder Research Farm near Ty Ty, GA (31.509°N, 83.648°W), the Southwest Georgia Research and Education Center (SWGREC) in Plains, GA (32.037°N, 84.372°W), and the USDA–ARS National Peanut Laboratory in Dawson, GA (31.734°N, 84.373°W). Carryover experiments for tobacco were located at the Bowen Farm in Tifton, GA (31.478°N, 83.408°W) and the USDA–ARS National Peanut Laboratory in Dawson, GA. Specific soils, taxonomy, pH, soil data, herbicide application, and peanut and tobacco planting dates are shown in Table 1. Before wheat was planted in 2014, all soils were disk harrowed and moldboard plowed 25- to 30-cm deep, then rotary tilled (Kelly Manufacturing, Tifton GA). Single plots were 3.6-m wide and 9.1-
Table 1 Location, soil types, physical characteristics of each soil, application timing, and planting dates to test the effect of pyrasulfotole carryover on peanut and tobacco.

a Soil pH was determined by the 1 kg L−1 soil–water method. A mechanical analysis is used to identify the textural classification of soil based on sand, silt, and clay using the hydrometer method.
b The organic matter content (C) is determined by the loss on ignition method and expressed as percent by weight.
c The CEC was determined by adding the milliequivalents of bases present in the Mehlich-1 extract and the milliequivalents of exchangeable hydrogen as determined by direct titration with 0.023M Ca(OH)2.
d Southwest Georgia Research & Education Center.
m long at all locations. The winter wheat cultivar ‘AGS 2027’ was conventionally drilled at 100 kg ha−1 in all plots on November 3, 2014, at Ty Ty Ponder Farm, December 4, 2014, at Tifton Bowen Farm, December 5, 2014, at Plains SWGREC, and December 1, 2014, at Dawson USDA–ARS. Total rainfall and irrigation for the period after herbicide application were recorded through crop harvest in 2015 (Table 2) (Flitcroft Reference Flitcroft2015).
Table 2 Averaged monthly 10-cm soil temperature, rainfall, and cumulative irrigation (IRR) for pyrasulfotole carryover to double-crop peanut and tobacco in Georgia in 2015.Footnote a

a Locations: Dawson/USDA–ARS, Dawson, GA; Plains/SWGREC, Southwest Georgia Research and Education Center, Plains, GA; Tifton/Bowen, Bowen Farm, Tifton, GA; Ty Ty/Ponder, Ponder Farm, Ty Ty, GA. Data from Flitcroft I (2015).
Four replications of five treatments were arranged in a randomized complete block design in each location of the peanut and tobacco trials. Complete treatment applications and descriptions are listed in Tables 1 and 3. Treatments included POST applications of pyrasulfotole formulated with bromoxynil and mefenpyr-diethyl in December 2014 to wheat at Feekes stage 1 or January 2015 at Feekes stage 2, at 300 g ai ha−1 and 600 g ai ha−1, respectively. Pyrasulfotole was applied with a nonionic surfactant at 0.25% (v/v) and an urea ammonium nitrate (UAN) (32%) at 2.3 L ha−1 in all treatments. Herbicides were applied with a CO2-pressurized sprayer calibrated to deliver 188 L ha−1 at 172 kPa to one half of each plot (1.8-m wide by 9.1-m long). This allowed for a nontreated buffer adjacent to each treated plot for the entire experiment. Glyphosate was applied to all experimental plots at 1,540 g ai ha−1 approximately 1 mo following pyrasulfotole autumn or winter treatment to allow for the maximum likelihood for carryover to occur. In early April, all plots were desiccated with glyphosate again to prepare seedbeds for peanut and tobacco planting. Glyphosate applications provided control of all grasses, including the wheat, and small-seeded broadleaf weeds that could have influenced pyrasulfotole dissipation, as previous research has shown that plants can extract herbicide residues from soil (Harper Reference Harper1994). All treated and nontreated adjacent beds were planted to their respective crop so as to have side-by-side comparisons for each plot.
