Introduction
Alcohol use disorder (AUD) is a widespread illness in the USA with estimated 12-month and lifetime prevalence rates of 13.9 and 29.1% (Grant et al., Reference Grant, Stinson, Dawson, Chou, Dufour, Compton, Pickering and Kaplan2015). AUD harms individuals by disrupting educational attainment and interpersonal functioning, and by increasing risk for negative physical and psychiatric outcomes (Rehm, Reference Rehm2011; Grant et al., Reference Grant, Stinson, Dawson, Chou, Dufour, Compton, Pickering and Kaplan2015). AUD is also costly to society through lower productivity, crime, and motor vehicle accidents. The prevalence of AUD increases steadily across adolescence followed by dramatic increases in early adulthood (Chassin et al., Reference Chassin, Flora and King2004; Grant et al., Reference Grant, Stinson, Dawson, Chou, Dufour, Compton, Pickering and Kaplan2015). Alcohol use commonly begins in adolescence (Johnston et al., Reference Johnston, O'Malley, Bachman, Schulenberg, Patrick and Miech2015), making this a critical period to investigate the developmental origins of AUD.
One way that alcohol use might increase risk for AUD is by impacting the developing brain, particularly subcortical and prefrontal regions, which undergo significant neurodevelopmental change during adolescence (Gogtay et al., Reference Gogtay, Giedd, Lusk, Hayashi, Greenstein, Vaituzis, Nugent, Herman, Clasen and Toga2004; Doremus-Fitzwater et al., Reference Doremus-Fitzwater, Varlinskaya and Spear2010; Casey et al., Reference Casey, Jones and Somerville2011). During adolescence, there is a pruning of dopamine receptors in reward-related areas of the brain, such as the ventral striatum (VS; Seeman, Reference Seeman1987, Teicher et al., Reference Teicher, Andersen and Hostetter1995). These neurodevelopmental changes are thought to be important for understanding the neural underpinnings of reward-driven behavior and risk for alcohol misuse (Spear, Reference Spear2016), especially because drugs of abuse enhance dopamine neurotransmission (Robinson and Berridge, Reference Robinson and Berridge1993). Thus, adolescence is thought to be a time when the brain may be particularly vulnerable to the neurotoxic effects of alcohol (Wiers et al., Reference Wiers, Bartholow, van den Wildenberg, Thush, Engels, Sher, Grenard, Ames and Stacy2007; Casey and Jones, Reference Casey and Jones2010). Animal research supports this hypothesis, showing that the effects of alcohol on behavioral and cognitive functioning, as well as brain structure, are more pronounced during adolescence relative to adulthood (Spear, Reference Spear2016). In humans, cross-sectional functional magnetic resonance imaging (fMRI) studies also document differences in the reactivity of reward-related neural systems among adolescents with AUD relative to healthy controls (Ewing et al., Reference Ewing, Sakhardande and Blakemore2014). Thus, greater exposure to alcohol during adolescence may result in altered neurocircuitry of the reward system, including sensitization of VS dopaminergic pathways, which could increase vulnerability for developing AUDs later in life.
However, the majority of studies that have examined associations between adolescent alcohol use and reward-related neural functioning are limited by being cross-sectional in design, utilizing small sample sizes, focusing only on clinical samples with extreme use (Tapert et al., Reference Tapert, Schweinsburg, Barlett, Brown, Frank, Brown and Meloy2004; Wetherill et al., Reference Wetherill, Squeglia, Yang and Tapert2013; Xiao et al., Reference Xiao, Bechara, Gong, Huang, Li, Xue, Wong, Lu, Palmer and Wei2013), and/or examining reward-related neural functioning only among children of alcoholics (Heitzeg et al., Reference Heitzeg, Cope, Martz and Hardee2010; Yau et al., Reference Yau, Zubieta, Weiland, Samudra, Zucker and Heitzeg2012). Longitudinal studies are needed to provide a better test of whether adolescent alcohol use impacts reward-related neural functioning. Moreover, evidence is needed from naturalistic samples beginning with alcohol use onset, rather than from youth who are already abusing alcohol. Thus, prospective studies of community youth using repeated assessments represent a powerful way to examine change in alcohol use over time (Duncan et al., Reference Duncan, Duncan and Hops1994; Duncan and Duncan, Reference Duncan and Duncan1995), and establish whether different (or even normative) rates of alcohol consumption across adolescence impact reward-related neural functioning and persistent alcohol misuse.
