Introduction
The Cape Verde Islands are situated off the west coast of Africa, c. 500 km W of the Cap Verde in Senegal, 14°48′ to 17°12′ N; 25°42′ to 22°41′W, in the North African arid and semiarid climatic regions. A checklist of lichens and allied fungi of these islands, published by Mies (Reference Mies1993), included four taxa of Rinodina: R. atrocinerea (Hook.) Körb., R. corticola (Arnold) Arnold, R. intermedia Bagl. and R. cf. roboris (Dufour ex Nyl.) Arnold. The material reported by Mies (Reference Mies1989, Reference Mies1993) as R. atrocinerea and R. intermedia, could not be located; that reported as R. corticola (coll. Mies CV-3745, GZU) and R. roboris (coll. Mies CV-936, GZU; CV-1265, B) does not correspond to these species (see also under R. capeverdeana). An additional specimen identified as Rinodina sp. (coll. Mies CV-4690, GZU) has proved to be Rinodina beccariana Bagl. var. lavicola (M. Steiner) Matzer & H. Mayrhofer, which is reported here from Cape Verde for the first time.
In an attempt to find additional Rinodina material on the Cape Verde Islands, the second author visited three islands, Santiago, São Vicente and Santo Antão, and collected c. 25 specimens. These specimens represented nine species of which three, R. capeverdeana, R. inspersoparietata and R. polymorphaespora, are described below as new to science. A further three species, R. algarvensis, R. colobinoides and R. guianensis, represent new records for Africa.
Materials and Methods
The study is based on specimens in the private herbarium of P.P.G. van den Boom, mostly collected in July 2006. They were examined using standard techniques with stereoscopic and compound microscopes. Current mycological terminology generally follows Kirk et al. (Reference Kirk, Cannon, David and Stalpers2001). Ascospores, mounted in water, were measured at ×1000 magnification; only free ascospores lying outside the asci were measured. The terminology used for the apothecia follows Dughi (Reference Dughi1952), for asci (Rambold et al. Reference Rambold, Mayrhofer and Matzer1994) and for ascospore types and ascospore ontogenies (Giralt Reference Giralt2001).
Chemical constituents were identified by the standard methods of thin-layer chromatography (TLC) (e.g. Culberson & Ammann Reference Culberson and Ammann1979; Culberson et al. Reference Culberson, Culberson and Johnson1981; Culberson & Johnson Reference Culberson and Johnson1982).
The following type specimens were studied for comparison: Rinodina geesteranii H. Mayrhofer: South-Africa: Western Cape: Wynberg Flats, SE of Cape Town, on granite outcrop near dusty road, 19 xii 1949, R. A. Maas Geesteranus (GZU, isotype).
Rinodina griseosquamosa Vain.: Brazil: Rio de Janeiro, in rupe granítica, 1885, E. Wainio 220, 172 (TUR-V 12480—lectotype!, 12478—isolectotype!, here designated).
Rinodina placynthielloides Aptroot: Taiwan: Taichung County: 30 km ENE of Taichung, 7 km NW of Kukwan, along mountain trail, 1000–1300 m, 51RTG9279, on granite of wall along path, 20 x 2001, Aptroot 53451 (B—holotype).
Rinodina punctosorediata Aptroot & Sparrius: Taiwan: Hualien County: 43 km WNW of Hualien, Meifeng, roadside with relict mature trees, 2250 m, 51RUG1666, on Castanopsis, 11 x 2001, Sparrius 6117 (B—holotype).
Rinodina scabridula Matzer & H. Mayrhofer: South Africa: Transvaal: Northern Province Dist. Zoutpansberg, Louis Trichard, near ‘The Punch-bowl’, ± 4500 ft, on sandstone rocks, 8 x 1953, O. Almborn 6169 (GZU— holotype).
The Species
Rinodina algarvensis Giralt, Barbero & van den Boom
Rinodina algarvensis is characterized by its bluish grey thallus composed of discrete to contiguous areolae which often become sorediate, its large Pachysporaria-type ascospores, 19–25 × 11–16 μm, with polygonal lumina when immature, often grading into the Mischoblastia- or Physcia-type when young and, especially, by its particular chemistry with stictic acid (and the related substances cryptostictic, norstictic and menegazziaic acid) in addition to atranorin, chloratranorin and zeorin. For additional information see Giralt et al. (Reference Giralt, Barbero and van den Boom1996a). Up to now, R. algarvensis has been known only as a saxicolous lichen.