Table 3 Effect of pyrasulfotole treatment applied to wheat on double-crop peanut stand, diameter, and yield in Georgia from 2014 to 2015.

a Herbicides were applied to wheat in Feekes stage 1 or 2 at Plains on December 19, 2014, and January 22, 2015; Ty Ty on December 9, 2014, and January 16, 2015; and Dawson December 15, 2014, and January 28, 2015, respectively. Peanut was planted May 6, 2015, April 22, 2015, and May 5, 2015, respectively. Replication, location, and the location by treatment interaction were considered as random factors for analyses; therefore, data were combined for presentation.
b Means within a column followed by the same letter are not significantly different from each other according to Fisher’s protected LSD test at P≤0.05.
c Pyrasulfotole formulated with bromoxynil and mefenpyr-diethyl.
On the day of peanut planting, land preparation was performed using a Brown strip-till implement (Brown Manufacturing, Ozark, AL). Rows were ripped with a single subsoiler shank, in tandem with fluted coulters to break up large clods, along with rolling crumblers (Brown Manufacturing) that served to smooth the seedbed. Strip-tillage rows were ripped 8-cm deep and 20-cm wide with 0.9 m between peanut row centers. Approximately 50% of the surface remained after the strip-tillage operation was performed. The peanut cultivar ‘GA-06G’ was then planted at 112 kg ha−1 in single rows spaced 0.9-m apart on April 22, 2015, at Ty Ty Ponder Farm, May 5, 2015, at USDA–ARS at Dawson, and May 6, 2015, at SWGREC at Plains. Standard culture practices for peanut production were incorporated for the entire area and followed University of Georgia recommendations for fertilizer, weed, plant pathogen, and insect control (Prostko Reference Prostko2015). Peanut was maintained weed-free via hand weeding when necessary to maintain uniformity among all treatments. Irrigation was applied to supplement rainfall at all locations when required to maintain peanut growth (Table 2).
For the areas transplanted to tobacco, treated beds were row marked, then soil was chisel plowed with a three-shank ripper to 40-cm depth. The bed was then rotary tilled to a 20-cm depth to allow for tobacco transplanting. The tobacco cultivar ‘NC196’ was then mechanically transplanted April 7, 2015, at USDA–ARS Dawson, and April 9, 2015, at the Tifton Bowen Farm. Tobacco transplant density was 2 plants m−1 row, with 2 rows per treated plot. Standard culture practices for transplanted tobacco production were followed using University of Georgia recommendations for fertilizer, weed, plant pathogen, and insect control (Moore et al. Reference Moore, Bertrand, Jones, Givan, Sumner, Harris, Kightlinger and Rains2013). Tobacco was maintained weed-free via hand weeding as needed, plus a single cultivation to 10-cm deep at 2 and wk after transplanting, to maintain uniformity among all treatments. Irrigation was applied to supplement rainfall at all locations when required to maintain tobacco growth (Table 2).
Visual estimates of peanut and tobacco injury were determined based on a combination of plant chlorosis, necrosis, and plant stunting with a scale of 0 (no injury relative to nontreated control [NTC]) to 100% (plant death). Crop injury was evaluated multiple times throughout the growing season at 10- to 14-d intervals after planting.
In the peanut trials, crop response was determined by comparing emerged plant stand counts up to 30 d after emergence, plant width taken multiple times during the growing season, and final peanut pod yield. In October 2015 for all locations, peanuts were dug and inverted based on mesocarp pod color (Williams and Drexler Reference Williams and Drexler1981). Field plots were then harvested 10 to 20 d later with conventional equipment, maintaining plot integrity for each sample by storing harvested material in cabbage bags. Peanuts in pod were then dried to 7% moisture and mechanically cleaned and shelled, and the final yield was determined. Crop response in the tobacco trials was measured by taking stand counts up to 30 d after planting, height on 5 random plants in each plot at 51, 64, and 85 d after transplanting (DAP), and then 5 random plants harvested for leaf number, width, plant height, and fresh weight of leaves and stalks cut to the soil surface at 65 and 85 DAP.