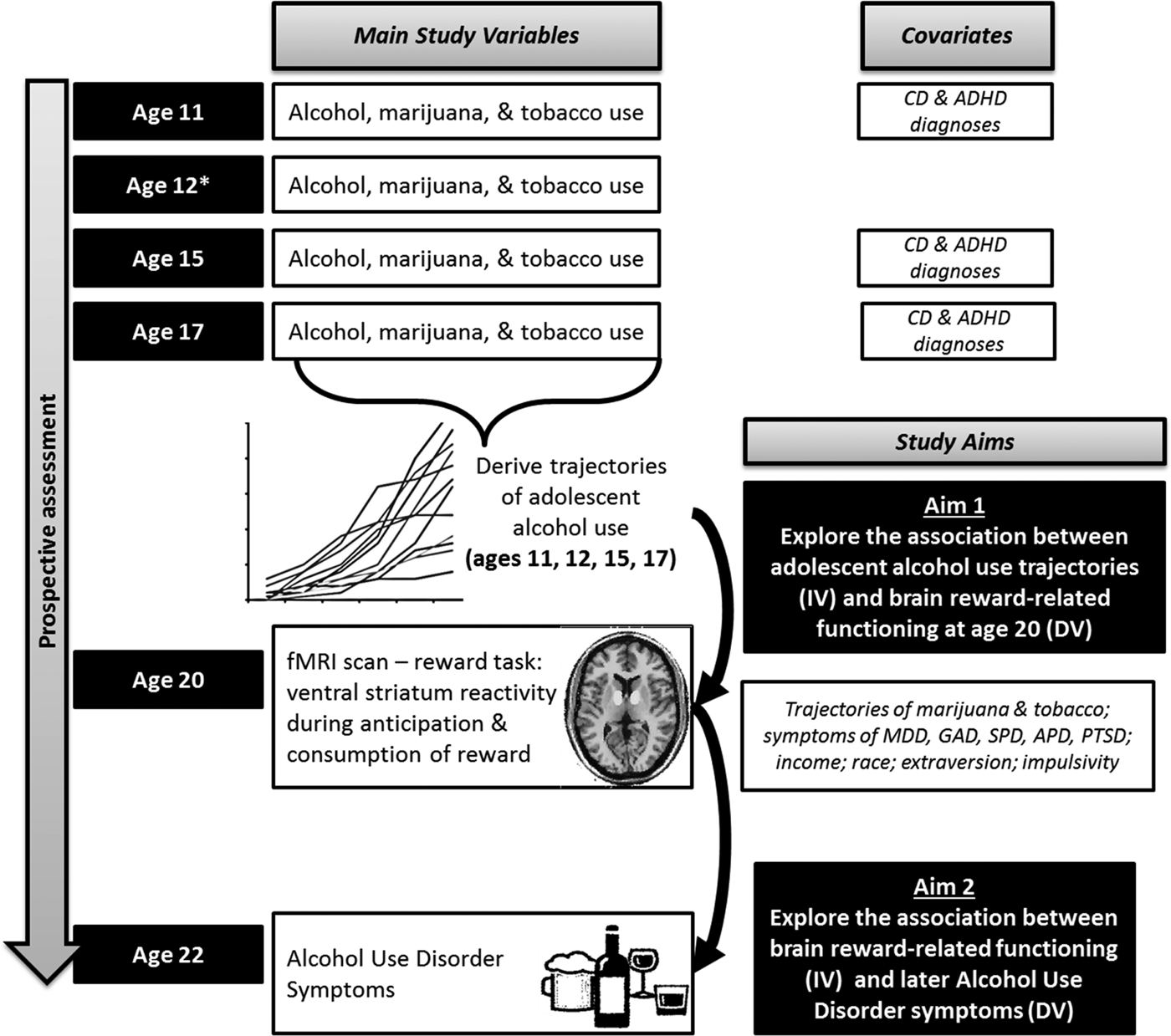
In the current study, we addressed two questions centered on alcohol use from adolescence to early adulthood via VS reward-related functioning. We focused on a low income, urban male sample because urban males are exposed to more risk factors linked to alcohol misuse (Elliott et al., Reference Elliott, Huizinga and Menard2012) and there are higher rates of AUD in males relative to females (Grant et al., Reference Grant, Stinson, Dawson, Chou, Dufour, Compton, Pickering and Kaplan2015). First, we examined whether the trajectory of alcohol use (i.e. the rate of increase in use) across adolescence predicted VS reward processing at age 20. We focused on a VS region-of-interest because of the centrality of the VS to reward processing (Haber and Knutson, Reference Haber and Knutson2010) and links between VS reactivity and AUD in adulthood (Heitzeg et al., Reference Heitzeg, Nigg, Yau, Zucker and Zubieta2015; Nikolova et al., Reference Nikolova, Knodt, Radtke and Hariri2016). We tested whether the relationship between adolescent alcohol use and VS reactivity was dependent on reward phase (i.e. reward anticipation v. receipt). We hypothesized that because of increased incentive anticipation and ‘wanting’ of reward linked to repeated alcohol exposure (Robinson and Berridge, Reference Robinson and Berridge1993; Silveri and Spear, Reference Silveri and Spear2002), greater adolescent alcohol use would be related to increased VS reactivity during reward anticipation, but not reward receipt. Second, we examined whether trajectories of alcohol use across adolescence were related to AUD symptoms at age 22 via VS reward processing at age 20, testing longitudinal, indirect pathways. We hypothesized that adolescent alcohol use would predict VS hypersensitivity during reward anticipation at age 20, which in turn, would predict increases in AUD symptoms at age 22 (Fig. 1).

Fig. 1. Hypothesized associations between trajectories of adolescent alcohol use, ventral striatum reactivity at age 20, and increases in symptoms of AUD at age 22. Note. * At age 12, clinician consensus data were not available for the KSADS, thus we did not include conduct disorder or ADHD diagnoses from this assessment point. ADHD, attention deficit hyperactivity disorder; MDD, major depressive disorder; GAD, generalized anxiety disorder; SPD, social phobia; PTSD, post-traumatic stress disorder; APD, antisocial personality disorder; AUD, alcohol use disorder; DUD, drug use disorder; DV, dependent variable; IV, independent variable. To address Aim 1, we tested whether individual differences in the rate of alcohol use across adolescence from ages 11, 12, 15 and 17, based on latent growth curve modeling, were related to ventral striatum reward reactivity at age 20 assessed via functional magnetic resonance imaging (fMRI). To address Aim 2, we tested whether individual differences in ventral striatum reward reactivity at age 20 were related to increases in symptoms of AUD at age 22 (i.e. controlling for symptoms of AUD at age 20 and overlapping DUD and APD symptoms at age 22). The indirect (mediated) effect of adolescent alcohol use on later symptoms of AUD at age 22 via ventral striatum reactivity at age 20 was also examined. Models controlled for other salient personality, psychiatric, and demographic confounds.