The other two Rinodina species known to possess stictic acid and atranorin/choratranorin as major secondary compounds are the North American R. stictica Sheard & Tønsberg (Sheard & Tønsberg Reference Sheard and Tønsberg1995) and R. verruciformis Sheard. The former species differs from R. algarvensis because its ascospores do not exhibit polygonal lumina when young and the latter species lacks soredia (Sheard & Mayrhofer Reference Sheard and Mayrhofer2002).
The holotype of R. punctosorediata Aptroot & Sparrius, a recently described, sorediate species from Taiwan, growing either on bark or rock (Aptroot & Sparrius Reference Aptroot and Sparrius2003) also contains atranorin and stictic acid. As with R. stictica, R. punctosorediata also differs exclusively from R. algarvensis by the absence of polygonal lumina during its ascospore ontogeny, a fact that makes it clearly conspecific with R. stictica.
Ecology and distribution. The Cape Verde specimens constitute additional records for the species hitherto known only from the type locality in southern Portugal. The Cape Verde habitats include a trail along a lightly cultivated mountain slope and a cultivated area with many terraces. In both situations, there were only a very few accompanying lichen species such as an Acarospora sp. (brown) and a Trapelia sp. The most remarkable record is v. d. Boom 36685, which was found corticolous in a rich lichen community on bark of Pinus (for additional data see under Rinodina colobinoides). The type collection (Portugal) is from a maritime lowland area, but the Cape Verde records are from c. 1000 m altitude and are new to Africa.
Material examined: Santo Antão: S of Ribeira Grande, SE of Corda, trail 203 from Cha de Mato to Losna, outcrops near Cha de Mato with shrubs and small trees, 1095 m, 25°04.7′W; 17°07.6′N, 2006, P. & B. van den Boom 36741 (hb. v.d. Boom); NW of Corda, trail 205 in NW direction, to Figueiral, cultivated area with many walls of stones, on S exposed vertical surface of stone, 970 m, 25°05.4′W; 17°08.3′N, 2006, P. & B. van den Boom 36637 (hb. v.d. Boom); Corda, centre of village, outcrops and roadside trees along main road, on Pinus, 1060 m, 25°05.3′W; 17°07.9′N, 2006, P. & B. van den Boom 36685 (hb. v.d. Boom).
Rinodina colobinoides (Nyl.) Zahlbr
Rinodina colobinoides is characterized by its entirely leprose thallus (blastidiate), the yellowish orange, K+ purple-rose pigment in the thalline and apothecial tissues, and by the Pachysporaria-type ascospores, 15–21 × 6·5–10 μm, which are constricted at the septum, smooth and with a well-developed torus. Further information may be found in Giralt et al. (Reference Giralt and Mayrhofer1995, Reference Giralt, van den Boom and Boqueras1996b).
Ecology and distribution. Rinodina colobinoides was found on the bark of a mature Pinus in a rather rich lichen community, associated with Heterodermia leucomelos (L.) Poelt, Lecanora sp., Normanina pulchella (Borrer) Nyl., Phaeophyscia hispidula (Ach.) Moberg, Ramalina farinacea (L.) Ach., Rinodina algarvensis, Teloschistes flavicans (Sw.) Norman and Usnea spp. In mainland Europe, it is known from two localities in Portugal (Giralt et al. Reference Giralt, van den Boom and Boqueras1996b) and also from the British Isles (Coppins Reference Coppins2002). This corticolous species seems to be an oceanic species with a tropical and subtropical distribution (Giralt et al. Reference Giralt and Mayrhofer1995, Reference Giralt, van den Boom and Boqueras1996b). New to Africa.
Material examined: Santo Antão: S of Ribeira Grande, Corda, centre of village, outcrops and roadside trees along main road, on Pinus, 1060 m, 25°05.3′W; 17°07.9′N, 2006, P. & B. van den Boom 36678, 36684 (hb. v.d. Boom).
Rinodina guianensis Aptroot
The Cape Verde specimens are poorly developed, damaged and not very fertile, but they show an isidiate thallus and Pachysporaria-type ascospores following a type B ontogeny. The ascospores are smooth, without a torus, and include minute, scattered and globular ‘inclusions’ within the spore wall. All these characters are diagnostic for R. guianensis. Nevertheless, in contrast to R. guianensis, these specimens lack the yellow pigment reacting K+ purple in the apothecium (Giralt et al. Reference Giralt and Mayrhofer1995). Although other species containing this or similar pigments (e.g. R. intermedia, R. canariensis and R. alba) do not consistently possess it, the specimens studied cannot be assigned with total certainty to R. guianensis.