Data were subjected to ANOVA using PROC GLMMIX (SAS Institute, Cary, NC) to determine interactions between main factors (P≤0.05). Treatment timing and herbicide rate were considered fixed effects, whereas random effects included location, repetitions, and the associated interactions. Visual estimates of injury, crop density, canopy diameter, and yields were analyzed.
Results and Discussion
There were differences for environmental measures taken during the course of each experiment. However, all experiments were conducted at times and locations when herbicide applications could normally occur in Georgia wheat production and are thus representative of producer practices. Cumulative rainfall and irrigation ranged from 105 to 145 cm between the time of herbicide application and peanut or tobacco harvest (Table 2), which are representative for the region. As bromoxynil has a half-life of 7 d with water solubility of 130 mg L−1 (Shaner Reference Shaner2014), it was considered to be dissipated by time of planting, as between 37 to 56 cm of rain had occurred between December 2014 and March 2015 for all locations (Table 2), and therefore, would not affect peanut or tobacco planted to rotation in April or May. As pyrasulfotole is formulated with other chemical compounds, its persistence may be associated with other compounds in the mixture.
Crop Response to Pyrasulfotole
There were no injury symptoms during the season in the form of visual evaluation for chlorosis, necrosis, or stunting for peanut or tobacco (unpublished data). There were no differences for final stand establishment for peanut or tobacco for any herbicide carryover treatment compared with the nontreated control (Tables 3 and 4). Initial peanut seedling emergence did not vary by experiment for peanut, and there was never any effect of pyrasulfotole treatment with respect to rate or timing compared with the nontreated control, indicating that pyrasulfotole did not limit peanut seed germination and emergence with final stands of 16 to 17 plant m−1 row (Table 3). Transplanted tobacco stand remained constant at 2 plant m−1 row for all treatments (Table 4). Given the 4- to 9-mo rotational restrictions for some broadleaf and legume crops listed on the pyrasulfotole label, it is assumed there is residual activity that can negatively impact specific crops (Anonymous 2016). Robinson et al. (Reference Robinson, Letarte, Cowbrough, Sikkema and Tardif2014) reported injury to red clover (Trifolium pratense L.) from pyrasulfotole applied to wheat, but this was applied after red clover was seeded. In reviewing the literature, the authors could find no information about residual effects of pyrasulfotole on other rotational crops.
Table 4 Effect of pyrasulfotole treatment applied to wheat on double-crop tobacco stand, leaf diameter, and plant height over time in Georgia from 2014 to 2015.
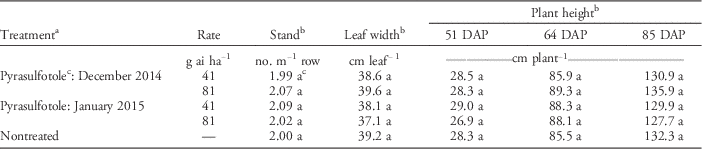
a Herbicides were applied to wheat in Feekes stage 1 (December) or 2 (January) at Tifton December 18, 2014, and January 5, 2015; and at Dawson December 15, 2014, and January 28, 2015, respectively. Tobacco was transplanted in Tifton and Dawson April 7 and April 9, 2015, respectively. Replication, location, and the location by treatment interaction were considered as random factors for analyses; therefore, data were combined for presentation.
b Means within a column followed by the same letter are not significantly different from each other according to Fisher’s protected LSD test at P≤0.05.
c Pyrasulfotole formulated with bromoxynil and mefenpyr-diethyl.