Methods and materials
Participants
139 participants were drawn from the Pitt Mother & Child Project, a longitudinal study of 310 racially diverse and low-income boys and their families (Shaw et al., Reference Shaw, Hyde and Brennan2012). The sample is at risk for externalizing outcomes based on being male, urban, and from low-income families (Shaw et al., Reference Shaw, Hyde and Brennan2012). Boys and their mothers were seen in person almost yearly from ages 1.5–22 in their home and/or the laboratory (assessments at 1½, 2, 3½, 5, 6, 7, 8, 9, 10, 11, 12, 15, 17, 20, and 22 years old). In the current study, we focused on the latter six assessment points that covered adolescence and early adulthood: 11, 12, 15, 17, 20, and 22 years old. Assessments included questionnaires and a psychiatric interview. At age 20, the assessment also included a fMRI scan at age 20. Participants were reimbursed after assessments and procedures were approved by the University of Pittsburgh IRB.
Attrition to the age 20 and 22 visits was low for such a long-term study (252 and 256 men participated at ages 20 and 22, respectively, 81 and 83% retention across more than 20 years) (Shaw et al., Reference Shaw, Hyde and Brennan2012; Murray et al., Reference Murray, Shaw, Forbes and Hyde2017). Of the 256 men retained at age 20, 144 had usable fMRI reward data and 139 had both fMRI and adolescent alcohol use data (online Supplementary Table S1 summarizes details on attrition). The 139 males with fMRI data at age 20 did not differ significantly from the full sample retained at age 20 based on self-reported alcohol (p > 0.39) or marijuana (p > 0.76) consumption via the Alcohol and Drug Consumption Questionnaire (Cahalan et al., Reference Cahalan, Cisin and Crossley1969) or self-reported antisocial behavior (p > 0.37) via the Self-Report of Delinquency Questionnaire (Elliott et al., Reference Elliott, Huizinga and Menard2012).
Measures
Adolescent alcohol use (ages 11, 12, 15, and 17)
We assessed adolescent alcohol use using items from the Self-Report Delinquency Questionnaire (Elliott et al., Reference Elliott, Huizinga and Menard2012), which assesses engagement in antisocial activities in the past year via a three-point scale (0 = never, 1 = once/twice, 2 = more often; α = 0.90). Three separate items assessing consumption of beer, liquor, or wine were summed to create alcohol frequency scores at each age, which were subjected to latent growth curve modeling to derive alcohol trajectories (see online Supplementary Table S2). Rates were similar to those reported via surveys of adolescents within community samples (Johnston et al., Reference Johnston, O'Malley, Bachman, Schulenberg, Patrick and Miech2015; Miech et al., Reference Miech, Johnston, O'Malley, Bachman and Schulenberg2015).
Symptoms of AUD (ages 20 and 22)
To assess AUD at ages 20 and 22, we used interviewer assessments from the Structured Clinical Interview for DSM-IV Axis I (SCID-I) (First et al., Reference First, Gibbon, Spitzer, Benjamin and Williams1995; American Psychiatric Association, Reference Association2000) (online Supplementary Methods outline the training and reliability procedures). Results are presented using an AUD symptom count combining the total number of alcohol use and dependence symptoms. To confirm these results within a DSM-5 framework, which removed the abuse-dependence distinction, we compared findings for a symptom count excluding ‘recurrent substance-related legal problems’ that does not appear in DSM-5 (American Psychiatric Association, Reference Association2013). We also found similar results using separate alcohol abuse v. dependence symptom counts. At age 22, approximately 21% of our sample met lifetime criteria for Alcohol Abuse or Dependence diagnoses. These estimates are consistent with epidemiological surveys of community samples (Grant et al., Reference Grant, Goldstein, Saha, Chou, Jung, Zhang, Pickering, Ruan, Smith and Huang2004).
Personality confounds (age 20)
To ensure that effects were not accounted for by stable personality traits linked to hypersensitivity to reward or alcohol misuse, we controlled for impulsivity, which has been linked to VS reward-related reactivity and alcohol use (Beck et al., Reference Beck, Schlagenhauf, Wüstenberg, Hein, Kienast, Kahnt, Schmack, Hägele, Knutson and Heinz2009), and extraversion, which predicts brain reward-related functioning (Cohen et al., Reference Cohen, Young, Baek, Kessler and Ranganath2005) and alcohol use (Kuntsche et al., Reference Kuntsche, Knibbe, Gmel and Engels2006). Impulsivity was assessed at age 20 via self-report using the Barratt Impulsiveness Scale-Version II, a 30 item measure tapping several aspects of impulsivity, including deficits in behavioral control (Patton and Stanford, Reference Patton and Stanford1995) (α = 0.79) Extraversion was assessed age 20 via self-report on the 12-item extraversion subscale of the NEO Personality Inventory-Revised (NEO PI-R Short Form) (α = 0.64) (Costa and McCrae, Reference Costa and McCrae1997).