The peculiar globular ‘inclusions’ within the spore wall have been observed in other species of Rinodina, such as R. dolichospora Malme and R. intermedia, that are closely related to R. guianensis. Photographs illustrating this feature may be found in Mayrhofer et al. (Reference Mayrhofer, Kantvilas and Ropin1999, under R. dolichospora) and in M. Giralt, K. Kalb and H. Mayrhofer (unpublished). The new saxicolous species R. inspersoparietata described below shares the same feature.
Ecology and distribution. In the study area R. guianensis has been found corticolous on a trunk of a ?Mangifera tree and on small trunks of Caffea shrubs. In the field, it is easily mistaken for Rinodina isidioides (Borrer) H. Olivier, an atlantic species occurring locally rather abundantly in the western Iberian Peninsula (Giralt Reference Giralt2001). The lichen community on Caffea (v.d. Boom 36865) was rather rich in Bacidia sp. and Physcia atrostriata Moberg. New to Africa.
Material examined. Santo Antão: SW of Vila das Pombas, Figueiral de Paúl, NE part of the valley, area of Lombo de Luzia, different plantations, scattered mixed trees, acidic outcrops and walls, on ?Mangifera, 180 m, 25°02.7′W; 17°07.8′N, 2006, P. & B. van den Boom 36889, 36906 (hb. v.d. Boom); SW of Vila das Pombas, Figueiral de Paúl, SW part of the valley, Cha de Padre, small coffee plantation, scattered mixed trees, acidic outcrops and walls, on Caffea, 195 m, 25°03.0′W; 17°07.0′N, 2006, P. & B. van den Boom 36865 (hb. v.d. Boom).
Rinodina intermedia Bagl
Rinodina intermedia is easily recognized by its crustose to subsquamulose, ochraceous to brownish thallus containing deoxylichesterinic acid and growing on soil or terricolous mosses, and by its large, non-ornamented, (4–)6–11(–12)-loculate submuriform ascospores with type A ontogeny. Detailed information of this species is given in Mayrhofer et al. (Reference Mayrhofer, Sheard, Grassler and Elix2001).
Ecology and distribution. The collecting site of Rinodina intermedia was rich in lichens, including members of the genera, Caloplaca, Lecidella, Parmotrema, Phaeophyscia, Physcia, Ramalina and Toninia. Rinodina intermedia has previously been reported from the island Fogo (Mies Reference Mies1989,Reference Mies1993).
Material examined. Santo Antão: S of Ribeira Grande, NW of Corda, trail 205 in NW direction to Figueiral, area with terrace cultivation, many walls, some outcrops and scattered trees, on N vertical soil among outcrop, 970 m, 25°05.4′W; 17°08.3′N, 2006, P. & B. van den Boom 36648 (hb. v.d. Boom).
Rinodina oleae Bagl
The crustose thallus lacking secondary lichen substances, the small, abundant and confluent apothecia with plane, dark brown to black discs together with the I+ blue apothecial cortex and the Dirinaria-type ascospores (= Physcia-type thickenings but ontogeny of type B), are diagnostic for this species. Detailed information of R. oleae is given in Giralt & Mayrhofer (Reference Giralt and Mayrhofer1995), Kaschik (Reference Kaschik2006) and Trinkaus et al. (Reference Trinkaus, Mayrhofer and Matzer1999, under R. gennarii Bagl.).
Ecology and distribution. Rinodina oleae has been found in a poorly developed lichen community with a sorediate Physcia sp. Although this species is very common in many places in Europe and occurs on a wide range of substrata, on the Cape Verde islands it seems to be rather rare.
Material examined. Santiago: W of Sao Domingos, Rui Vaz, centre of village, mixed trees and outcrops along small road, on ?Mimosa, 825 m, 23°36.0′W; 15°02.1′N, 2006, P. & B. van den Boom 36337 (hb. v.d. Boom).
Rinodina oxydata (A. Massal.) A. Massal. s. lat
Under Rinodina oxydata s. lat. we have included saxicolous specimens with a thallus containing atranorin, cryptolecanorine to lecanorine apothecia, which often become pseudolecanorine, and large Mischoblastia-type ascospores. As has already been noted by several authors (e.g. Matzer & Mayrhofer Reference Matzer and Mayrhofer1996; Giralt Reference Giralt2001), R. oxydata should be treated as a species aggregate until a thorough worldwide revision is carried out.
Ecology and distribution. All three collecting sites of R. oxydata s. lat. were rather rich in Trentepohlia-containing lichens, especially species of Bactrospora, Lecanographa, Opegrapha, as well as species of Caloplaca, Dirinaria and Pyxine.