Pyrasulfotole at 41 or 81 g ha−1 applied in either December or January did not affect peanut when planted in late April or early May (Table 1) compared with the nontreated control. At 20 to 27 DAP, peanut width was 16 to 17 cm with no visible signs of stunting (Table 3). This measurement timing represents approximately 120 (December) to 150 (January) days after pyrasulfotole applications. Multiple measures taken at 34 to 42 DAP for peanut width indicated no differences for any treatment when compared with the nontreated control (unpublished data). Tobacco transplanted in April at 105 (December) to 90 (January) d after pyrasulfotole applications exhibited no differences in leaf width or plant height at 51, 64, or 85 DAP (Table 4). At the time of tobacco transplanting in April, the Tifton Bowen Farm had received more than 47 cm of rainfall, while the Dawson USDA–ARS facility received 37 cm. For the benzoylpyrazole herbicide topramezone, peas (Pisum sativum L.) were injured when planted within 2 wk of application (Rahman et al. Reference Rahman, Dowsett, Trolove and James2014). Topramezone has a half-life of approximately 14 d (Gorsic et al Reference Gorsic, Baric, Galzina, Scepanovic and Ostojic2008; Shaner Reference Shaner2014).
Peanut pod yield was not affected by any pyrasulfotole rate or timing when harvested at least 246 (December) to 284 (January) d after application. Yield was 5,220 to 5,490 kg ha−1 compared with the nontreated control’s yield of 5,340 kg ha−1 (Table 3). Tobacco harvested at 65 DAP exhibited no difference in leaf number per plant or fresh weight for leaf and stalk biomass per plant for pyrasulfotole compared with the nontreated control, with similar results at 85 DAP (Table 5).
Table 5 Effect of pyrasulfotole treatment applied to wheat on double-crop tobacco leaf number, and leaf and stalk biomass in Georgia from 2014 to 2015.
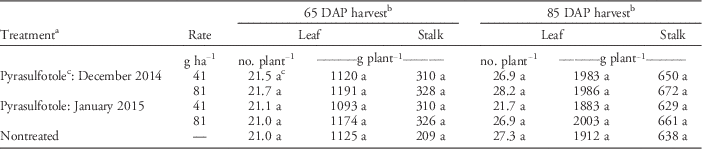
a Herbicides were applied to wheat in Feekes stage 1 (December) or 2 (January) at Tifton December 18, 2014, and January 5, 2015; and at Dawson December 15, 2014, and January 28, 2015, respectively. Tobacco was transplanted in Tifton and Dawson April 7 and 9, 2015, respectively. Replication, location, and the location by treatment interaction were considered as random factors for analyses; therefore, data were combined for presentation.
b Means within a column followed by the same letter are not significantly different from each other according to Fisher’s protected LSD test at P≤0.05.
c Pyrasulfotole formulated with bromoxynil and mefenpyr-diethyl.
These results indicate that autumn (December) or winter (January) applications of pyrasulfotole in wheat had no adverse effects on rotational planting of peanut or tobacco in Georgia. Adequate moisture in terms of rainfall plus irrigation (Table 2) provided a dissipation mechanism for pyrasulfotole to occur. Kaune et al. (Reference Kaune, Sliege, Lenz and Riggerger2008) reported half-life of 11 d for a Pikeville loamy sand with 1.2% organic matter content. They noted that once pyrasulfotole is adsorbed to soil, it does not readily desorb back into the aqueous phase. For that research, when soil pH was less than 5.7, pyrasulfotole desorption was negligible. In the current research, pH for three of the four locations was less than 5.7, except for the Dawson USDA–ARS soil pH, which was 6.4. Based on Kaune et al (Reference Kaune, Sliege, Lenz and Riggerger2008), having adequate moisture in soil would provide a mechanism for pyrasulfotole to interact with soil clay mineral and organic matter colloids. This should facilitate pyrasulfotole soil adsorption and limit any potential carryover to rotational peanut and tobacco. Additional research on pyrasulfotole soil adsorption in southeastern U.S. soils is an area for potential future research.