Adolescent comorbid psychiatric disorders
To confirm that relationships between adolescent alcohol use and brain reward-related functioning were not due to earlier attention deficit hyperactivity disorder (ADHD) or conduct disorder (CD), both well-established risk factors for AUD (van Emmerik-van Oortmerssen et al., Reference van Emmerik-van Oortmerssen, van de Glind, van den Brink, Smit, Crunelle, Swets and Schoevers2012), we included ADHD and CD diagnoses from the Schedule for Affective Disorders and Schizophrenia for School-Age Children (Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao, Flynn, Moreci, Williamson and Ryan1997), a semi-structured psychiatric interview using DSM-IV criteria. At ages 11, 15, and 17 years old, examiners administered interviews to boys and their mothers and diagnoses were made through clinician consensus. Training and reliability procedures are described in online Supplemental Methods. Earlier ADHD or CD was included as a present/absent dichotomous variable (i.e. present = diagnosis at any prior assessment).
Other comorbid psychiatric disorders
Finally, to ensure that effects were not accounted for by psychiatric comorbidities related to hypersensitivity to reward or engagement in alcohol misuse, we controlled for symptoms of the following DSM-IV disorders at age 20 as covariates based on SCID-I (First et al., Reference First, Gibbon, Spitzer, Benjamin and Williams1995) and SCID-II (First et al., Reference First, Spitzer, Gibbon and Williams1997) interviews: antisocial personality disorder (APD; Compton et al., Reference Compton, Conway, Stinson, Colliver and Grant2005), major depressive disorder (MDD; Hasin et al., Reference Hasin, Goodwin, Stinson and Grant2005), generalized anxiety disorder (GAD; Kushner et al., Reference Kushner, Abrams and Borchardt2000, Grant et al., Reference Grant, Goldstein, Saha, Chou, Jung, Zhang, Pickering, Ruan, Smith and Huang2004), post-traumatic stress disorder (PTSD) (Kushner et al., Reference Kushner, Abrams and Borchardt2000), and social phobia (SP; Kushner et al., Reference Kushner, Abrams and Borchardt2000). Although we included symptom counts as covariates in models, the findings were similar if we included diagnoses. Finally, in addition to including comorbid psychiatric disorders as covariates at age 20, we explored the specificity of pathways to AUD, by including substance use disorder (SUD) and APD symptoms as dependent variables at age 22.
Other covariates
To ensure that results relating to differences in reward functioning were not due to race or socioeconomic status, we accounted for the effects of race and income (Murray et al., Reference Murray, Shaw, Forbes and Hyde2017). As alcohol use tends to co-occur with marijuana and tobacco use (Moss et al., Reference Moss, Chen and Yi2014), we also accounted for rates of comorbid adolescent marijuana and tobacco use assessed via the same method as alcohol use (i.e. trajectories across adolescence; online Supplementary Table S2). Finally, all models included the age at which participants first reported drinking as a covariate. Descriptive statistics for all study variables are presented in Table 1 and bivariate correlations between study variables in online Supplementary Table S3.
Table 1. Descriptive data for other study variables for the subsample of men for whom imaging data were available (n = 139)

Note. Findings were similar when we accounted for psychiatric comorbidities using diagnoses instead of symptom count. The number of participants meeting diagnostic criteria was as follows at age 20: major depressive disorder, N = 12 (9%), generalizing anxiety disorder, N = 1 (1%), social phobia, N = 10 (7%), Antisocial personality disorder, N = 11 (8%), post-traumatic stress disorder, N = 2 (1%), and alcohol use and dependence, N = 18 (13%). At age 22, the number of participants meeting criteria for Alcohol Use and Dependence was N = 28 (20%).
Neuroimaging procedures
After participants completed questionnaires and clinical interviews, they underwent a fMRI scan. The fMRI reward paradigm was a slow event-related card-guessing game that evaluates neural response to the anticipation and receipt of monetary reward (Nusslock et al., Reference Nusslock, Almeida, Forbes, Versace, Frank, Labarbara, Klein and Phillips2012). During trials, participants guessed via button press whether the values of visually presented cards, with possible values of 1–9, were higher or lower than 5 (4 s), learned the trial type (possible-win) to anticipate feedback (6 s), and receive feedback (e.g. win money/no change; 1s plus 9s inter-trial interval) (Nusslock et al., Reference Nusslock, Almeida, Forbes, Versace, Frank, Labarbara, Klein and Phillips2012). Participants were told that their performance would determine a monetary reward after the scan ($1 per win). Trials were presented in pseudorandom order with predetermined outcomes in a single, 8-min run, with 24 trials and a balanced number of trial types. This task has previously been shown to differentiate anticipation v. receipt phases of reward processing with large task-based effect sizes in the VS (Hasler et al., Reference Hasler, Dahl, Holm, Jakubcak, Ryan, Silk, Phillips and Forbes2012; Nusslock et al., Reference Nusslock, Almeida, Forbes, Versace, Frank, Labarbara, Klein and Phillips2012; see online Supplementary Fig. S1 for a fuller task description).
Bold fMRI acquisition parameters
As described previously (Murray et al., Reference Murray, Shaw, Forbes and Hyde2017), participants were scanned with a research-dedicated Siemens 3-T Trio scanner with a 12-channel head coil at the University of Pittsburgh. Blood oxygenation level–dependent (BOLD) functional images were acquired with a gradient-echo echoplanar imaging sequence repetition time (TR)/echo time (TE)/flip angle = 2000/29 milliseconds/90°, field of view = 200 × 200 mm, matrix = 64 × 64), that covered 34 interleaved axial slices (3 mm slice thickness) aligned with the AC–PC plane and encompassing the entire cerebrum and most of the cerebellum to maximize coverage of limbic structures.