Material examined. Santiago: W of Sao Domingos, WNW of Rui Vaz, “Monte Tchopa”, near telecommunication station, hilly area with low exposed outcrops on slope, steep N exposed outcrops and mixed trees, S sloping outcrops, 1085 m, 23°37.5′W; 15°02.2′N, 2006, P. & B. van den Boom 36389 (hb. v.d. Boom). Santo Antão: SW of Ponta do Sol, Fontainhas, S side above village, N exposed slope with acidic outcrops along trail, N vertical rock, 245 m, 25°06.2′W; 17°11.2′N, 2006, P. & B. van den Boom 36821 (hb. v.d. Boom); SW of Ribeira Grande, Ribeira de Duque, trail 205a, southern part, between Cha de Rocho and Meio de Questin, north-south trail with steep outcrops, E exposed steep (80°) rock, 160 m, 25°05.3′W; 17°09.8′N, 2006, P. & B. van den Boom 36933 (hb. v.d. Boom).
The New Species
Rinodina capeverdeana Giralt & van den Boom sp. nov
Rinodina dalmatica similis, sed differt thallo pannarino destituto, apotheciis minoribus c. 0·2–0·5 mm latis et ascosporis minoribus c. 14–22 × 7–10·5 μm. Conidia 3–5 × 1 μm.
Typus: Cape Verde Islands, Santo Antão, SW of Ribeira Grande, Ribeira de Duque, trail 205a, southern part, between Cha de Rocho and Meio de Questin, north-south trail with steep outcrops, N exposed steep (70°) rock and N vertical outcrop, 160 m, 25°05.3′W; 17°09.8′N, 22 July 2006, P. & B. van den Boom 36927 (B—holotypus; LG, hb. v.d. Boom—isotypi).
(Figs 1 & 2)
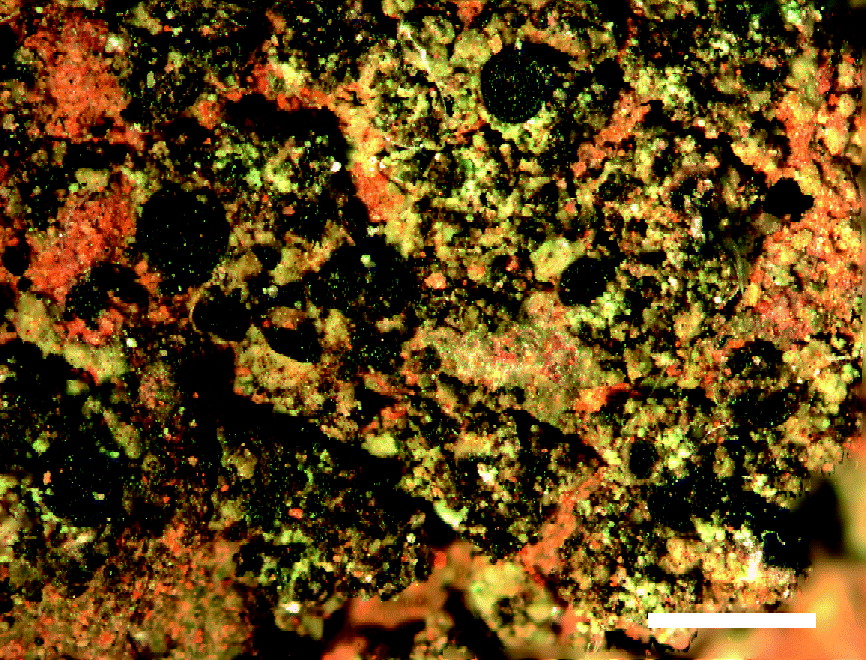
Fig. 1. Rinodina capeverdeana, habitus (holotype). Scale = 1 mm.
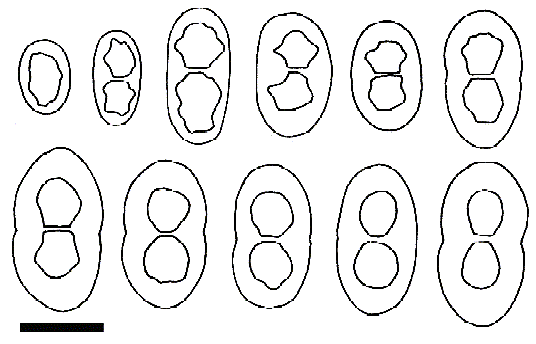
Fig. 2. Rinodina capeverdeana, ascospore ontogeny and variability (holotype). Scale = 10 μm.
Thallus crustose, composed of areolae with the appearance of minute sublobate squamules, up to 150 μm thick, greenish to olivaceous. Areolae discrete to usually contiguous, becoming partly or entirely blastidiate and then forming a rather continuous leprose crust. Thalline cortex paraplectenchymatous, 20–25 μm, covered by an epinecral layer of 5–10 μm. Algal layer continuous, 50–100 μm, photobiont cells 7–13 μm diam. Medulla indistinct to 10 μm thick. Blastidia pale greenish, olivaceous to brownish, 25–50 μm diam.
Apothecia lecanorine, immersed to subimmersed, becoming pseudolecanorine, adnate to sessile, prominent and constricted at the base, 0·2–0·3(–0·55) mm wide. Thalline exciple 60–70(–100) μm thick, with a well-developed, paraplectenchymatous cortex. Proper exciple nearly indistinct laterally, expanded above to 30–50 μm. Disc dark brown to black, flat to rarely subconvex. Thalline margin thin, blastidiate or not, usually excluded. Proper margin thin, not persistent. Hymenium 80–100 μm high, colourless. Hypothecium 70–120 μm high. Asci of Lecanora-type, 8-spored. Epihymenium dark-brown. Apical cells of the paraphyses 3–5·5 μm diam., not separating easily, with dark-brown caps. Ascospores Pachysporaria-type, (14–)16–19(–22) × (7–)8–10·5 μm, not ornamented, when mature with a well-developed torus and slightly constricted; young ascospores with very characteristic polygonal lumina. Ascospore ontogeny of type A.
Pycnidia abundant, globose and prominent, dark-brown. Conidia bacilliform, 3–5 × 1 μm.
Chemistry. All spot tests negative. No lichen substances detected by TLC.
Ecology and distribution. Rinodina capeverdeana is known only from the type locality, where it was growing abundantly in association with Caloplaca sp, Endocarpon pusillum Hedw., Peltula sp., Pertusaria sp., Pyxine cf. cocoes (Sw.) Nyl., R. inspersoparietata, Trentepohlia sp. and cyanobacteria, on steep (70°) N-exposed outcrops of rather soft rock, in a small valley not particularly rich in lichens.
A corticolous specimen examined (coll. Mies CV-1265, hb. Follmann, B), identified by Mies as R. cf. roboris, probably belongs to R. capeverdeana (but see below). An additional specimen of R. cf. roboris (coll. Mies CV-936, GZU), also collected on the Island of Santiago, does not belong to this species. The specimen mentioned from Fogo Island by Mies (Reference Mies1989, Reference Mies1993) could not be located.
Observations. Rinodina capeverdeana is characterized by its thallus lacking secondary lichen substances and composed of partially to entirely blastidiate areolae and a well-developed paraplectenchymatous cortex, its small lecanorine apothecia often losing the thalline margin and becoming pseudolecanorine, its abundant, globose pycnidia and its Pachysporaria type ascospores, when young with typical polygonal lumina.
The corticolous specimen (coll. Mies CV-1265, hb. Follmann, B) differs from the saxicolous one in possessing a better developed not so continuously leprose thallus, more and larger usually persistently lecanorine apothecia (up to 0·7 mm diam.) and smaller pycnidia. Although microscopically both specimens are very similar, there are too many thalline and apothecial differences to include them with certainty within the same taxon, particularly when R. capeverdeana is saxicolous and its habit variability still unknown. The specimens reported in Mies (Reference Mies1989, Reference Mies1993) as R. cf. roboris (coll. Mies CV-936, GZU) and R. corticola (coll. Mies CV-3745, GZU) both belong to a species with Physcia-type ascospores close to R. exigua, but lacking atranorin.
The blastidiate thallus, the lecanorine apothecia becoming pseudolecanorine and, especially, the Pachysporaria-type ascospores when young with polygonal lumina, are all characters close to those of R. dalmatica Zahlbr. However R. dalmatica is only known to be corticolous, contains pannarin and has larger apothecia and ascospores, 17–26 × 8–13 μm (Giralt et al. Reference Giralt and Mayrhofer1995). The ascospores of R. capeverdeana are similar in appearance to those of R. dalmatica in Giralt et al. (Reference Giralt and Mayrhofer1995, fig. 2, p. 7). According to Sheard & Mayrhofer (Reference Sheard and Mayrhofer2002), the recently described North American R. perreagens Sheard, is closely related to R. dalmatica, but differs mainly by its slightly larger ascospores, 21·8–31·8 × 13·4–16·2 μm (Sheard & Mayrhofer Reference Sheard and Mayrhofer2002). It differs from the new species by the same characters as R. dalmatica.