MRI data analysis
Image processing and analysis
Analyses were completed using the general linear model of SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Images for each participant were segmented, realigned to the first volume in the time series, unwarped to correct for head motion, co-registered to high-resolution structural scans, spatially normalized into Montreal Neurological Institute space using a 12-parameter affine model, and smoothed to minimize noise and residual difference in gyral anatomy with a 6 mm FWHM Gaussian filter. Voxelwise signal intensities were ratio-normalized to the whole-brain global mean. After preprocessing, Artifact detection Tools software (http://www.nitrc.org/projects/artifact_detect/) was used by detecting global mean intensity and translation or rotational motion outliers (>4.5 s.d. from the mean global brain activation, >2 mm movement, or 2o translation in any direction) by creating a regressor within each participant's first-level analysis to account for confounding effects of volumes with large motion deflections or intensity spikes. Single-subject BOLD fMRI data were only included in analyses if there was a minimum of 85% VS coverage using a bilateral, anatomically-based VS region of interest (ROI). Participants with less than 80% task responding were excluded from analysis (Murray et al., Reference Murray, Shaw, Forbes and Hyde2017).
BOLD fMRI data analysis
Linear contrasts employing canonical hemodynamic response functions were used to estimate condition-specific BOLD activation for each individual. Individual contrast images were used in second-level random effects models to determine mean reward-related reactivity using one-sample t tests for: reward anticipation>baseline and reward outcome>baseline. Baseline was defined as the last 3 s of the 9-s inter-trial interval as previously described (Nusslock et al., Reference Nusslock, Almeida, Forbes, Versace, Frank, Labarbara, Klein and Phillips2012). As the VS is central to reward processing (Haber and Knutson, Reference Haber and Knutson2010), we examined results within the VS ROI masking for main effects of the task using a Family-Wise Error (FWE) correction. A bilateral VS ROI was constructed in the WFU PickAtlas Tool v2.4 using two spheres of 10 mm radius around MNI coordinates x = ± 12, y = 12, z = −10.
Analytic strategy
Deriving trajectories of AUD adolescence
To measure trajectories of alcohol use across adolescence, we used latent growth curve modeling (LGCM) in Mplus version 7.2 (Muthén and Muthén, Reference Muthén and Muthén2014) using alcohol frequency scores at ages 11, 12, 15, and 17 (online Supplementary Table S2). We estimated latent factors for the intercept, slope, and quadratic slope. Comparative Fit Index (CFI: cut-off value 0.95), and Root Mean Square Error of Approximation (RMSEA: cut-off value 0.06) were used to assess model fit. We compared a no-change (intercept-only) model, linear model, and quadratic model, judging the best-fitting model based on CFI and RMSEA values and whichever had the lowest Bayesian Information Criterion (Hu and Bentler, Reference Hu and Bentler1999).
Adolescent alcohol use, brain reward-related functioning, and AUD in adulthood
First, regression analysis was performed in SPM8 to determine whether adolescent alcohol use was associated with neural response during reward anticipation or outcome. We first ran a model where we included only starting levels of alcohol, race, and income (i.e. ‘no covariates models’). Second, we ran a model including the following covariates: race, income, alcohol starting levels, impulsivity, extraversion, psychiatric diagnoses, and adolescent marijuana and nicotine use (Table 1 presents descriptive data).
To link differences in neural response to reward to later alcohol use, we used two approaches. First, we employed conjunction analysis in SPM8 (Nichols et al., Reference Nichols, Brett, Andersson, Wager and Poline2005) to determine whether AUD symptoms at age 22 were related to reward responsivity in overlapping regions associated with adolescent alcohol use. Second, as conjunction analyses cannot test indirect effects, we tested a path model in Mplus to examine whether adolescent alcohol use was related to AUD symptoms at age 22 via brain reward-related functioning at age 20. To avoid double correlation issues inherent in extracting data from SPM8 based on our variables of interest (i.e. alcohol use) (Kriegeskorte et al., Reference Kriegeskorte, Simmons, Bellgowan and Baker2009), we extracted left and right VS peaks within an anatomical VS mask from the main effects of the reward task using the VOI tool (online Supplementary Fig. S2) (for examples of this approach, see Hyde et al., Reference Hyde, Byrd, Votruba-Drzal, Hariri and Manuck2014, Waller et al., Reference Waller, Corral-Frias, Vannucci, Bogdan, Knodt, Hariri and Hyde2016). The indirect pathway from adolescent alcohol use to AUD symptoms at age 22 via VS reactivity at age 20 was estimated as the product of the coefficients (AB) (‘Sobel test’). To test the specificity of effects to AUD, we included direct and indirect pathways via the brain from adolescent alcohol use to SUD and APD symptoms at age 22.
Results
Main effects of task in the VS
The reward task yielded robust bilateral activity within the VS ROI with significant clusters emerging for reward anticipation>baseline (online Supplementary Fig. S2) and reward receipt>baseline (online Supplementary Fig. S3) (Murray et al., Reference Murray, Shaw, Forbes and Hyde2017).