Other saxicolous, blastidiate species, R. blastidiata Matzer & H. Mayrhofer, R. furfurea H. Mayrhofer & Poelt, R. obnascens (Nyl.) H. Olivier and R. scabridula Matzer & H. Mayrhofer, apart from other differential characters, never possess polygonal lumina in the young ascospores (see Matzer & Mayrhofer Reference Matzer and Mayrhofer1994, Reference Matzer and Mayrhofer1996).
The holotype of R. placynthielloides, a saxicolous, isidiate species recently described from Taiwan (Aptroot & Sparrius Reference Aptroot and Sparrius2003), has been studied for comparison. Although in the envelope containing the type specimen there is a drawing of an ascospore, we were unable to find apothecia corresponding to the isidiate thallus of R. placynthielloides. We did however, identify small, K+ yellow thalli (atranorin) with pseudolecanorine apothecia belonging to R. oxydata mixed up with the thallus of R. placynthielloides. As the thalli of R. placynthielloides and R. capeverdeana are clearly different and, according to Aptroot & Sparrius (Reference Aptroot and Sparrius2003), the apothecia of R. placynthielloides are lecideine and its asci 4-spored, we reject the possibility that this species is conspecific with R. capeverdeana with lecanorine apothecia (often becoming pseudolecanorine) and 8-spored asci.
Rinodina capeverdeana is somewhat similar in habit to poorly developed specimens of Hyperphyscia and Phaeophyscia species.
Additional material examined. Santiago: Milho Branco, 300 m, an Borke eines alten Ziziphus spec., 1986, B. Mies 322b (coll. Mies CV- 1265, hb. Follmann, B).
Rinodina inspersoparietata Giralt & van den Boom sp. nov
Rinodina dolichospora similis, sed differt thallo saxicola, apotheciis pseudolecanorinis et ascosporis minoribus, c. 17–23 × 7·5–12 μm, ontogenia typo B. Conidia 3–4·5 × 1 μm.
Typus: Cape Verde Islands, Santo Antão, SW of Ribeira Grande, Ribeira de Duque, trail 205a, southern part, between Cha de Rocho and Meio de Questin, north-south trail with steep outcrops, N exposed steep (70°) rock and N vertical outcrop, 160 m, 25°05.3′W; 17°09.8′N, 22 July 2006, P. & B. van den Boom 36926 (B—holotypus; LG, hb. v.d. Boom—isotypi; 36919, 36927a, 36928—topotypi).
(Figs 3 & 4)

Fig. 3. Rinodina inspersoparietata, habitus (holotype). Scale = 1 mm.

Fig. 4. Rinodina inspersoparietata, ascospore ontogeny and variability (holotype). Scale = 10 μm.
Thallus crustose to subsquamulose, ochraceous, olivaceous to brownish, areolate to rimose-areolate. Photobiont cells usually 5–7 μm diam., with some larger, up to 12 μm.
Apothecia from the beginning pseudolecanorine, sessile, prominent, up to 0·7 mm diam. Proper exciple (40–)70–100 μm, basal part including algal cells, external part brown-pigmented, internal part colourless, paraplectenchymatous. Proper margin not persistent, black. Disc flat to convex, dark-brown to black. Hymenium colourless, (80–)100–120 μm high. Hypothecium colourless to pale yellowish brown, up to 150 μm thick. Epihymenium orange-brown. Asci Lecanora-type, 8-spored. Oil paraphyses abundant; apical cells 3–4 μm diam., with brown caps. Ascospores Pachysporaria-type or ± Physconia-type, (17–)19–21(–23) × (7·5–) 9–10(–12) μm, with young intermediates of the Physcia-type, including globular inclusions within the spore wall, not ornamented, torus absent or poorly developed, ontogeny of type B.
Pycnidia immersed, rare. Conidia bacilliform, 3–4·5 × 1 μm.
Chemistry. All spot tests negative. No lichen substances detected by TLC.
Ecology and distribution. Known from the same type locality as R. capeverdeana, with which R. inspersoparietata was growing abundantly on steep (70°) N exposed outcrops of rather loose-textured (soft) rocks, in a rather small valley, not particularly rich in lichens. Closely associated with Caloplaca sp., Endocarpon pusillum, Peltula sp., Pertusaria sp., Pyxine cf. cocoes, Trentepohlia sp. and cyanobacteria, It seems to be rather widely distributed in the Cape Verde archipelago, being known from the two largest islands, Santiago in the south and Santo Antão in the north.
Observations: Rinodina inspersoparietata is characterized by its saxicolous crustose to subsquamulose, ochraceous to olivaceous thallus lacking secondary lichen substances, its pseudolecanorine and sessile apothecia and its Pachysporaria-type non-ornamented ascospores, 17–23 × 7·5–12 μm, with a poorly developed torus, including globular inclusions in the spore wall and following a type B ontogeny.
The presence of the globular inclusions within the spore wall, clearly allies this species with the corticolous R. dolichospora and R. guianensis and with the terricolous R. intermedia. Among all species of Rinodina hitherto known they are unique in possessing this character. This group of species also shares other particular characters (e.g. ochraceous thallus, small photobiont cells, epihymenium orange-brown, etc.) (M. Giralt, K. Kalb & H. Mayrhofer, unpublished). Apart from the different habitat, these species can be separated from R. inspersoparietata: R. dolichspora by its larger ascospores with type A ontogeny; R. guianensis by the presence of isidia and R. intermedia by its submuriform ascospores.
The type material of the South African R. geesteranii and the Brazilian R. griseosquamosa were studied for comparison. In contrast to R. inspersoparietata, both taxa lack globular inclusions in the ascospores. Furthermore, R. geesteranii has finely ornamented ascospores, which are intermediate between the Milvina- and the Pachysporaria-type and develop after a type-A ontogeny, and R. griseosquamosa possesses a thallus composed of thicker, larger, imbricate, whitish grey squamules and larger, crytolecanorine apothecia. For more details see also Matzer & Mayrhofer (Reference Matzer and Mayrhofer1996), Wainio (Reference Wainio1890) and Malme (Reference Malme1902).
Rinodina inspersoparietata could be mistaken for R. oxydata in the study area because of its pseudolecanorine apothecia, but the latter species possesses atranorin and has Mischoblastia-type ascospores, developing after a type A ontogeny.
Additional material examined. Santiago: SE of Tarrafal, along road to Fundura, N side of serra Malagueta, vertical shaded and overhanging rocks along road, E & NW exposed vertical rock, 640 m, 23°42.0′W; 15°12.5′N, 2006, P. & B. van den Boom 36536, 36528 (hb. v.d. Boom); SE of Tarrafal, along road to Fundura, S rim of Serra Malagueta, N of Fundura, vertical outcrops on W exposed slope, W exposed vertical, 675 m, 23°41.6′W; 15°10.5′N, 2006, P. & B. van den Boom 36553 (hb. v.d. Boom); W of Sao Domingos, S side of Rui Vaz, low exposed outcrops along field and steep N exposed outcrops on top of hill, N vertical outcrop, 815 m, 23°35.8′W; 15°01.7′N, 2006, P. & B. van den Boom 36376 (hb. v.d. Boom).
Rinodina polymorphaespora Giralt & van den Boom sp. nov
Thallus corticola, crustacea, effusa, grisea ad griseo-fusca, granulosa. Apothecia lecanorina, sessilia, 0·2–0·3(–0·5) mm diametro, disco plano vel subconvexo, nigrobrunneo. Ascosporae (14–)15·5–19(–23) × (6–)7–9(–10·5) μm, typo Physcia-, Physconia- et Pachysporaria, 1(–2–3)-septata, toro non vel vix evoluto. Pycnidia non visa.
Typus: Cape Verde Islands, Santiago, W of Sao Domingos, Rui Vaz, centre of village, mixed trees and outcrops along small road, on Casuarina, 825 m, 23°36.0′W; 15°02.1′N, 7 July 2006, P. & B. van den Boom 36334 (B—holotypus; hb. v.d. Boom—isotypus).
(Figs 5 & 6)

Fig. 5. Rinodina polymorphaespora, habitus (holotype). Scale = 0.5 mm.

Fig. 6. Rinodina polymorphaespora, ascospore ontogeny and variability (holotype). Scale = 10 μm.
Thallus crustose, thin, continuous, smooth to minutely granulose, pale greyish to greyish brown. Photobiont cells up to 25 μm diam. Prothallus absent.
Apothecia lecanorine, sessile, 0·2–0·3(–0·5) mm diam. Thalline exciple well developed, up to 60 μm. Proper exciple indistinct to 10 μm wide laterally, expanded up to 30 μm above. Thalline margin thin, smooth, usually persistent, concolorous with thallus. Proper margin often visible as a ring within the thalline margin. Disc dark-brown to black, flat to subconvex. Hymenium colourless. Hypothecium colourless. Epihymenium dark-brown. Asci Lecanora-type, 8-spored. Oil paraphyses often present; apical cells of the paraphyses dark-brown pigmented, 4–4·5 μm diam. Ascospores (14–)15·5–19(–23) × (6–)7–9(–10·5) μm (mean = 17·3 ±1.9 × 8·2 ± 0.9; n = 118), with internal wall thickenings of the Physcia-, Physconia- or Pachysporaria-type, some with tendencies to the Callispora-type, often with lumina protruding towards the spore ends, rarely becoming 2–3-septate, torus absent or poorly developed, usually not ornamented but a few overmature ones showing a scabrid surface, ontogeny mostly of type B, sometimes seemingly of type A.