Trajectories of alcohol use across adolescence
A quadratic LGCM showed the best fit to the adolescent alcohol use data (Fig. 2). The model included significant intercept and quadratic terms, as well as a significant variance for the quadratic term. This model suggests that alcohol use increased at a non-linear, accelerating rate (Grimm et al., Reference Grimm, Ram and Hamagami2011) (Fig. 2). We extracted intercept (i.e. to control for starting level) and quadratic factor scores to examine effects on brain reward-related functioning. Similar to the alcohol LGCM, the best fitting models for adolescent marijuana and tobacco use were quadratic (results available on request). We included the quadratic factor scores for marijuana and tobacco as covariates within models to establish unique effects of alcohol use.

Fig. 2. Alcohol use accelerates across adolescence in a sample of low-income males. Note. We used latent growth curve modeling (LGCM) in Mplus version 7.2 and robust maximum likelihood (MLR) estimation based on alcohol frequency scores at ages 11, 12, 15, and 17 (see online Supplementary Table S2). We estimated latent factors for the intercept (mean starting level), slope (linear change over time), and quadratic slope (nonlinear change over time/acceleration). A quadratic LGCM showed the best fit to the alcohol use data across adolescence: χ2 = 1.52, df = 1, p = 22, CFI = 0.99, RMSEA = 0.04). The model included significant intercept (B = 0.06, s.e. = .02, p = 0.002) and quadratic (B = 0.04, s.e. = 0.01, p < 0.001) terms, including significance variance for the quadratic term (B = 0.004, s.e. = 0.001, p = 0.01). The red line represents estimated mean accelerated rate of alcohol use across time in the sample, which was estimated separately and superimposed onto individual curves to aid interpretation of findings. We examined whether latent classes existed within this overall sample trajectory, but found no evidence for distinct classes represented within either a 2- or 3- class solution.
Association between adolescent alcohol use and brain reward-related functioning
During reward anticipation, greater acceleration (i.e. quadratic factor scores) in adolescent alcohol use was associated with higher response in the left, but not right, VS at age 20 (t = 4.21, k = 7; x = −14, y = 18, z = −12, p < 0.05FWE). This association emerged in a model controlling only for initial alcohol use (intercept), race, and income (Figs 3a, b). We also found a significant association between adolescent alcohol use and higher response in the left VS in a stringent model that accounted for adolescent marijuana and tobacco use, race, income, adolescent ADHD and CD diagnoses, extraversion and impulsivity at age 20, and MDD, PTSD, GAD, SP, and APD symptoms at age 20 (t = 4.21, k = 1; x = −14, y = 18, z = −12, p < 0.05FWE). Results were specific to reward anticipation as there was no association between adolescent alcohol use and VS reactivity during reward outcome.

Fig. 3. Greater acceleration in alcohol use across adolescence predicts increased ventral striatum (VS) reactivity during the anticipation of rewards at age 20. Note. Greater acceleration in adolescent alcohol use is related to increased left VS reactivity in the left VS region of interest (ROI) (centered at peak voxel: t = 4.21, k = 7; x = −14, y = 18, z = −12, p < 0.05FWE), controlling for alcohol starting level, race, and income. A smaller cluster was also significant (not shown in figure) centered at peak voxel: t = 3.82, k = 5, x = −12, y = 10, z = −12, p < 0.05FWE). The relationship between adolescent alcohol use and increased left VS reactivity also emerged after controlling for alcohol starting level, marijuana and tobacco use across adolescence, race, family income, earlier attention deficit hyperactivity disorder (ADHD) and control disorder (CD), extraversion and impulsivity at age 20, and major depressive disorder (MDD), post-traumatic stress disorder (PTSD), generalized anxiety disorder (GAD), social phobia (SP), and antisocial personality disorder (APD) symptoms at age 20 (t = 4.21, k = 1; x = −14, y = 18, z = −12, p < 0.05FWE).
Association between brain reward-related functioning and later AUD symptoms
Conjunction analyses suggested that more symptoms of AUD at age 22 were associated with increased left VS response to reward anticipation in an overlapping region that had been associated with adolescent alcohol use based on the more stringent model and even after controlling for AUD symptoms at age 20 (t = 1.80, k = 1; x = −14, y = 18, z = −12, p < 0.05FWE).
We also tested a path model in Mplus v. 7.2 focusing on three peaks that had emerged within anatomical VS ROIs from the task main effects. There were significant direct pathways from adolescent alcohol use to increased reactivity in all three VS ROIs during reward anticipation at age 20, even controlling for their overlap (Fig. 4). From a peak in the left VS ROI, there was also a pathway from the higher response to reward anticipation to increases in AUD symptoms at age 22, but not SUD or APD symptoms at age 22, controlling for symptoms of all three disorders at age 20. The indirect path from adolescent alcohol use to AUD symptoms at age 22 through left VS reactivity at age 20 was significant at a trend level, confirming the results of the conjunction analysis within a more conservative model (Fig. 4). Finally, because traditional significance testing using p values represents a weaker form of inference, we computed a log-transformed Bayes factor (BF 10) for the indirect pathway (Dienes and Mclatchie, Reference Dienes and Mclatchie2017). The BF 10 was 3.92 indicating ‘substantial’ evidence for the alternative hypothesis (Jeffreys, Reference Jeffreys1998). That is, the data from the indirect pathway we reported are 3.92 times more likely to have occurred under the alternative hypothesis than the null hypothesis. Thus, all three approaches for testing the indirect effect converged in supporting the hypothesis that accelerated adolescent alcohol use predicted symptoms of AUD in early adulthood via reward-related VS reactivity.