Pycnidia and conidia not seen.
Chemistry. All spot tests negative. No lichen substances detected by TLC.
Ecology and distribution. Rinodina polymorphaespora is known from a rather wide range of substrata such as Acacia and Eucalyptus trees and also various shrubs. At the type locality it was growing on a Casuarina tree, together with a Bacidia sp., a sorediate Caloplaca sp., and a sorediate Physcia, probably P. poncinsii Hue. Rinodina polymorphaespora is widely distributed in the Cape Verde archipelago, from the southern island Santiago to the northern island Santo Antão.
Observations: Rinodina polymorphaespora is characterized by its crustose thallus lacking secondary lichen substances, its small, lecanorine apothecia and its peculiar ascospores, (14–)15·5–19(–23) × (6–)7–9(–10·5) μm. In contrast to the constant macroscopical thalline and apothecial characters, the ascospores of R. polymorphaespora show a wide variation in internal wall thickenings. They can be assigned to the Physcia-, Physconia- or Pachysporaria-type, and may also show tendencies towards the Callispora-type. Furthermore, they can develop after an ontogeny of type A or B, may become 2–3-septate and can be smooth or ornamented. Nevertheless they always present lumina protruding towards the spore ends. This variability can be observed in a single apothecial section.
Similar, remarkable variability in the spore thickenings has been reported in the corticolous species, R. confusa H. Mayrhofer & Kantvilas and R. abolescens H. Magn. (cf. Mayrhofer et al. Reference Mayrhofer, Kantvilas and Ropin1999; Giralt & Mayrhofer Reference Giralt and Mayrhofer1995). The sporadic presence of 2–3-septate ascospores also allies R. polymorphaespora with species of Rinodina with 3-septate ascospores, such as R. conradii Körb., R. connectens Malme, R. homobola (Nyl.) Vain. and R. homoboloides Vain. However, all these taxa possess larger ascospores that, except in R. conradii, develop only after a type A ontogeny.
Rinodina polymorphaespora seems to be related to R. oleae, R. boleana, R. pyrina and even to R. conradii, since, apart from being very similar in habit, it has all ascospore-types of these species. However, its ascospores are significantly larger than in the first three taxa and smaller and more rarely 2–3-septate than in R. conradii (cf. Kaschik Reference Kaschik2006; Giralt & Mayrhofer Reference Giralt and Mayrhofer1991, Reference Giralt and Mayrhofer1995; Mayrhofer et al. Reference Mayrhofer, Kantvilas and Ropin1999).
Additional material examined. Santiago: W of Sao Domingos, N of Rui Vaz, along village, on rocky mountain, with some shrubs, on ?Acacia, 855 m, 23°35.7′W; 15°02.3′N, 2006, P. & B. van den Boom 36509 (hb. v.d. Boom); W of Sao Domingos, WNW of Rui Vaz, “Monte Tchopa”, near telecommunication station, hilly area with low exposed outcrops on slope, steep N exposed outcrops and mixed trees, on Eucalyptus, 1085 m, 23°37.5′W; 15°02.2′N, 2006, P. & B. van den Boom 36404 (hb. v.d. Boom). Santo Antão: S of Ribeira Grande, SE of Corda, trail 203 from Cha de Mato to Losna, outcrops near Cha de Mato with shrubs and small trees, on shrub on N slope, 1095 m, 25°04.7′W; 17°07.6′N, 2006, P. & B. van den Boom 36751, 36752 (hb. v.d. Boom); S of Ribeira Grande, NW of Corda, trail 205 in NW direction to Figueiral, area with terrace cultivation, many walls, some outcrops and scattered trees, on Acacia, 970 m, 25°05.4′W; 17°08.3′N, 2006, P. & B. van den Boom 36641 (hb. v.d. Boom).
The authors are indebted to M. Barbero for carrying out the TLC analyses, H. Sipman for correcting the Latin diagnoses and for comments on the manuscript and H. Mayrhofer for locating material from Cape Verde in GZU. We thank the curators of B, GZU and TUR-V for the loan of specimens and the first author also thanks the ‘Comissionat per a la Recerca’ (Catalan Government) for support.