Fig. 4. Accelerated alcohol use in adolescence predicts increased ventral striatum (VS) reactivity during the anticipation of monetary reward at age 20, and in turn, left VS reactivity predicts increases in alcohol use disorder (AUD) symptom severity from ages 20 to 22. Note. **p < 0.01, p < 0.05. The model shows only significant pathways but we modeled all pathways from predictors to outcomes and within-time covariance (i.e. between all measures at ages 20 and 22; see online Supplementary Table S4). We tested whether accelerated adolescent alcohol use was related to AUD symptoms at age 22 via brain reward-related reactivity (Fig. 1). The model included a quadratic factor of adolescent alcohol use extracted from latent growth curve modeling (LGCM) (Fig. 2) and three significant peaks that emerged within the anatomic VS region of interest (ROI) from the main effects of the reward task in the left and right VS (online Supplementary Fig. S1). The main variables of interest are shown in bold text. Covariates and correlated outcomes (i.e. to test specificity and uniqueness of effects) are shown in smaller, italicized, non-emphasized text. Direct paths were examined for statistical significance. Indirect pathways were estimated as the product of the coefficients (‘Sobel test’) as an index of effect size but we also present bootstrapped CI of the effects. To examine whether VS reward-related reactivity uniquely predicted increases in AUD symptoms at age 22, we controlled for AUD symptoms at age 20. To isolate effects of adolescent alcohol use, we also accounted for symptoms of other drug use disorders and antisocial personality disorder both when the functional magnetic resonance imaging (fMRI) scan was completed at age 20 and concurrently to the outcome of AUD symptoms at age 22. The model accounted for the effects of race and income. Results were unchanged when we included concurrent marijuana and tobacco use across adolescence, impulsivity and extraversion at age 20, and other comorbid psychiatric disorders at age 20.
Discussion
We provide evidence that greater acceleration in alcohol use from ages 11 to 17 is related to increased VS reactivity during reward anticipation at age 20, which in turn predicted symptoms of AUD at age 22. We accounted for a host of covariates, suggesting that adolescent alcohol use independently contributes to reward-related neural function and is not attributable to comorbid psychopathology. Moreover, the association between alcohol use and VS reactivity in early adulthood was maintained after adjusting for adolescent marijuana and tobacco use and co-occurring drug use and APD, emphasizing specificity in the effects on AUD. These findings highlight a mechanistic neural pathway through which greater adolescent alcohol use is associated with reward responsivity at age 20, leading to risk for persistent, clinically-significant AUD symptoms at age 22, a time of heightened vulnerability for AUD development (Chassin et al., Reference Chassin, Flora and King2004; Grant et al., Reference Grant, Stinson, Dawson, Chou, Dufour, Compton, Pickering and Kaplan2015).
Consistent with prior findings from community samples (Duncan et al., Reference Duncan, Duncan and Hops1994; Duncan and Duncan, Reference Duncan and Duncan1995), we found escalation in alcohol consumption across adolescence within an urban, low-income male sample, a group known to be at risk for AUD (Elliott et al., Reference Elliott, Huizinga and Menard2012; Grant et al., Reference Grant, Stinson, Dawson, Chou, Dufour, Compton, Pickering and Kaplan2015). The greater escalation in the rate of adolescent alcohol use was related to brain reward-related functioning during the period of emerging adulthood involving ongoing brain maturation (Casey and Jones, Reference Casey and Jones2010; Casey et al., Reference Casey, Jones and Somerville2011). Results are consistent with animal findings suggesting that adolescent drinking primes the brain for alcohol-related psychopathology through increased reward sensitivity (Spear, Reference Spear2016). For example, alcohol exposure during adolescence was linked to persistence of an ‘adolescent-like phenotype’ in adult rodents characterized by greater wanting of alcohol (Spear and Swartzwelder, Reference Spear and Swartzwelder2014). Several receptor systems are thought to underpin increased reward sensitivity to alcohol (Silveri and Spear, Reference Silveri and Spear2002), including the N-methyl-d-aspartate (NMDA) receptor system, expressed in striato-cortical structures, such as the VS (Schramm et al., Reference Schramm, Egli and Winder2002). This system undergoes significant developmental change during adolescence, providing a molecular mechanism through which adolescents may experience increased responsiveness to the positive aspects of alcohol (Silveri and Spear, Reference Silveri and Spear2002; Spear and Swartzwelder, Reference Spear and Swartzwelder2014). Thus, via changes to NMDA signaling, greater adolescent alcohol exposure may confer hypersensitivity of striato-cortical reward circuits, promoting adolescent-like, impulsive alcohol misuse that continues into adulthood, including AUD symptomatology (Conrod and Nikolaou, Reference Conrod and Nikolaou2016).
We also found an association between higher VS reactivity to reward anticipation at age 20 and increases in AUD symptom severity from ages 20 to 22. Although neurobiological models of psychopathology emphasize that individual differences in brain reactivity precede changes in symptomatology, only a handful of empirical studies have established such ‘neuroprediction’ pathways, including for depression (Mattson et al., Reference Mattson, Hyde, Shaw, Forbes and Monk2016) and criminal recidivism (Aharoni et al., Reference Aharoni, Vincent, Harenski, Calhoun, Sinnott-Armstrong, Gazzaniga and Kiehl2013). Our results suggest that hypersensitive reward-related neural reactivity represents a predictive biomarker of risk for AUD. At the same time, caution is warranted as our results do not preclude the possibility that pre-existing neural alterations in reward circuity predisposed individuals to adolescent alcohol misuse or AUD. This possibility is supported by studies of adolescents with a family history of alcoholism who show functional and structural differences in reward circuitry relative to healthy controls prior to alcohol use onset (Yau et al., Reference Yau, Zubieta, Weiland, Samudra, Zucker and Heitzeg2012). Although a limitation of this study is that we did not measure parent history of AUD, we did control for the effects of impulsivity and extraversion, which could be proxies for stable biologically-based predispositions for adolescent alcohol use or neural hypersensitivity to reward. Future prospective studies capable of identifying individual differences in reward circuity prior to initiation of alcohol use would help to address these issues. Notably, these issues do not apply to the neuroprediction pathway we established during early adulthood, where VS hypersensitivity to reward at age 20 predicted increases in AUD symptoms at age 22.
Finally, we found a relationship between adolescent alcohol use and increased VS reactivity to reward anticipation, but not reward receipt. The anticipation phase is associated with appetitive processing, or ‘wanting’ reward, whereas the receipt phase is linked to consumption, or ‘liking’ a gained reward (Robinson and Berridge, Reference Robinson and Berridge1993). Our results are consistent with a recent study that used a similar task, reporting increased VS activation during the anticipation of monetary reward among alcohol-dependent adults v. healthy controls (Becker et al., Reference Becker, Kirsch, Gerchen, Kiefer and Kirsch2016). However, findings differ in direction from studies of adult alcoholics, who are typically reported to show reduced VS activation during monetary reward anticipation (Wrase et al., Reference Wrase, Schlagenhauf, Kienast, Wüstenberg, Bermpohl, Kahnt, Beck, Ströhle, Juckel and Knutson2007; Beck et al., Reference Beck, Schlagenhauf, Wüstenberg, Hein, Kienast, Kahnt, Schmack, Hägele, Knutson and Heinz2009). Thus, a developmental dissociation may exist, such that adolescent drinking initially primes the brain for increased response to non-substance and substance-associated reward cues. However, following chronic alcohol exposure associated with alcohol dependency, a ‘hijacking’ of the reward system via dopaminergic modulation of signals in the VS may occur that ‘flips’ motivational responses specifically to non-substance related rewards (Robinson and Berridge, Reference Robinson and Berridge1993; Forbes et al., Reference Forbes, Rodriguez, Musselman and Narendran2014; Deserno et al., Reference Deserno, Beck, Huys, Lorenz, Buchert, Buchholz, Plotkin, Kumakara, Cumming and Heinze2015). Future studies employing multiple follow-up scans of participants across adolescence and adulthood are needed to test this developmental dissociation hypothesis.
The current study had a number of strengths, including a prospective, naturalistic design, well-established task for eliciting VS reward-related reactivity, and sophisticated quantitative modeling, all within a relatively large community sample. However, several limitations are worth noting. First, our sample consisted only of males raised in low-income, urban homes. Thus, the findings may not generalize to other populations, particularly women and/or those not living in low-income, urban environments. We focused on males because they are more likely to engage in risky behavior and drug use (Compton et al., Reference Compton, Conway, Stinson, Colliver and Grant2005; Grant et al., Reference Grant, Stinson, Dawson, Chou, Dufour, Compton, Pickering and Kaplan2015). However, there are important sex-specific volumetric developmental changes that occur in the VS across adolescence in the VS (cubic for males and linear for females) (Goddings et al., Reference Goddings, Mills, Clasen, Giedd, Viner and Blakemore2014). Accordingly, future studies of sex-balanced samples at risk for AUD are needed to explore potentially sex-specific neural pathways that impact VS reward-related responsivity and differentially increase risk for AUD. Second, the measure we used to assess alcohol and marijuana use across time had a limited frequency range (i.e. 0–2), which may have led to an underestimation of effect sizes. Future prospective studies are needed with measures that can more precisely assess alcohol consumption (e.g. AUD identification test; Bush et al., Reference Bush, Kivlahan, McDonell, Fihn and Bradley1998). Finally, although we assessed symptoms of AUD using a standardized clinical interview with extensive training and oversight of interviewers, inter-rater reliability of interviews was not assessed.
The current study is the first to use a large community sample of low-income, racially diverse males to examine how individual differences in the rate of alcohol use across adolescence impact later functioning of mesolimbic reward circuitry and risk for AUD. We found that greater acceleration in adolescent alcohol use was related to increased VS reactivity during reward anticipation. In turn, this pathway predicted increases in AUD symptoms during early adulthood. Results provide evidence to support the hypothesis that greater acceleration in adolescent alcohol consumption is related to hypersensitivity of the brain to reward anticipation and in turn, clinically-significant alcohol use, which persists into adulthood. Thus, increased VS reward-related reactivity may represent a biological mechanism to be targeted within novel strategies for more effective treatment of AUD.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003329171800137X
Acknowledgements
This research was supported by Grant R01 MH050907 from the National Institutes of Health to Daniel S. Shaw, Grant R01 DA02622 to Daniel S. Shaw and Erika E. Forbes. Rebecca Waller was supported by a NIAAA T32 Fellowship in the Addiction Center, Department of Psychiatry, University of Michigan (2T32 AA007477-24A1). The authors thank the staff and study families of the Pitt Mother and Child Project.
Financial support
All authors report no biomedical financial interests or potential conflicts of interest.